Overview (Steven B. Heymsfield)
The American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Research Workshop, “Advances in the Science and Application of Body Composition Measurement,” was held on January 29, 2011, in Vancouver, British Columbia. The conference brought together experts across the spectrum of the rapidly advancing field of body composition and human metabolism research. The 1-day meeting was organized to cover developments in the 3 key areas of body composition research, methodology, models, and clinical observations/ applications.1,2 Each speaker highlighted the respective field's current status, limitations, and future research directions. This report provides a summary of each speaker's presentation with selected references.
Advances in Methodology
Air Displacement Plethysmography (David Fields)
Air displacement plethysmography (ADP) measures body volume using Boyle's law, which describes the inverse relationship between volume and pressure under isothermal conditions. In 1995, seminal work by Dempster and Aitkens3 described the physical structure and basic operating principles of the first commercially available ADP device (ie, Bod Pod; COSMED USA, Inc, Concord, CA). ADP was first validated in an adult population in 1995,4 and in 2000, the device was validated in children (aged ≈10–12 years).5 In the late 1990s and early 2000s, numerous studies began to appear in the literature reporting on the validity, reliability, and feasibility of ADP in a wide spectrum of populations (eg, obese, pediatric, athletic, and elderly) against more commonly used techniques (eg, hydrostatic weighing, total body water, dual energy X-ray absorptiometry [DXA], and multicompartment models). To date, 4 reviews have been written on ADP, and in each case, the consensus is that ADP is a valid tool for the determination of body composition. ADP is a reliable and valid technique for many populations, including children, the elderly, obese subjects, and athletes. More studies using multicompartment models as a reference standard are needed. Sources of variation between ADP and other methods remain unknown and should be studied further.
In 2003, a new and potentially exciting development occurred in the field. COSMED USA, Inc developed an ADP (ie, Pea Pod) device that can measure body composition in infants starting at birth going up to 8 kg.6 The Pea Pod is still relatively new, but it holds promise as a viable tool in measuring whole-body composition in infants. More studies using multicompartment models as a reference standard are needed. Potential sources of variation (eg, movement and crying) at this time remain unknown.
ADP is an attractive tool in the assessment of body composition for the following 3 reasons: (1) accommodates both obese (≈159 kg) and very tall subjects (≈2 m), (2) technology covers the life span (birth to adulthood), and (3) compliance is generally high, even in pediatric populations. In conclusion, ADP is a valuable technique in the evaluation of body composition in a wide spectrum of populations.
Dual-Energy X-Ray Absorptiometry (John A. Shepherd)
DXA is primarily used to derive the mass of one material in the presence of another through knowledge of their unique X-ray attenuation at different energies. Two images are made from the attenuation of low and high average X-ray energy. DXA is a special imaging modality that is not typically available in general-use X-ray systems because of the need for special beam filtering and near-perfect spatial registration of the 2 attenuations.7 DXA's primary commercial application has been to measure bone mineral density as an assessment of fracture risk and to diagnose osteoporosis, and the X-ray energies used are optimized for bone density assessment. However, the whole body can also be scanned to measure whole-body bone mass and soft tissue body composition.8,9 Reference populations have been scanned and defined by sex, ethnicity, and age. The largest study for body composition in the United States was the National Health and Nutrition Survey (NHANES) that scanned 22,000 participants from 8–85 years old.10 Currently, there are estimated to be >50,000 whole-body DXA systems in use worldwide.
Current state of the art
DXA systems are currently capable of scanning a very broad range of weights, from neonates (approximately 1 kg) to morbidly obese (205 kg). The repeatability is also very high for all reported total body measures. The repeatability for percent fat measures is typically better than 1% (standard deviation) and 2% (coefficient of variation) for total fat and lean mass measures.11 In addition, whole-body DXA scans can be subdivided into arms, legs, trunk, head, android, and gynoid soft tissue regions to report all bone and soft tissue measures within the region. The dose of a DXA whole-body scan is very low in comparison to other X-ray imaging models. One whole-body DXA is <10 μSv (8 μSv = 1 day's background radiation). However, DXA systems do not currently provide accurate tissue compartmental measures. For example, in the abdomen, DXA can only report abdominal fat and cannot distinguish between visceral and subcutaneous fat because they overlay and have the same X-ray attenuation properties.12
Another unresolved issue is the soft tissue calibration standard for DXA. Currently, there isn't a phantom that can be used to cross-calibrate DXA systems between manufacturers or a standard of accuracy of percent fat. There has been some success at representing muscle mass as appendicular lean mass in just the legs and arms.13 However, it has yet to be shown as a reasonable surrogate of muscle strength or function. Another area of keen interest is the low-dose measure of breast composition in young girls and women to study breast cancer risk.14 With the ease of use, availability, and safety of DXA, there is much interest in using the technology for studies of catabolic diseases, obesity, and bone density. Future directions for DXA may be to develop more sophisticated models of visceral and muscle fat.
In summary:
Provides direct measure of fat and lean mass through X-ray attenuation
Is in broad clinical use worldwide in a variety of settings from radiology departments to exercise/physiology labs
Is clinically useful in a variety of patient body sizes, including neonates to the morbidly obese up to 450 lbs
Is one of the few methods with a large amount of reference population data, including a 22,000-person random sampling of the entire U.S. population by zip code in the NHANES
Has a very high test-retest precision of approximately 1% for most body composition measures
Future directions for DXA may include the development of more sophisticated models of visceral and muscle fat. Currently, the image profile of muscle and subcutaneous fat is not used for modeling the 3-dimensional nature of these compartments. With 3-dimensional modeling, separation between overlapping compartments, such as visceral and subcutaneous fat, may be possible.
Bioimpedance Spectroscopy (Carrie P. Earthman)
Bioimpedance spectroscopy (BIS) and multifrequency bio-electrical impedance analysis (MF-BIA) can theoretically provide estimates of fluid compartments (extracellular [ECW], intracellular [ICW], and total body water [TBW]) and body cell mass (derived from ICW), which may be used by clinicians as part of nutrition assessment.15
BIS and MF-BIA devices measure impedance to the flow of a weak current applied to the body.16 At low frequencies, impedance is purely resistive, reflecting ECW. At higher frequencies, the current can completely penetrate cells, and the impedance measured reflects TBW.15 BIS devices apply the current over a spectrum of frequencies. Impedance data are then fit to the Cole model, and extracellular and intracellular resistance (Re and Ri) may be applied to prediction equations (BIS Cole), or they can be applied to equations derived from Hanai mixture theory (BIS Cole/Hanai) to generate ECW and ICW estimates.17,18 MF-BIA applies impedance data from 2–7 frequencies to equations originally derived through statistical regression of impedance and other variables against multiple-dilution reference data. Both BIS and MF-BIA methods involve assumptions that may be violated under certain clinical conditions, and thus they must be validated against reference methods. A number of studies have evaluated BIS Cole/Hanai, BIS Cole, and various MF-BIA methods against reference methods for measuring fluid volumes in clinical populations.19-21 Errors have been observed to correlate with body mass index (BMI); thus, a modification of the BIS Cole/Hanai method that adjusts for BMI, termed body composition spectroscopy, was developed and evaluated in healthy individuals and individuals on dialysis, with subsequent evaluation and development.19 Although some studies have demonstrated that BIS and MF-BIA methods can produce estimates of fluid volumes that are in reasonable agreement with reference estimates in healthy individuals and some clinical populations, more variable results have been observed in studies of individuals with alterations in fluid distribution and body geometry. Some have observed that the BIS Cole/Hanai method can detect changes in fluid volumes in HIV-infected individuals and in critically ill patients, but it was unable to accurately detect fluid changes in obese individuals undergoing weight loss.
Although variability at the individual level observed in most studies makes these methods seem less useful in the clinic for measurement of absolute volumes, they continue to hold promise. Development and cross-validation of population-specific constants and equations may improve individual estimates by BIS and MF-BIA. Novel applications of these techniques include the use of the ratio of impedance at 200 KHz to 5 KHz for the prediction of disease severity, clinical outcomes, and the evaluation of dry weight, as well as the use of segmental measurements to evaluate fluid volume changes after dialysis and to evaluate lymphedema. It is certainly possible that the use of these bioimpedance techniques can contribute useful information to the total clinical picture, thus facilitating patient management. BIS and MF-BIA methods remain of significant interest to clinicians and researchers for the assessment of body composition parameters that may reflect nutrition status or clinical outcomes, and ongoing research and development of these methods are warranted.
In summary:
Although MF-BIA (with an appropriate equation) and BIS techniques can provide reasonably accurate whole-body fluid measures in healthy normal-weight people, there have been mixed results in clinical populations (variability at individual level particularly problematic for clinic use).
- BIS Cole/Hanai method:
- Has best potential but needs refinement particularly for populations with abnormal body geometry (eg, obesity)
- May be better for measuring changes (>2 kg) in patients with stable fluid and electrolyte balance (eg, HIV)
- Improved results with BMI correction (body composition spectroscopy20)
Both MF-BIA and BIS are being used by some to monitor fluid status and dry weight
Both MF-BIA and BIS are being used by some fluid status and dry weight
Future directions:
Further refinement of the BIS Cole/Hanai method is needed.
Population-specific resistivity constants and other adjustments may improve the accuracy of the BIS Cole/Hanai method.
Segmental approach may improve estimates in patients with abnormal body geometry or hydration status.
Additional research is needed to evaluate use of the impedance ratio Z200/Z5 for assessing dry weight and for predicting disease severity.
Development and validation of algorithms for using MF-BIA or BIS data are needed to identify malnourished patients.21
With refinement, these methods can provide information that may be used to enhance nutrition assessment.
Quantitative Magnetic Resonance (Antonella Napolitano)
Quantitative magnetic resonance (QMR) is a technique that has been validated in rodents to measure body composition precisely and accurately.22 Differences in the nuclear magnetic resonance properties of hydrogen atoms in organic and nonorganic environments allow the fractionation of signals originating from fat and lean tissue and free water. In 2006, this technology was scaled for adult human application (QMR Echo-MRI; Echo Medical, Houston, TX).23 It has been shown that fat mass measurements are highly correlated with those estimated by the 4-compartment (4C) model, and QMR measurements underestimated fat mass in all subjects. The discrepancies were higher for male subjects with higher BMI. This reduced accuracy, however, is balanced by the high precision of the repeated measurement of fat mass that is possible with this technology (coefficient of variation [CV] <0.5%24; SD ±0.13 kg24), surpassing all the other available methodologies. Recently, QMR has been validated also for pediatric use.25
An important open question relates to the performance of this novel methodology when measuring TBW. Several experimental paradigms were investigated: (1) comparison to D2O dilution, (2) ability to detect a volume of infused saline, and (3) ability to detect water removal by hemodialysis. When TBW was compared against D2O dilution measurements in healthy volunteers, QMR measurements underestimated absolute values, and this bias appeared to be related to fat mass (greater bias for subjects with greater fat mass). In addition, the current version of the instrument cannot detect fluid shifts ≤1 L. Thus, QMR is a valuable method for quantifying small changes of fat mass in longitudinal interventions,26 but it is yet not capable of detecting modest changes in TBW.
In summary:
QMR has been validated in adults and children as a precise methodology to assess body fat mass changes, and the system's precision is better than other body composition methodologies.
The QMR is a simple method for measuring body composition, is convenient for subjects, and can be performed very rapidly (<3 minutes).
The high precision can be exploited to reduce substantially the cohort numbers and duration of clinical trials.
TBW and lean mass measurements, however, do not show the same degree of precision (and accuracy) and appear to be biased; more studies of these relations are needed.
On the base of the data acquired so far, it is still uncertain whether fat and water mass measurements are completely independent of each other.
Magnetic Resonance Imaging/Spectroscopy (Wei Shen)
Magnetic resonance imaging and spectroscopy (MRI and MRS) has been increasingly used to study human body composition and related physiological and pathological conditions. MRI can measure the volume of body components, including adipose tissue, skeletal muscle, organs, and bone. Recent advances suggest that adipose tissue is not a homogeneous depot but rather contains distinct adipose tissue components with different metabolic activities. Advances in MRI technology have made it possible to quantify subregions of adipose tissue depots such as visceral adipose tissue (ie, omental, mesenteric adipose tissue, and extra-peritoneal adipose tissue), intermuscular adipose tissue, and bone marrow adipose tissue.27 Standardizing the protocols and testing the reproducibility of each MRI measurement method is important to develop reliable MRI quantification methods.28,29
Because whole-body MRI scans are time-consuming to analyze, it is advantageous to optimize single-slice protocols, especially for clinical studies.30,31 Recent studies have shown that a single slice in the upper abdomen not only provides the best representation of total volume of visceral adipose tissue but also correlates with health risks even more closely than the traditionally used slice located at the L4–L5 level.
MRI measured body composition has been used to answer a wide spectrum of clinical and research questions, including those related to obesity, osteoporosis, resting energy expenditure, and sarcopenia. Both water-fat imaging and 1H MRS methods can measure organ fat, including fat content in muscle, liver, and pancreas. When there is elevated adipose tissue infiltration, MRS imaging provides a more accurate measurement of intramyocellular lipid than single-voxel MRS. The advantage of multinuclei MRS is its ability to measure many chemical compounds and metabolites in brain, skeletal muscle, or liver concurrently and therefore may possess the potential to answer unique questions.
Short-Term Changes: Balance Methods (Leanne M. Redman)
Energy balance or weight maintenance occurs when energy intake is equal to energy expenditure. Therefore, in energy balance, body energy stores (fat mass and fat-free mass [FFM]) are not changing. The macronutrient balance theory proposed by Flatt,32 however, suggests that energy balance or long-term weight maintenance is achieved when protein, carbohydrate, and fat balances are all close to zero. This corresponds to a situation not only where energy intake equals energy expenditure but also when the composition of the fuel mix oxidized (ie, the respiratory quotient) is equal to the composition of the fuel mix consumed in the diet (ie, the food quotient). Because protein balance is achieved on a daily basis (except during severe protein restriction or high protein intake in addition to strength training) and carbohydrate and fat provide the majority of energy intake, weight maintenance is primarily a function of carbohydrate and fat metabolism. Short- and long-term studies of carbohydrate and fat balance measured in a respiratory chamber show that consumption of dietary carbohydrates induces a proportionate increase in carbohydrate oxidation,33 whereas consumption of dietary fat does not promote an analogous increase in fat oxidation.34,35 Therefore, the presence of even a small amount of carbohydrate in a high-fat meal spares the oxidation of fat, leading to deposition of the excess dietary fat intake in fat stores. Twenty-four energy balance studies in normal-weight men and women thus reveal that energy balance is positively correlated with fat balance even though energy balance is not related to either carbohydrate or protein balances.36 Therefore, the inability of the body to oxidize excess dietary fat over time (a positive fat balance) can lead to increased body fat stores and body weight.37 Studies in Pima Indians show that independent of energy expenditure, a low ratio of fat to carbohydrate oxidation (leading to a positive fat balance) is associated with subsequent weight gain.38 Furthermore, physical activity can attenuate the positive fat balance observed in response to increased dietary fat intake.39 Given the tight association between fat balance and energy balance, short-term changes in body composition can therefore be measured with indirect calorimetry with assessments of fat and carbohydrate balances from precise measures of dietary macronutrient intake, carbohydrate and fat oxidation rates, and protein balance from urinary nitrogen production.
Short-Term Changes: Balance Techniques (Manfred J. Müller)
A major challenge for current in vivo body composition analysis (BCA) techniques is the valid assessment of small body composition changes in response to changes in energy balance. Acute changes in energy balance are associated with an unstable (ie, a nonsteady state) condition of body composition that is mainly attributed to shifts in fluid or glycogen balance as shown by water, sodium, and carbohydrate balance. However, 2-compartment or criterion methods such as densitometry or DXA require a constant density and hydration of lean mass. The measurement of energy balance from energy/macro-nutrient intake, together with energy expenditure and macronutrient oxidation, combined with urinary nitrogen excretion, aims at assessing small and short-term changes in body composition and may serve as a “gold standard” for in vivo BCA techniques.
The validation of in vivo BCA techniques against energy and nitrogen balance has been attempted in only a few studies.40-43 When used to estimate body composition changes, balance techniques had high accuracy; errors in estimates of fat oxidation assessed within a respiration chamber were around 9.5 g/d.43 Balance techniques were most sensitive to changes in fat mass, with a precision of about 0.030 kg,41,43 0.26 kg,42 and 0.71 kg.40 Estimates of fat loss in response to diet in obese women were similar— that is, 2.77 kg (from calorimetry with correction for nitrogen loss), 2.83 kg (based on densitometry), 2.37 kg (from determination of total body water by deuterium oxide), and 2.90 kg (based on measurements of total body potassium).40 When comparing balance data against in vivo BCA,43 the bias in estimates of fat mass was similar in magnitude with differences in the direction (eg, –0.275 kg for densitometry, +0.330 kg for total body water, –1.00 kg for total body potassium), whereas the bias of 2 different 3-compartment models was 0.008 or 0.045 kg, suggesting the value of multicomponent models.
More recently, we performed controlled feeding studies in a group of 10 healthy, normal-weight men (aged 24.9 years) participating in 2 cycles of controlled 7-day periods of caloric restriction and refeeding and overfeeding, as well as caloric restriction at ±60% energy requirement.44 During caloric restriction, mean cumulative body weight changes over the 7-day periods were –3.0 kg, with subjects returning to their baseline body weight at the end of subsequent refeeding (+3.1 kg). These changes were accompanied by a mean 2.2-kg decrease in fat mass (as assessed by densitometry), with values approximating baseline values following refeeding (+1.4 kg). During overfeeding, weight gain of 1.6 kg (P < .01) was followed by a 3.4-kg decrease in body weight. Fat mass trended toward similar changes. Cumulative 7-day energy balance was similarly negative during both underfeeding periods (–38.6 MJ vs –40.2 MJ) and positive during both over-feeding periods (54.1 MJ vs 52.5 MJ), respectively. Nitrogen balance was –28 and –143 g/7 days during caloric restriction and +140 and +108 g/7 days during refeeding and overfeeding, respectively. Changes in energy balance correlated with changes in fat mass (r = 0.70, P < .001). In addition, changes in FFM correlated with changes in nitrogen balance (r = 0.59, P < .001).
However, during undernutrition, densitometry-derived estimates of fat mass exceeded changes in fat mass predicted from energy balance (assuming that 100%, 75%, or 50% of body energy content lost or gained is lost or gained as body fat). By contrast, changes during overfeeding were underestimated. The minimal detectable change in fat mass was 1.8 kg using densitometry. The exceptionally high precision of QMR technology provides a great potential for quantifying small changes in body composition in protocols following over- and underfeeding. Although the decrease in fat mass with underfeeding correlated with the change in energy balance (r = 0.97, P < .001), the absolute changes in fat mass were unsound, suggesting that further validation studies are needed. Following the individual courses of energy balance and estimates of fat mass (either by densitometry or QMR), there was high inter- and intraindividual variance in the data.
In summary, balance studies seem to be more accurate when measuring small changes in body composition but are also cumbersome and limited by labor intensity. These studies can be used to assess short-term changes in body composition, but current in vivo BCA techniques should be referred to the steady-state situation only (ie, in a weight-stable or weight-stabilized situation).
Advances in Models
Energy Expenditure (Dympna Gallagher)
The use of FFM as a single and homogeneous tissue, or compartment, ignores the fact that the multiple organs and tissues that comprise FFM each have a different metabolic rate. Compared to the resting metabolic rate of skeletal muscle (14.5 kcal/kg/d), the metabolic rate of heart and kidneys is 33-fold higher (440 kcal/kg/d), brain is 18-fold higher (240 kcal/kg/d), and liver is 15-fold higher (200 kcal/kg/d). The presented data highlight the important contributions that these high metabolic rate organs have on resting energy expenditure (REE) and support the notion that although they comprise a minor portion of total FFM, much of the variation in REE commonly thought attributable to sex, race, and even age can be explained by variation in the components of FFM, specifically these select high metabolic rate organs.
REE prediction equations are typically modeled based on the energy requirements of 2 distinct body composition compartments: fat or adipose tissue and FFM or adipose tissue–free mass, which have markedly different specific energy requirements. In brief, FFM is the principal contributor to energy requirements and is commonly used as a surrogate for metabolically active tissue. However, this practice is inherently flawed as it pools together numerous organs and tissues that differ significantly in metabolic rate. The brain, liver, heart, and kidneys alone account for approximately 60% of REE in adults, but their combined weight is <6% of total body weight or 7% of FFM.45-48 The skeletal muscle component of FFM comprises 40%–50% of total body weight (or 51% of FFM) and accounts for only 18%–25% of REE.45,47,48 REE varies in relation to body size across mammalian species.49,50 Within humans, REE/kg of body weight or FFM is highest in newborns (~56 kcal/kg weight/d51) and declines sharply until 4 years and slowly thereafter, reaching adult values (~25 kcal/kg weight/d51). Among adults, REE is lower in the later adult years, to an extent beyond that explained by changes in body composition.52,53 That is, the loss of FFM cannot fully explain the decrease (5%–25%) in REE in healthy elderly.
Elucidating the extent of organ and tissue atrophy has important implications for understanding REE changes with age and REE-related diseases such as obesity.54,55 Autopsy data have shown a linear decline in organ weight with increasing age for the brain, liver, and kidneys, whereas weight for the heart increased with age.56 We confirmed these findings in healthy African American and white adults (aged 19–88 years) using in vivo MRI-derived organ measures (ie, older people have a smaller mass of brain, kidneys, liver, and spleen but not the heart compared to younger subjects).57 Our findings demonstrate that age has a significant effect on these organs, and the effect of age is consistent across sex and across the race groups studied.
A question of importance to understanding the determinants of REE is how much additional variability in REE can be accounted for by distinguishing between high (brain, heart, liver, kidneys, spleen, or skeletal muscle) and low metabolic rate tissue components vs a measure of undifferentiated FFM as a single component. In a study of healthy adults, we found that 5% of the 30% variability in REE that remains unexplained by models using undifferentiated FFM as a single component can be accounted for by distinguishing between select high and low metabolic rate tissue components.58 Moreover, these data showed that the significant race and age effects present in the undifferentiated FFM model become statistically nonsignificant when the mass of high metabolic rate organs (HMRO) is taken into consideration. The latter demonstrates that differences in the mass of these HMRO with increasing age and across race groups are important independent determinants of REE. A novel finding of this study was that adding brain mass to the prediction of REE explained an additional 2% of the variance and rendered the age effect statistically nonsignificant.
A perplexing and implausible finding from published FFM-derived REE prediction equations has been the positive intercept that exists, thereby inferring that a component of REE remains when FFM or body mass is extrapolated to zero. Specifically, the positive intercept can vary from 186–662 kcal/d with slopes varying from 19.7–24.5 kcal/kg FFM/d as previously summarized.59 We investigated whether the prediction of REE with specific tissue/organ measures included in the REE prediction models rendered the intercept not different to zero.58 Only with the inclusion of brain mass was the REE prediction equation not different from zero, implying that when body mass is zero, REE is zero. The addition of brain mass reduced the intercept from 560 kcal/kg/d (REE = Age + Sex + Race + Fat + FFM + HMRO – trunk) to 69 kcal/kg/d (REE = Age + Sex + Race + Fat + FFM + HMRO – trunk + brain), thereby highlighting the importance of this single organ to the prediction of REE. The brain has one of the highest specific metabolic rates (240 kcal/kg/d) and is thus a good representation of a high metabolic rate organ.60
Specific to changes in REE with weight loss, evidence suggests that weight loss leads to a reduction in REE beyond that explainable by losses in fat and FFM.61,62 Bosy-Westphal and colleagues (2009)63 found that a 10% weight loss in young overweight and obese women was associated with changes in high metabolic activity organ weights (liver, heart, and kidneys) of between 4% and 6%, which exceeded the loss in total FFM (2.6%). In contrast, no change was observed for brain mass. With respect to changes observed in REE, this 10% weight loss in overweight and obese women resulted in a significant decrease in REE (7.7%), of which 47% was explained by losses in FFM and FM and an additional 13% to changes in individual organ and tissue mass (60% total explained by body composition). The authors ascribed the remaining 40% decrease in REE to be due to adaptive thermogenesis.
There is also a growing interest in understanding the REE of overweight and obese individuals because the overweight and obese groups constitute an increasing proportion of the population. Even though adipose tissue has a low rate of energy expenditure (4.5 kcal/kg/d), its mass varies more than all other major tissues in the body.64 Despite the low metabolic rate of adipose tissue, it is notable that fat mass remained a significant contributor to REE in all REE prediction models, including those with all organs.58 In studies examining changes in REE for subjects undergoing significant weight or fat loss, the relative variance induced by this tissue component alone needs to be considered.
Dynamic Energy Balance Models: Health (Diana Thomas)
This report analyzes the impact of various FFM–fat mass models on the resulting half-life and steady state yielded by an energy balance model. Although the significance of body composition influence on weight loss has been experimentally observed and examined quantitatively by considering static amounts of weight change,65,66 this is the first attempt to analyze the combined effects of body composition on time-varying weight loss. Through this analysis, we establish that the half-life is highly sensitive to baseline body composition.
Current state of the art
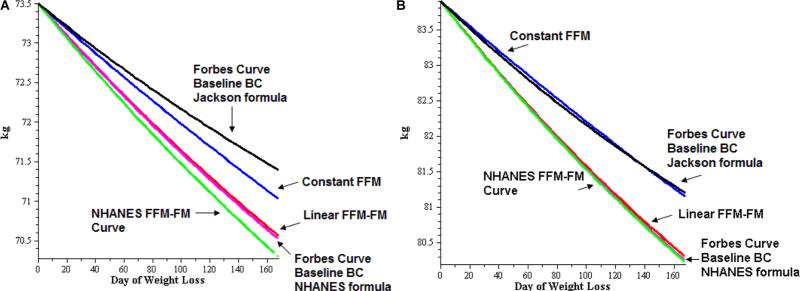
Four different FFM–fat mass models have been employed within an energy balance equation67—specifically, constant, linear, the Forbes relationship, and a model derived from the 1999–2004 NHANES data. It was found that half-life was sensitive to the choice of model used to determine baseline body composition (Table 1, Figure 1). The weight loss curves in Figure 1 are generated for the average NHANES woman (Figure 1A) and man (Figure 1B) using different body composition formulas within the core energy balance equation.
Table 1.
Eigenvalues, Half-Life, and Steady States Resulting From Different Choices of FFM-FM Model Within a Core Energy Balance Equation67
| Eigenvalue |
Half-Life, d |
Steady State, kg |
|||||
|---|---|---|---|---|---|---|---|
| FFM-FM Model | M | F | M | F | M | F | Characteristics |
| Constant FFM | –0.00126 | –0.00126 | 551 | 551 | 74.8 | 65.3 | Constant eigenvalue |
| Linear FFM | –0.00323 | –0.00323 | 215 | 215 | 75.1 | 65.5 | Constant eigenvalue |
| Forbes curve66 with baseline body composition90 | –0.00328 | –0.00245 | 211 | 283 | 75.2 | 65.5 | Eigenvalues dependent on gender |
| Forbes curve66 with baseline body composition89 | –0.00344 | –0.00254 | 202 | 273 | 77.6 | 67.9 | Eigenvalues dependent on gender |
| NHANES curve90 | –0.00334 | –0.00287 | 208 | 242 | 74.9 | 64.7 | Eigenvalues dependent on age, height, and gender |
F, female; FFM, fat-free mass; FM, fat mass; M, male; NHANES, National Health and Nutrition Examination Survey.
Figure 1.
Weight loss curves generated for the average National Health and Nutrition Examination Survey (NHANES). (A) woman and (B) man using different body composition formulas within the core energy balance equation. The Forbes curves apply baseline body composition (BC) estimates from the Jackson89 and NHANES formulas.90,91 The NHANES fat-free mass (FFM)-fat mass (FM) formula,90,91 the linear FFM-FM formula, and the constant FFM formula curves are from Thomas et a1..90,91 Weight (kg) appears on the y-axis, and day of weight loss is on the x-axis.
Unresolved questions and future directions
The 2 advanced models, Forbes and the NHANES-based models, are developed from relatively sedentary subject data. FFM– fat mass models in highly active individuals should be compared to longitudinal data where physical activity is increased or decreased. It should be determined whether longitudinal data travel along a cross-sectional relationship, and if not, the reasons should be investigated.
Dynamic Models of Macronutrient Metabolism and Body Composition Change (Kevin D. Hall)
We know a great deal about how diet and physical activity affect various aspects of energy metabolism, body weight, and body composition. But integrating this knowledge to make quantitative predictions is a formidable task given the complex interactions between the metabolism of fat, carbohydrate, and protein, as well as adaptations of energy expenditure and nonlinear changes of body composition.
Current state of the art
Several mathematical models have recently been developed by my research group to integrate whole-body metabolism data with body composition data in an attempt to better understand these complex interactions and make quantitative predictions about how diet modifications result in adaptations of fuel selection and energy expenditure.68-71 These models accurately predict changes of body weight and body composition in both obese and nonobese men and women and provide several useful insights regarding human metabolism and body weight regulation.
Unresolved questions and future directions
Although mathematical models of metabolism and body composition change are becoming increasingly realistic, several aspects of these models remain phenomenological rather than mechanistic. For example, the mechanisms underlying the metabolic adaptation to reduced energy diets require elucidation. Furthermore, although cross- sectional data exist describing the relationship between organ size and FFM, longitudinal relationships are required to quantify the impact of changes in organ size on resting metabolic rate during weight gain and loss.
Advances in Clinical Observations/ Applications
Aging (Paolo Caserotti)
Aging is associated with profound changes in body composition, including loss of skeletal muscle mass (sarcopenia), progressive increase in total body fat during adulthood followed by a loss later in life, and remodeling of fat distribution.72 The latter includes an increase in intermuscular fat, lipid content in the muscles identified by lower Hounsfield units during computed tomography (CT) imaging (ie, muscle attenuation), visceral fat, and a progressive loss of subcutaneous fat.72,73 In addition, weight gain and weight loss over time have recently been associated with preserved muscle mass and accelerated muscle atrophy, respectively; however, both weight gain and loss are associated with the increase in fatty infiltration of muscle.73 Overall, these changes seem to be jointly or independently associated with negative health and functional outcomes such as insulin resistance and impaired mechanical muscle function.74
Since the work of Baumgartner et al in 199872 that demonstrated the association of low muscle mass and functional impairment, the role of sarcopenia in understanding the pathway to physical disability has been critical and has received considerable attention. Nevertheless, recent evidence suggests that the correlation between muscle “quantity” and “muscle function” (eg, muscle strength) is relatively weak, and in contrast to muscle strength, muscle mass has been demonstrated to be a poor predictor of functional limitation, gait speed, and even mortality.74,75
It is becoming increasingly clear that muscle quality (force per unit of muscle mass) and neural function play an essential role in the disabling pathway and that a new end point incorporating these aspects in addition to muscle mass is needed.76,77
Chronic Disease/Cancer (Vickie Baracos)
Noting the acquisition of some 30,000 CT images per year in a typical cancer center, we proposed the opportunistic use of these high-quality images that are readily available to provide accurate and practical studies of body composition across the cancer trajectory. Recent work by our group exploiting CT images for body composition analysis has revealed the natural history of cancer cachexia, including progressive alterations in skeletal muscle, adipose tissue, organs, and tumor mass.78,79 Our research group has undertaken a prospective cohort study of body composition in patients with advanced cancers of the lung and gastrointestinal tract at a cancer center that serves northern Alberta, Canada.80,81 Consecutive patients (n = 1473) have been assessed by CT at a standardized vertebral landmark (third lumbar vertebra). This cross-sectional analysis provides population demographics of body composition at referral for cancer treatment. At referral, mean BMI was surprisingly high when framed against conventional notions of cachexia in advanced cancer: 25.6 kg/m2 with 52% of patients over-weight or obese. Only 7.5% overall were underweight as conventionally understood (BMI <18.5 kg/m2). Analysis of CT images revealed extremely high heterogeneity of muscle mass within all strata of BMI. The overall prevalence of severe muscle depletion (sarcopenia) was 41% and was present in patients in all BMI categories. Wasting of skeletal muscle is a prominent feature of lung cancer patients, despite normal or heavy body weights. The significance of muscle wasting in normal-weight, overweight, and obese patients as a nutrition risk factor, as a prognostic factor, and as a predictor of cancer treatment toxicity is discussed.82
Analysis of repeated images over time in 388 patients (a total of 1279 CT images) provides information on the natural history of the changes in muscle and adipose tissue over time. Overall, both muscle and adipose tissues were lost, and this increased in magnitude overtime until death, in an exponential fashion. Although muscle loss was common, the overall frequency of muscle gain was 15.4%, and muscle was stable in 45.6% of intervals between any 2 scans. Multinomial logistic regression revealed that being within 90 days (vs >90 days) from death was the principal risk factor for muscle loss (odds ratio [OR] = 2.68; P < .002), and muscle gain was correspondingly less likely (OR = 0.49; P < .009) at this time. Sex, age, BMI, and tumor group were not significant predictors of muscle loss or gain.
We conclude that cancer patients in contemporary populations are likely to simultaneously have high body weight and skeletal muscle wasting. Studies of the progression over time suggest a clear possibility of anabolic potential, but that anabolic potential wanes dramatically during the last 90 days of life, a period dominated by intense catabolism. This is consistent with the idea of refractory cachexia, which evolves during the terminal stages of cancer, which is no longer responsive to antineoplastic therapies.83
Catabolic Diseases (Claude Pichard)
Acute illnesses often result in a catabolic state because of metabolic stress and reduced physical activity, leading to major muscle and adipose tissue wasting and organ dys-functions.84-88 These alterations, which are characteristic of protein calorie malnutrition, increase morbidity and duration of hospital stay, as well as delay and prolong the recovery phase.
Lean tissues, also named FFM, and adipose tissues (ie, fat mass) are altered by catabolic conditions. Variations in fluid status during acute illness and related changes in body weight are difficult to evaluate and interpret during treatment. Body weight poorly reflects the size and the evolution of FFM and fat mass during catabolic diseases. Therefore, an optimal nutrition assessment should include the evaluation of FFM and fat mass changes during metabolic stress and catabolism.
Significant progress has been made in the past decade in measuring body composition, allowing the quantification of body compartments at different levels of definition: from simple 2-compartment (FFM vs fat mass) to sophisticated multiple-compartment models (molecular quantification such as body nitrogen, potassium, etc). Despite these advances, the clinical measurement of body composition remains limited to the determination of TBW, FFM, fat mass, and bone mass by bioelectrical impedance analysis, DXA, and CT, mainly because other technologies are still either too complex and expensive or too imprecise.
In conclusion, we believe that a systematic evaluation of body composition parameters (ie, TBW, FFM, and fat mass) could significantly contribute to determining patients’ overall status, improve the tailoring of diet intake or nutrition support to patients’ specific needs, and thereby significantly improve the global quality of care and the cost-effectiveness ratio.
Acknowledgments
The 2011 A.S.P.E.N. Research Workshop, “Current Advances in the Science and Application of Body Composition Measurement,” was supported by award number 5U13DK064190 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Financial disclosure: Support provided by grants DFG Bo1-1 and Mü 8-1 (Dr Müller) and grants from the Herman and Margret Sokol Institute for Pharmaceutical Life Sciences Fellowship (Dr Thomas).
References
- 1.Wang Z, Pierson R, Heymsfield S. The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr. 1992;56(1):19–28. doi: 10.1093/ajcn/56.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527–558. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- 3.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27(12):1692–1697. [PubMed] [Google Scholar]
- 4.McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27(12):1686–1691. [PubMed] [Google Scholar]
- 5.Fields DA, Goran MI. Body composition techniques and the four-compartment model in children. J Appl Physiol. 2000;89(2):613–620. doi: 10.1152/jappl.2000.89.2.613. [DOI] [PubMed] [Google Scholar]
- 6.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53(3):486–492. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 7.Kelly TL, Slovik DM, Neer RM. Calibration and standardization of bone mineral densitometers. J Bone Miner Res. 1989;4(5):663–669. doi: 10.1002/jbmr.5650040504. [DOI] [PubMed] [Google Scholar]
- 8.Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996;12(1):45–51. doi: 10.1016/0899-9007(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 9.Kelly TL, Berger N, Richardson TL. DXA body composition: theory and practice. Appl Radiat Isot. 1998;49(5-6):511–513. doi: 10.1016/s0969-8043(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 10.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard CM, Roza MA, Barr RD, Webber CE. Reproducibility of DXA measurements of bone mineral density and body composition in children. Pediatr Radiol. 2009;39(2):148–154. doi: 10.1007/s00247-008-1067-7. [DOI] [PubMed] [Google Scholar]
- 12.Snijder MB, Visser M, Dekker JM, et al. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord. 2002;26(7):984–993. doi: 10.1038/sj.ijo.0801968. [DOI] [PubMed] [Google Scholar]
- 13.Modlesky CM, Cavaiola ML, Smith JJ, Rowe DA, Johnson DL, Miller F. A DXA-based mathematical model predicts midthigh muscle mass from magnetic resonance imaging in typically developing children but not in those with quadriplegic cerebral palsy. J Nutr. 2010;140(12):2260–2265. doi: 10.3945/jn.110.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepherd JA, Malkov S, Fan B, Laidevant A, Novotny R, Maskarinec G. Breast density assessment in adolescent girls using dual-energy X-ray absorptiometry: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1709–1713. doi: 10.1158/1055-9965.EPI-08-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19(5):433–446. doi: 10.1177/0115426504019005433. [DOI] [PubMed] [Google Scholar]
- 16.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27(9):921–933. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- 18.Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys. 2008;30(10):1257–1269. doi: 10.1016/j.medengphy.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Matthie JR. Bioimpedance measurements of human body composition: critical analysis and outlook. Expert Rev Med Devices. 2008;5(2):239–261. doi: 10.1586/17434440.5.2.239. [DOI] [PubMed] [Google Scholar]
- 20.Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27(1):75–80. doi: 10.1159/000167013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wieskotten S, Heinke S, Wabel P, et al. Bioimpedance-based identification of malnutrition using fuzzy logic. Physiol Meas. 2008;29(5):639–654. doi: 10.1088/0967-3334/29/5/009. [DOI] [PubMed] [Google Scholar]
- 22.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res. 2004;12(1):150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- 23.Napolitano A, Miller SR, Murgatroyd PR, et al. Validation of a quantitative magnetic resonance method for measuring human body composition. Obesity (Silver Spring) 2008;16(1):191–198. doi: 10.1038/oby.2007.29. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher D, Thornton JC, He Q, et al. Quantitative magnetic resonance fat measurements in humans correlate with established methods but are biased. Obesity (Silver Spring) 2010;18(10):2047–2054. doi: 10.1038/oby.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andres A, Mitchell AD, Badger TM. QMR: validation of an infant and children body composition instrument using piglets against chemical analysis. Int J Obes (Lond) 2010;34(4):775–780. doi: 10.1038/ijo.2009.284. [DOI] [PubMed] [Google Scholar]
- 26.Swe Myint K, Napolitano A, Miller SR, et al. Quantitative magnetic resonance (QMR) for longitudinal evaluation of body composition changes with two dietary regimens. Obesity (Silver Spring) 2010;18(2):391–396. doi: 10.1038/oby.2009.272. [DOI] [PubMed] [Google Scholar]
- 27.Shen W, Wang Z, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11(1):5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen W, Chen J. Application of imaging and other noninvasive techniques in determining adipose tissue mass. Methods Mol Biol. 2008;456:39–54. doi: 10.1007/978-1-59745-245-8_3. [DOI] [PubMed] [Google Scholar]
- 29.Heshka S, Puryanitya M, Shen W, et al. Inter-reader reliability in reconstructing tissue volumes from magnetic resonance images. FASEB J. 2004;18(4):A177. [Google Scholar]
- 30.Shen W, Punyanitya M, Chen J, et al. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks. Int J Obes (Lond) 2007;31(5):763–769. doi: 10.1038/sj.ijo.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80(2):271–278. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flatt JP. McCollum Award Lecture, 1995: diet, lifestyle, and weight maintenance. Am J Clin Nutr. 1995;62(4):820–836. doi: 10.1093/ajcn/62.4.820. [DOI] [PubMed] [Google Scholar]
- 33.Snitker S, Larson DE, Tataranni PA, Ravussin E. Ad libitum food intake in humans after manipulation of glycogen stores. Am J Clin Nutr. 1997;65(4):941–946. doi: 10.1093/ajcn/65.4.941. [DOI] [PubMed] [Google Scholar]
- 34.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr. 1997;66(2):276–282. doi: 10.1093/ajcn/66.2.276. [DOI] [PubMed] [Google Scholar]
- 35.Schutz Y, Flatt JP, Jequier E. Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Am J Clin Nutr. 1989;50(2):307–314. doi: 10.1093/ajcn/50.2.307. [DOI] [PubMed] [Google Scholar]
- 36.Abbott WG, Howard BV, Christin L, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol. 1988;255(3, pt 1):E332–E337. doi: 10.1152/ajpendo.1988.255.3.E332. [DOI] [PubMed] [Google Scholar]
- 37.Swinburn B, Ravussin E. Energy balance or fat balance? Am J Clin Nutr. 1993;57(5)(suppl):766S–770S. doi: 10.1093/ajcn/57.5.766S. discussion770S-771S. [DOI] [PubMed] [Google Scholar]
- 38.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259(5, pt 1):E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 39.Smith SR, de Jonge L, Zachwieja JJ, et al. Concurrent physical activity increases fat oxidation during the shift to a high-fat diet. Am J Clin Nutr. 2000;72(1):131–138. doi: 10.1093/ajcn/72.1.131. [DOI] [PubMed] [Google Scholar]
- 40.Garrow JS, Stalley S, Diethelm R, Pittet P, Hesp R, Halliday D. A new method for measuring the body density of obese adults. Br J Nutr. 1979;42(2):173–183. doi: 10.1079/bjn19790105. [DOI] [PubMed] [Google Scholar]
- 41.Yang MU, Wang J, Pierson RM, Jr, Van Itallie TB. Estimation of composition of weight loss in man: a comparison of methods. J Appl Physiol. 1977;43(2):331–338. doi: 10.1152/jappl.1977.43.2.331. [DOI] [PubMed] [Google Scholar]
- 42.Almond DJ, King RF, Burkinshaw L, Oxby CB, McMahon MJ. Measurement of short-term changes in the fat content of the body: a comparison of three methods in patients receiving intravenous nutrition. Br J Nutr. 1984;52(2):215–225. doi: 10.1079/bjn19840090. [DOI] [PubMed] [Google Scholar]
- 43.Jebb SA, Murgatroyd PR, Goldberg GR, Prentice AM, Coward WA. In vivo measurement of changes in body composition: description of methods and their validation against 12-d continuous whole-body calorimetry. Am J Clin Nutr. 1993;58(4):455–462. doi: 10.1093/ajcn/58.4.455. [DOI] [PubMed] [Google Scholar]
- 44.Bosy-Westphal AL, Morgenstern A, Later W, Muller MJ. Specifics of refeeding vs. overfeeding in a strictly controlled nutritional intervention study: implications for weight regain after weight loss. Obes Rev. 2010;11:218. [Google Scholar]
- 45.Holliday MA. Metabolic rate and organ size during growth from infancy to maturity and during late gestation and early infancy. Pediatrics. 1971;47(1):169–179. [PubMed] [Google Scholar]
- 46.Grande F. Energy expenditure of organs and tissues. In: Kinney JM, editor. Assessment of Energy Metabolism in Health and Disease: Report of the First Ross Conference on Medical Research. Ross Laboratories; Columbus, OH: 1980. pp. 88–92. [Google Scholar]
- 47.Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN, editors. Energy Metabolism: Tissue Determinants and Cellular Corollaries. Raven Press; New York: 1992. pp. 61–77. [Google Scholar]
- 48.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275(2, pt 1):E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 49.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–351. [Google Scholar]
- 50.Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27(4):511–541. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- 51.Holliday MA, Potter D, Jarrah A, Bearg S. The relation of metabolic rate to body weight and organ size. Pediatr Res. 1967;1(3):185–195. doi: 10.1203/00006450-196705000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Fukagawa NK, Bandini LG, Young JB. Effect of age on body composition and resting metabolic rate. Am J Physiol. 1990;259(2, pt 1):E233–E238. doi: 10.1152/ajpendo.1990.259.2.E233. [DOI] [PubMed] [Google Scholar]
- 53.Piers LS, Soares MJ, McCormack LM, O'Dea K. Is there evidence for an age-related reduction in metabolic rate? J Appl Physiol. 1998;85(6):2196–2204. doi: 10.1152/jappl.1998.85.6.2196. [DOI] [PubMed] [Google Scholar]
- 54.Bosy-Westphal A, Eichhorn C, Kutzner D, Illner K, Heller M, Muller MJ. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J Nutr. 2003;133(7):2356–2362. doi: 10.1093/jn/133.7.2356. [DOI] [PubMed] [Google Scholar]
- 55.Puggaard L, Bjornsbo KS, Kock K, Luders K, Thobo-Carlsen B, Lammert O. Age-related decrease in energy expenditure at rest parallels reductions in mass of internal organs. Am J Hum Biol. 2002;14(4):486–493. doi: 10.1002/ajhb.10066. [DOI] [PubMed] [Google Scholar]
- 56.Garby L, Lammert O, Kock KF, Thobocarlsen B. Weights of brain, heart, liver, kidneys, and spleen in healthy and apparently healthy adult Danish subjects. Am J Hum Biol. 1993;5(3):291–296. doi: 10.1002/ajhb.1310050307. [DOI] [PubMed] [Google Scholar]
- 57.He Q, Heshka S, Albu J, et al. Smaller organ mass with greater age, except for heart. J Appl Physiol. 2009;106(6):1780–1784. doi: 10.1152/japplphysiol.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Javed F, He Q, Davidson LE, et al. Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fat-free mass. Am J Clin Nutr. 2010;91(4):907–912. doi: 10.3945/ajcn.2009.28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Heshka S, Gallagher D, Boozer CN, Kotler DP, Heymsfield SB. Resting energy expenditure–fat-free mass relationship: new insights provided by body composition modeling. Am J Physiol Endocrinol Metab. 2000;279(3):E539–E545. doi: 10.1152/ajpendo.2000.279.3.E539. [DOI] [PubMed] [Google Scholar]
- 60.Gallagher D, Albu J, He Q, et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr. 2006;83(5):1062–1067. doi: 10.1093/ajcn/83.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leibel RL, Hirsch J. Diminished energy requirements in reduced-obese patients. Metabolism. 1984;33(2):164–170. doi: 10.1016/0026-0495(84)90130-6. [DOI] [PubMed] [Google Scholar]
- 62.Weigle DS, Brunzell JD. Assessment of energy expenditure in ambulatory reduced-obese subjects by the techniques of weight stabilization and exogenous weight replacement. Int J Obes. 1990;14(suppl 1):69–77. discussion 77-81. [PubMed] [Google Scholar]
- 63.Bosy-Westphal A, Kossel E, Goele K, et al. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am J Clin Nutr. 2009;90(4):993–1001. doi: 10.3945/ajcn.2008.27402. [DOI] [PubMed] [Google Scholar]
- 64.Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17(5):608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 65.Dulloo AG, Jacquet J, Girardier L. Autoregulation of body composition during weight recovery in human: the Minnesota Experiment revisited. Int J Obes Relat Metab Disord. 1996;20(5):393–405. [PubMed] [Google Scholar]
- 66.Hall KD. Body fat and fat-free mass inter-relationships: Forbes’s theory revisited. Br J Nutr. 2007;97(6):1059–1063. doi: 10.1017/S0007114507691946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chow CC, Hall KD. The dynamics of human body weight change. PLoS Comput Biol. 2008;4(3):e1000045. doi: 10.1371/journal.pcbi.1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hall KD. Mechanisms of metabolic fuel selection: modeling human metabolism and body-weight change. IEEE Eng Med Biol Mag. 2010;29(1):36–41. doi: 10.1109/MEMB.2009.935465. [DOI] [PubMed] [Google Scholar]
- 69.Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab. 2010;298(3):E449–E466. doi: 10.1152/ajpendo.00559.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall KD, Guo J, Dore M, Chow CC. The progressive increase of food waste in America and its environmental impact. PLoS One. 2009;4(11):e7940. doi: 10.1371/journal.pone.0007940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–37. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 73.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 75.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 76.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20(1):49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- 77.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13(3):271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan BHL, Birdsell LA, Martin L, Baracos VE, Fearon KCH. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15(22):6973–6979. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 79.Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CMM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr. 2009;89(4):1173–1179. doi: 10.3945/ajcn.2008.27273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prado CMM, Liefers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 81.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non–small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91(4):1133S–1137S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 82.Prado CMM, Antoun S, Sawyer MB, Baracos VE. Two faces of drug therapy in cancer: drug-related lean tissue loss and its adverse consequences to survival and toxicity. Curr Opin Clin Nutr Metab Care. 2011;14(3):250–254. doi: 10.1097/MCO.0b013e3283455d45. [DOI] [PubMed] [Google Scholar]
- 83.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 84.Kyle UG, Genton L, Pichard C. Hospital length of stay and nutritional status. Curr Opin Clin Nutr Metab Care. 2005;8(4):397–402. doi: 10.1097/01.mco.0000172579.94513.db. [DOI] [PubMed] [Google Scholar]
- 85.Thibault R, Pichard C. Nutrition and clinical outcome in intensive care patients. Curr Opin Clin Nutr Metab Care. 2010;13(2):177–183. doi: 10.1097/MCO.0b013e32833574b9. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Ying Z, Bosy-Westphal A, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010;92:1369–1377. doi: 10.3945/ajcn.2010.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barbosa-Silva MC, Barros AJ, Post CL, Waitzberg DL, Heymsfield SB. Can bioelectrical impedance analysis identify malnutrition in preoperative nutrition assessment? Nutrition. 2003;19(5):422–426. doi: 10.1016/s0899-9007(02)00932-2. [DOI] [PubMed] [Google Scholar]
- 88.Kyle UG, Pichard C. Dynamic assessment of fat-free mass during catabolism and recovery. Curr Opin Clin Nutr Metab Care. 2000;3(4):317–322. doi: 10.1097/00075197-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 89.Jackson AS, Pollock ML. Practical assessment of body-composition. Phys Sportsmed. 1985;13(5):76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 90.Thomas D, Das SK, Levine JA, et al. New fat free mass–fat mass model for use in physiological energy balance equations. Nutr Metab (Lond) 2010;7:39. doi: 10.1186/1743-7075-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr. 2010;92(6):1326–1331. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]