Abstract
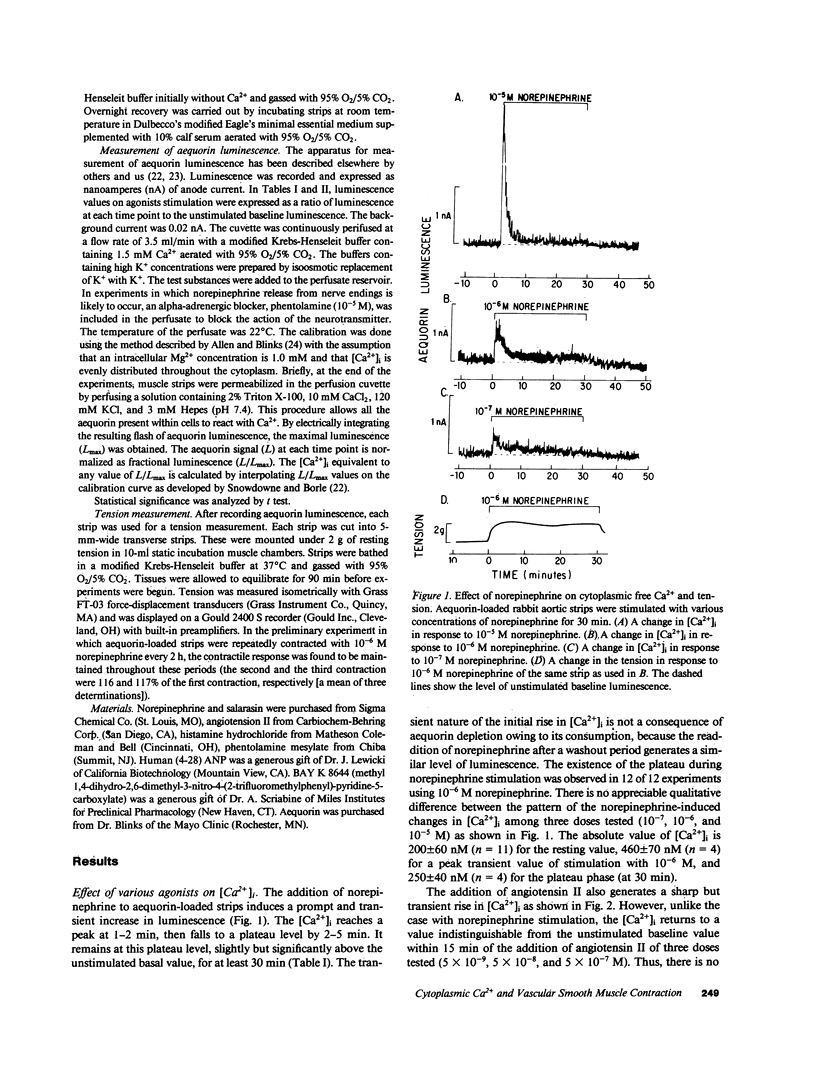
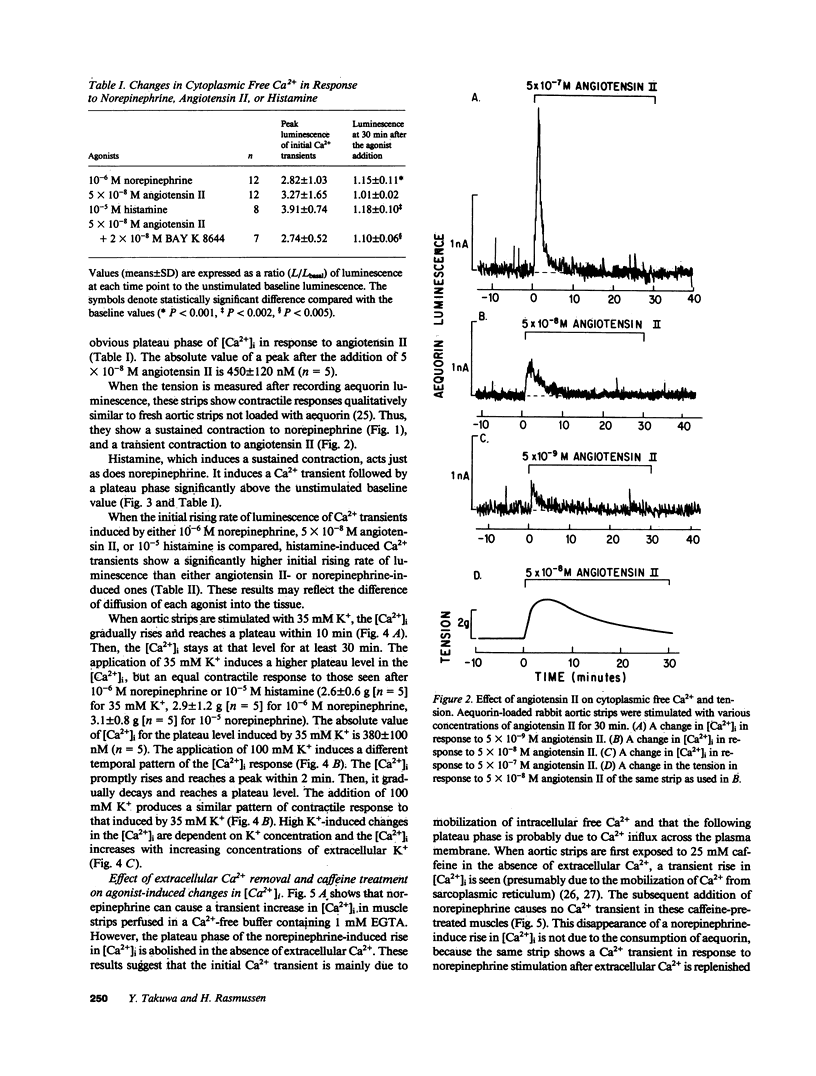
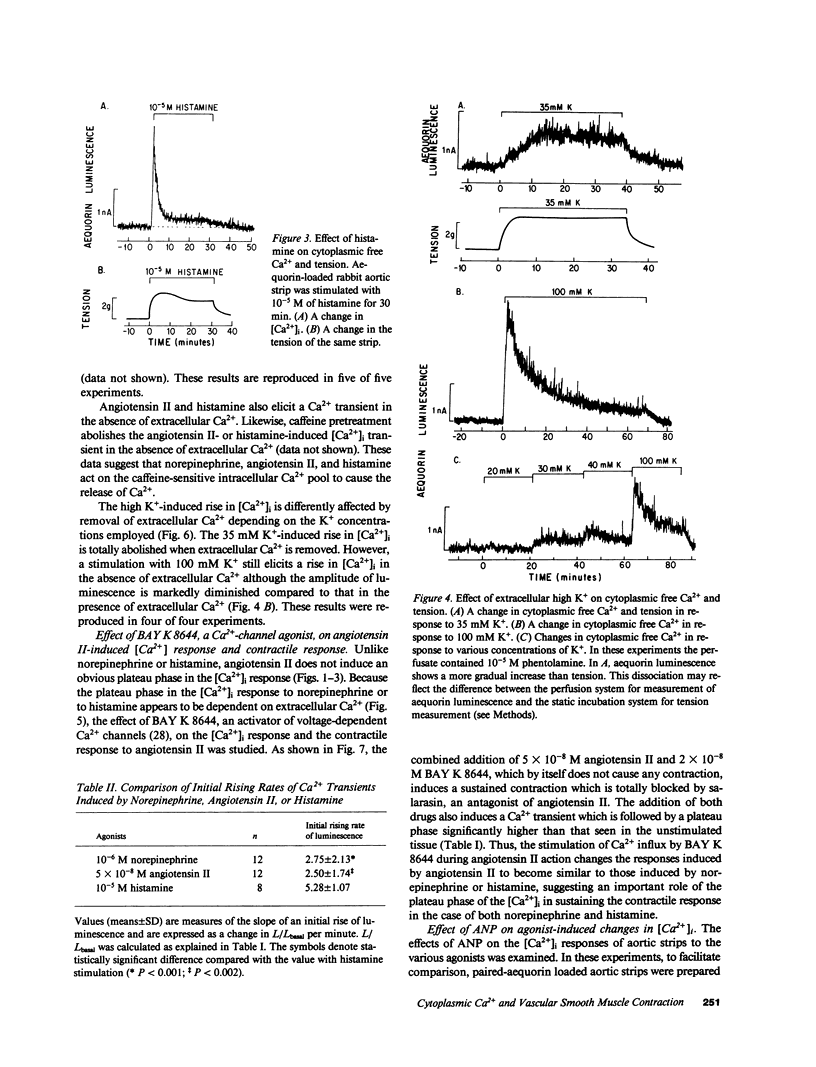
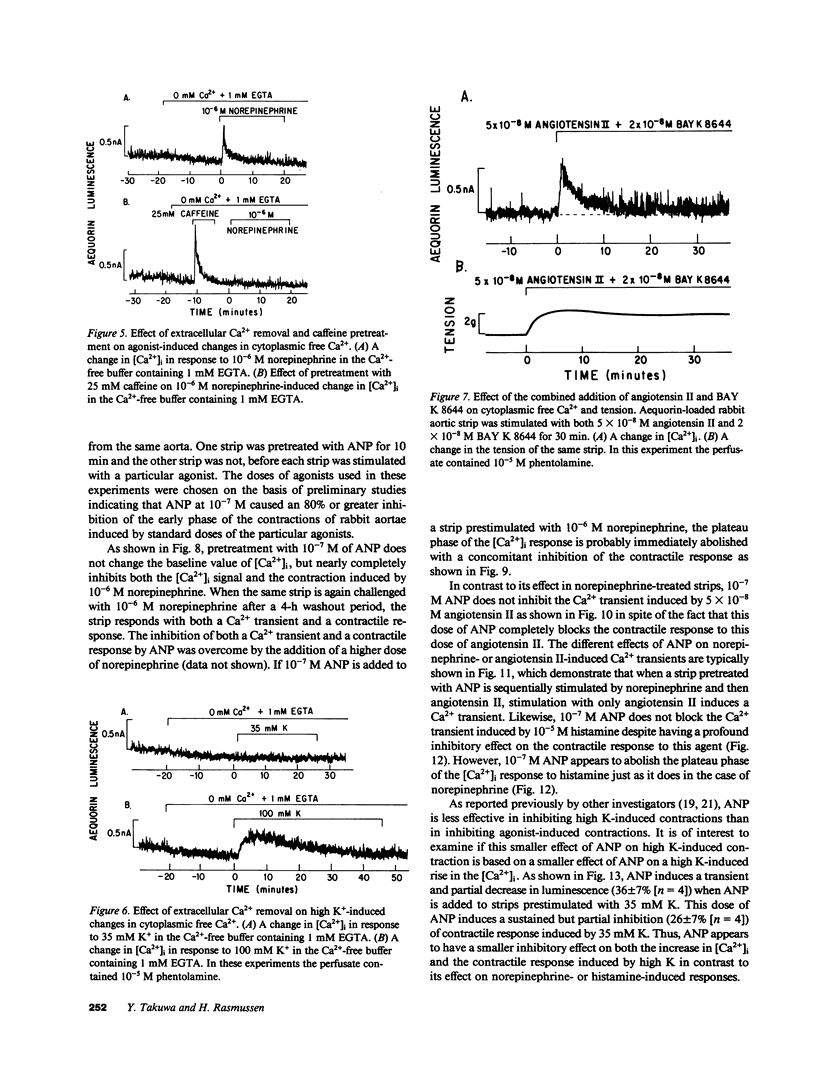
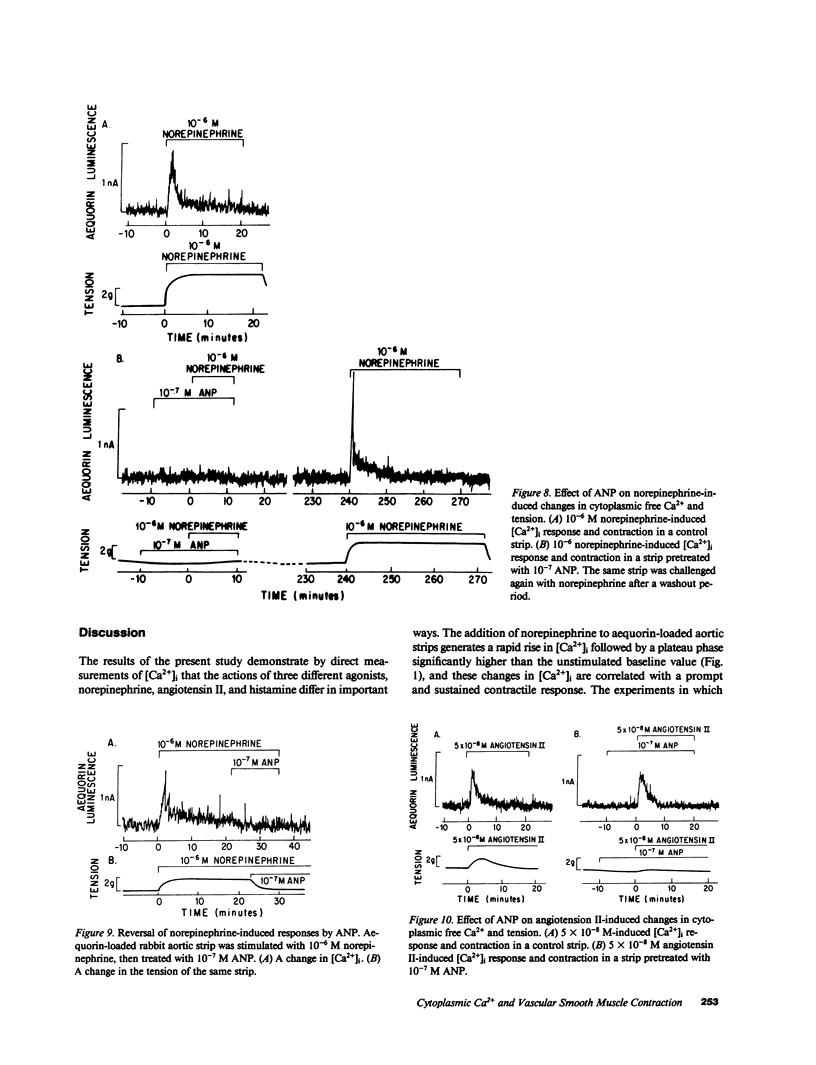
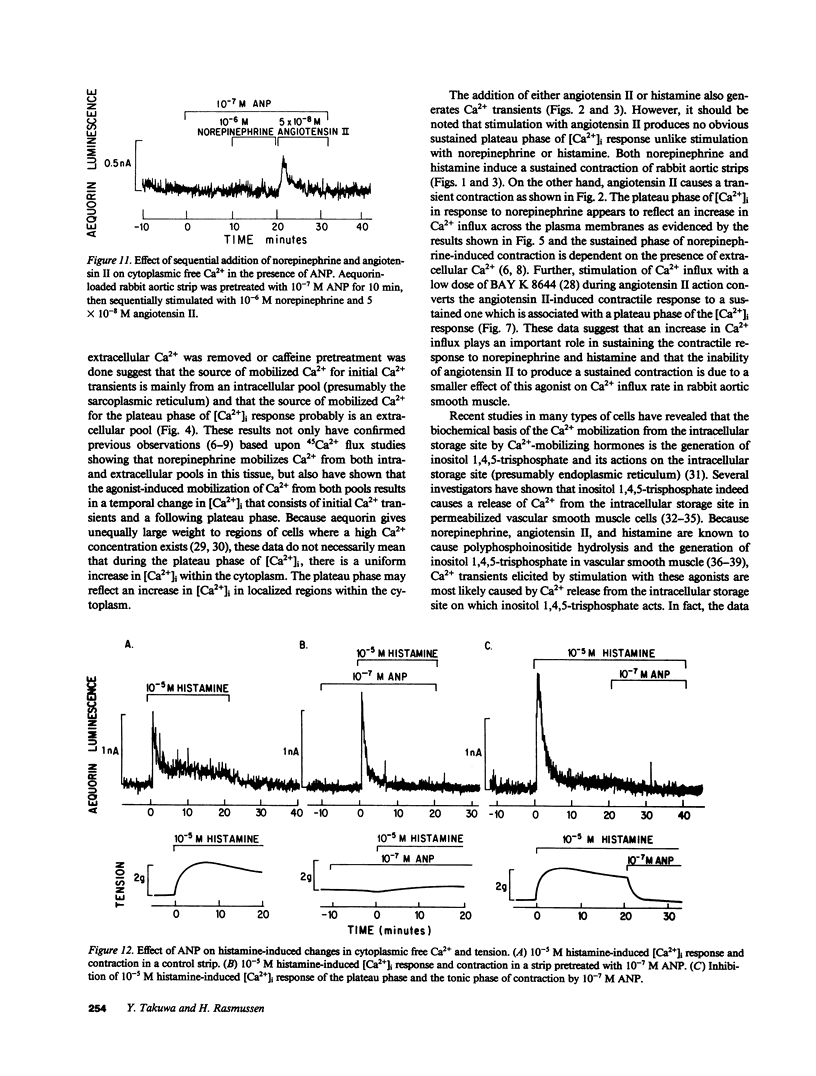
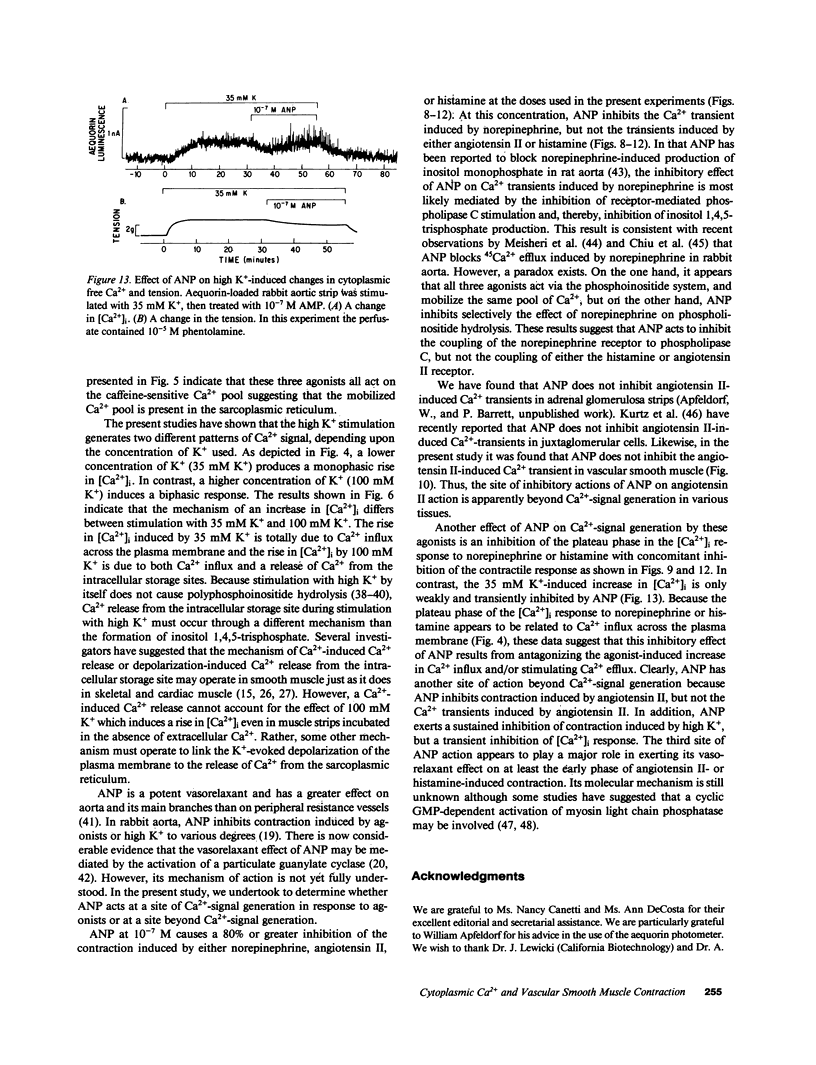
Addition of norepinephrine, angiotensin II, or histamine leads to a transient rise in the cytoplasmic Ca2+ concentration ([Ca2+]i), as measured with aequorin, in rabbit aortic strips. Each induces a [Ca2+]i transient which peaks in 2 min and then falls either back to baseline (angiotensin II) or to a plateau (norepinephrine and histamine). The [Ca2+]i transient is due to the mobilization of Ca2+ from a caffeine-sensitive, intracellular pool. An elevation of [K+] to 35 mM leads to a monotonic sustained rise in [Ca2+]i which depends entirely on extracellular Ca2+, but an increase to 100 mM leads to a [Ca2+]i transient from the mobilization of intracellular Ca2+. Atrial natriuretic peptide does not alter basal [Ca2+]i nor inhibit the [Ca2+]i transient induced by either histamine or angiotensin II, but blocks that induced by norepinephrine, and blocks the plateau phase induced by either histamine or norepinephrine. The peptide inhibits the contractile response to all three agonists and to K+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Rittenhouse S. E., Powers C. W., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Phorbol ester and 1-oleoyl-2-acetylglycerol inhibit angiotensin activation of phospholipase C in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 15;260(26):14158–14162. [PubMed] [Google Scholar]
- Campbell M. D., Deth R. C., Payne R. A., Honeyman T. W. Phosphoinositide hydrolysis is correlated with agonist-induced calcium flux and contraction in the rabbit aorta. Eur J Pharmacol. 1985 Oct 8;116(1-2):129–136. doi: 10.1016/0014-2999(85)90193-1. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Vallotton M. B. Cytosolic free calcium levels in monolayers of cultured rat aortic smooth muscle cells. Effects of angiotensin II and vasopressin. J Biol Chem. 1985 Jul 5;260(13):7836–7842. [PubMed] [Google Scholar]
- Chiu P. J., Tetzloff G., Sybertz E. J. The effects of atriopeptin II on calcium fluxes in rabbit aorta. Eur J Pharmacol. 1986 May 27;124(3):277–284. doi: 10.1016/0014-2999(86)90228-1. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Relative contributions of Ca2+ influx and cellular Ca2+ release during drug induced activation of the rabbit aorta. Pflugers Arch. 1974 Apr 4;348(1):13–22. doi: 10.1007/BF00587735. [DOI] [PubMed] [Google Scholar]
- Ebashi S. The Croonian lecture, 1979: Regulation of muscle contraction. Proc R Soc Lond B Biol Sci. 1980 Mar 21;207(1168):259–286. doi: 10.1098/rspb.1980.0024. [DOI] [PubMed] [Google Scholar]
- Fox A. W., Abel P. W., Minneman K. P. Activation of alpha 1-adrenoceptors increases [3H]inositol metabolism in rat vas deferens and caudal artery. Eur J Pharmacol. 1985 Oct 8;116(1-2):145–152. doi: 10.1016/0014-2999(85)90195-5. [DOI] [PubMed] [Google Scholar]
- Garcia R., Thibault G., Cantin M., Genest J. Effect of a purified atrial natriuretic factor on rat and rabbit vascular strips and vascular beds. Am J Physiol. 1984 Jul;247(1 Pt 2):R34–R39. doi: 10.1152/ajpregu.1984.247.1.R34. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kanaide H., Nakamura M. Cytosolic-free calcium transients in cultured vascular smooth muscle cells: microfluorometric measurements. Science. 1985 Aug 9;229(4713):553–556. doi: 10.1126/science.3927484. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kanaide H., Nakamura M. K+-depolarization induces a direct release of Ca2+ from intracellular storage sites in cultured vascular smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1985 Jun 28;129(3):877–884. doi: 10.1016/0006-291x(85)91973-4. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Della Bruna R., Pfeilschifter J., Taugner R., Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4769–4773. doi: 10.1073/pnas.83.13.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisheri K. D., Taylor C. J., Saneii H. Synthetic atrial peptide inhibits intracellular calcium release in smooth muscle. Am J Physiol. 1986 Jan;250(1 Pt 1):C171–C174. doi: 10.1152/ajpcell.1986.250.1.C171. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol. 1984 Dec;357:539–551. doi: 10.1113/jphysiol.1984.sp015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., de Lanerolle P., Lincoln T. M., Adelstein R. S. Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase. J Biol Chem. 1984 Jul 10;259(13):8429–8436. [PubMed] [Google Scholar]
- Pfitzer G., Merkel L., Rüegg J. C., Hofmann F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflugers Arch. 1986 Jul;407(1):87–91. doi: 10.1007/BF00580726. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M. Cyclic guanosine monophosphate inhibition of contraction may be mediated through inhibition of phosphatidylinositol hydrolysis in rat aorta. Circ Res. 1986 Mar;58(3):407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- Reynolds E. E., Dubyak G. R. Agonist-induced calcium transients in cultured smooth muscle cells: measurements with fura-2 loaded monolayers. Biochem Biophys Res Commun. 1986 May 14;136(3):927–934. doi: 10.1016/0006-291x(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Saida K. Intracellular Ca release in skinned smooth muscle. J Gen Physiol. 1982 Aug;80(2):191–202. doi: 10.1085/jgp.80.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Smith L., Brown E. R., Barnes D., Sabir M. A., Davis J. S., Farese R. V. Angiotensin II rapidly increases phosphatidate-phosphoinositide synthesis and phosphoinositide hydrolysis and mobilizes intracellular calcium in cultured arterial muscle cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7812–7816. doi: 10.1073/pnas.81.24.7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdowne K. W., Borle A. B. Measurement of cytosolic free calcium in mammalian cells with aequorin. Am J Physiol. 1984 Nov;247(5 Pt 1):C396–C408. doi: 10.1152/ajpcell.1984.247.5.C396. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Louis J., Regoli D., Barabé J., Park W. K. Myotropic actions of angiotensin and noradrenaline in strips of rabbit aortae. Can J Physiol Pharmacol. 1977 Oct;55(5):1056–1069. doi: 10.1139/y77-145. [DOI] [PubMed] [Google Scholar]
- Stull J. T. Phosphorylation of contractile proteins in relation to muscle function. Adv Cyclic Nucleotide Res. 1980;13:39–93. [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Sumimoto K., Kuriyama H. Mobilization of free Ca2+ measured during contraction-relaxation cycles in smooth muscle cells of the porcine coronary artery using quin2. Pflugers Arch. 1986 Feb;406(2):173–180. doi: 10.1007/BF00586679. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Bollag W. E., Rasmussen H. The effects of bombesin on polyphosphoinositide and calcium metabolism in Swiss 3T3 cells. J Biol Chem. 1987 Jan 5;262(1):182–188. [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. Carbachol induces a rapid and sustained hydrolysis of polyphosphoinositide in bovine tracheal smooth muscle measurements of the mass of polyphosphoinositides, 1,2-diacylglycerol, and phosphatidic acid. J Biol Chem. 1986 Nov 5;261(31):14670–14675. [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]
- Winquist R. J., Faison E. P., Nutt R. F. Vasodilator profile of synthetic atrial natriuretic factor. Eur J Pharmacol. 1984 Jun 15;102(1):169–173. doi: 10.1016/0014-2999(84)90353-4. [DOI] [PubMed] [Google Scholar]
- Winquist R. J. The relaxant effects of atrial natriuretic factor on vascular smooth muscle. Life Sci. 1985 Sep 23;37(12):1081–1087. doi: 10.1016/0024-3205(85)90351-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Aaronson P., Loutzenhiser R., Meisheri K. Ca2+ movements in smooth muscle. Chest. 1980 Jul;78(1 Suppl):157–165. doi: 10.1378/chest.78.1_supplement.157. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Siegel B. The mechanism of alpha-adrenergic activation of the dog coronary artery. Circ Res. 1980 Mar;46(3):426–429. doi: 10.1161/01.res.46.3.426. [DOI] [PubMed] [Google Scholar]


