Abstract
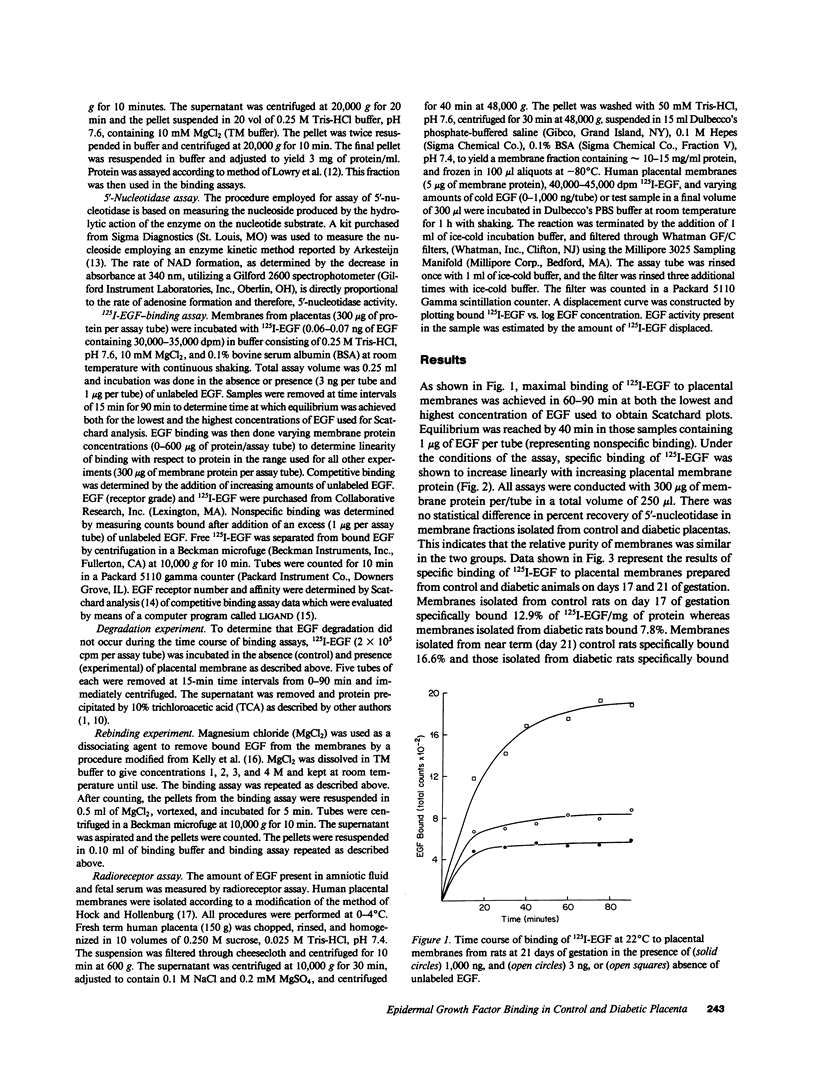
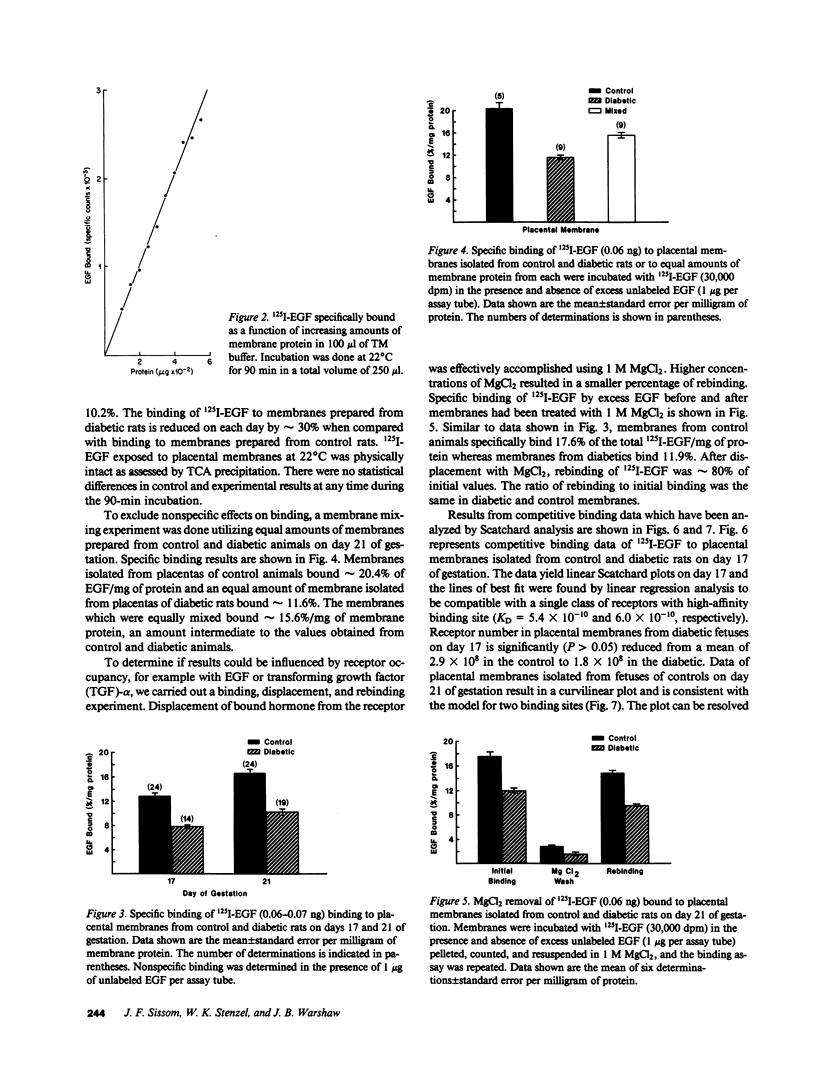
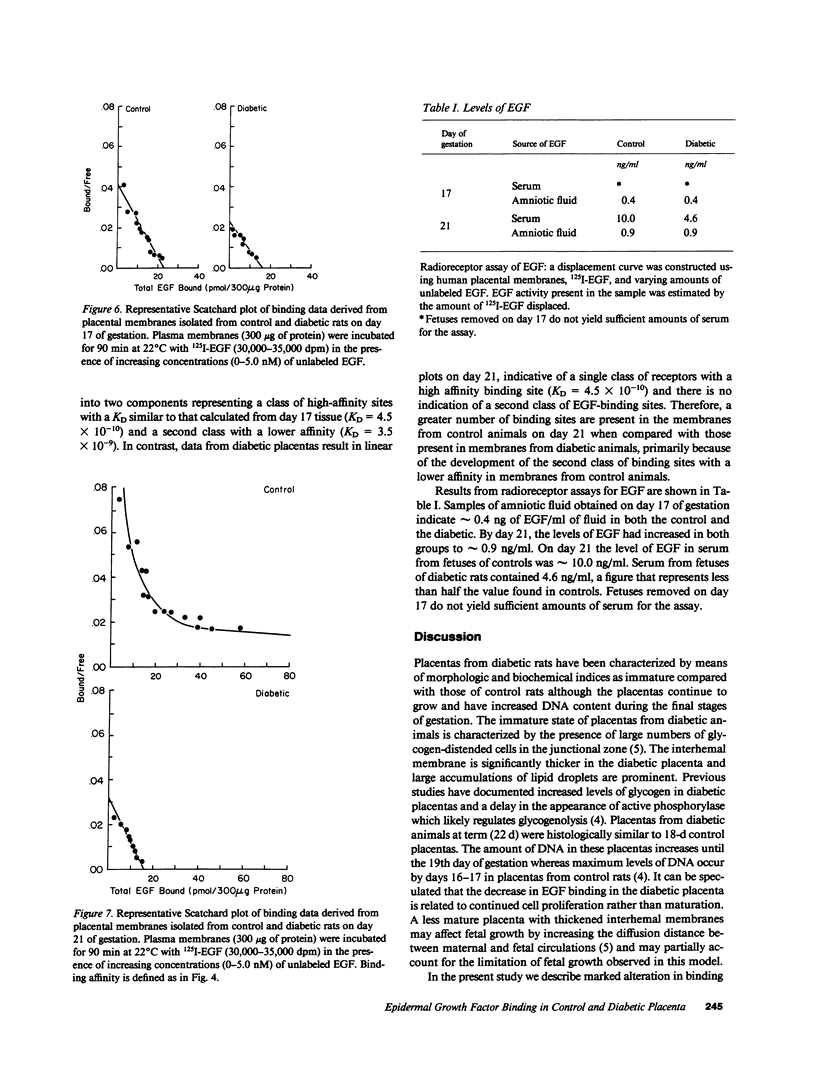
Placentas from streptozotocin-diabetic rats have previously been shown to be morphologically and biochemically immature when compared with those of control rats. The binding of epidermal growth factor (EGF) to plasma membranes prepared from placentas of control and streptozotocin-diabetic fetuses has been characterized on days 17 and 21 of gestation. Results from competitive binding data analyzed by Scatchard analysis indicate the presence of a single class of receptors on day 17 (KD = 5.4 X 10(-10)) and the appearance of a second class of binding sites for 125I-EGF by day 21 (Kd = 3.5 X 10(-9)) in membranes from control fetuses. Placental membranes from diabetic fetuses show decreased specific binding (approximately 30%) on both days and the absence of a second class of binding sites on day 21 of gestation. Results from a radioreceptor assay indicate that the quantity of EGF in the serum of fetuses removed from control rats on day 21 is twofold greater than the quantity in serum of fetuses from diabetic rats. These data reveal a developmental increase in EGF-binding sites in the placenta of normal, near-term fetal rats, largely because of the appearance of a second class of binding sites with a lower affinity for EGF. The failure (or delay) of this second class to develop in the diabetic may be important for the control of maturation and growth of this tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Meek J. The ontogeny of epidermal growth factor receptors during mouse development. Dev Biol. 1984 May;103(1):62–70. doi: 10.1016/0012-1606(84)90007-1. [DOI] [PubMed] [Google Scholar]
- Adamson E. D., Warshaw J. B. Down-regulation of epidermal growth factor receptors in mouse embryos. Dev Biol. 1982 Apr;90(2):430–434. doi: 10.1016/0012-1606(82)90392-x. [DOI] [PubMed] [Google Scholar]
- Arkesteijn C. L. A kinetic method for serum 5'-nucleotidase using stabilised glutamate dehydrogenase. J Clin Chem Clin Biochem. 1976 Mar;14(3):155–158. doi: 10.1515/cclm.1976.14.1-12.155. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Frolik C. A., Roberts A. B., Miller D. M., Sporn M. B. Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell. 1984 Jan;36(1):35–41. doi: 10.1016/0092-8674(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Earp H. S., Austin K. S., Blaisdell J., Rubin R. A., Nelson K. G., Lee L. W., Grisham J. W. Epidermal growth factor (EGF) stimulates EGF receptor synthesis. J Biol Chem. 1986 Apr 15;261(11):4777–4780. [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewolb I. H., Barrett C., Warshaw J. B. Placental growth and glycogen metabolism in streptozotocin diabetic rats. Pediatr Res. 1983 Jul;17(7):587–591. doi: 10.1203/00006450-198307000-00014. [DOI] [PubMed] [Google Scholar]
- Gewolb I. H., Merdian W., Warshaw J. B., Enders A. C. Fine structural abnormalities of the placenta in diabetic rats. Diabetes. 1986 Nov;35(11):1254–1261. doi: 10.2337/diab.35.11.1254. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Hollenberg M. D. Characterization of the receptor for epidermal growth factor-urogastrone in human placenta membranes. J Biol Chem. 1980 Nov 25;255(22):10731–10736. [PubMed] [Google Scholar]
- Kelly P. A., Leblanc G., Djiane J. Estimation of total prolactin-binding sites after in vitro desaturation. Endocrinology. 1979 Jun;104(6):1631–1638. doi: 10.1210/endo-104-6-1631. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladda R. L., Bullock L. P., Gianopoulos T., McCormick L. Radioreceptor assay for epidermal growth factor. Anal Biochem. 1979 Mar;93(2):286–294. doi: 10.1016/s0003-2697(79)80153-0. [DOI] [PubMed] [Google Scholar]
- Lai W. H., Guyda H. J. Characterization and regulation of epidermal growth factor receptors in human placental cell cultures. J Clin Endocrinol Metab. 1984 Feb;58(2):344–352. doi: 10.1210/jcem-58-2-344. [DOI] [PubMed] [Google Scholar]
- Morishige W. K., Uetake C. A., Greenwood F. C., Akaka J. Pulmonary insulin responsivitiy: in vivo effects of insulin on the diabetic rat lung and specific insulin binding to lung receptors in normal rats. Endocrinology. 1977 Jun;100(6):1710–1722. doi: 10.1210/endo-100-6-1710. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Neufeld N. D., Corbo L. M., Kaplan S. A. Plasma membrane insulin receptors in fetal rabbit lung. Pediatr Res. 1981 Jul;15(7):1058–1062. doi: 10.1203/00006450-198107000-00017. [DOI] [PubMed] [Google Scholar]
- Neufeld N. D., Kaplan S. A., Lippe B. M., Scott M. Increased monocyte receptor binding of [125I]insulin in infants of gestational diabetic mothers. J Clin Endocrinol Metab. 1978 Sep;47(3):590–595. doi: 10.1210/jcem-47-3-590. [DOI] [PubMed] [Google Scholar]
- Robert M. F., Neff R. K., Hubbell J. P., Taeusch H. W., Avery M. E. Association between maternal diabetes and the respiratory-distress syndrome in the newborn. N Engl J Med. 1976 Feb 12;294(7):357–360. doi: 10.1056/NEJM197602122940702. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Talamantes F. Characterization of the mouse placental epidermal growth factor receptor: changes in receptor number with day of gestation. Placenta. 1986 Nov-Dec;7(6):511–522. doi: 10.1016/s0143-4004(86)80137-0. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Kimball E. S., Sherwin S. A., Ranchalis J. E., Todaro G. J. Comparison of growth factors functionally related to epidermal growth factor in the urine of normal and human tumor-bearing athymic mice. Cancer Res. 1985 May;45(5):1934–1939. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]


