Abstract
Current neurocircuitry models of PTSD focus on the neural mechanisms that mediate hypervigilance for threat and fear inhibition/extinction learning. Less focus has been directed towards explaining social deficits and heightened risk of revictimization observed among individuals with PTSD related to physical or sexual assault. The purpose of the present study was to foster more comprehensive theoretical models of PTSD by testing the hypothesis that assault-related PTSD is associated with behavioral impairments in a social trust and reciprocity task and corresponding alterations in the neural encoding of social learning mechanisms. Adult women with assault-related PTSD (n=25) and control women (n=15) completed a multi-trial trust game outside of the MRI scanner. A subset of these participants (15 with PTSD and 14 controls) also completed a social and non-social reinforcement learning task during 3T fMRI. Brain regions that encoded the computationally modeled parameters of value expectation, prediction error, and volatility (i.e., uncertainty) were defined and compared between groups. The PTSD group demonstrated slower learning rates during the trust game and social prediction errors had a lesser impact on subsequent investment decisions. PTSD was also associated with greater encoding of negative expected social outcomes in perigenual anterior cingulate cortex and bilateral middle frontal gyri, and greater encoding of social prediction errors in the left temporoparietal junction. These data suggest mechanisms of PTSD-related deficits in social functioning and heightened risk for re-victimization in assault victims; however, comorbidity in the PTSD group and the lack of a trauma-exposed control group temper conclusions about PTSD specifically.
Keywords: PTSD, assault, fMRI, reinforcement learning, trust, social deficits
Introduction
There have been considerable efforts to understand the cognitive and neural mechanisms mediating posttraumatic stress disorder (PTSD) symptoms in order to boost treatment efficacy and ameliorate the poor quality of life associated with PTSD. Neurocircuitry models of PTSD1–4 have focused on identifying the neural mechanisms that mediate the clinical and behavioral observations of hypervigilance for threat and impaired fear extinction / fear inhibition. These models have ample empirical support and powerfully explain critical phenomena among PTSD populations. For example, hyperactive amygdala5–6 and insular cortex responses52 during threat processing and anticipation explains attentional bias towards threat7 and heightened interoceptive monitoring52; altered structure and function of the hippocampus4, 8–11 explain the impaired ability to extinguish learned fear responses10, 12–16; weaker recruitment of perigenual anterior cingulate cortex (ACC)17–18 explains observed deficits in emotion regulation19–20. While these neurocircuitry models represent mechanisms of hypervigilance for threat, fear extinction, and emotion regulation, they do not account for observed PTSD-related deficits in social domains.
A less widely-known literature21–25 demonstrates significant deficits in risk perceptions for social situations among violence victims and individuals with PTSD. For example, one study found that the latency with which victimized women decided to escape hypothetical risky social situations escalating towards rape significantly predicted subsequent revictimization21. A related line of research has demonstrated both among adolescents27 and adults28 that greater baseline histories of assault exposure and PTSD symptoms prospectively predict increased rates of revictimization. These data suggest that 1) violence victims have lower danger perceptions of risky social situations, and 2) assaultive violence exposure and PTSD severity predict heightened risk for future victimization. Critically, both of these observations cannot be explained by existing neurocircuitry models of PTSD or trauma exposure. For example, given the known findings of amygdala hyper-reactivity and attentional bias towards threat, one would predict greater risk perceptions in social situations when in fact the opposite is observed.
We previously demonstrated among adolescent girls that assaultive violence exposure is associated with less behavioral slowing as well as decreased ACC and bilateral anterior insula responses to unexpected negative social behavior during a social contingency learning task29. These preliminary data supported a hypothesis of altered social learning mechanisms among adolescent assault victims and ostensibly suggest mediating mechanisms to explain their decreased social risk perceptions and increased risk for revictimization. Here, we sought to elaborate this model by investigating the neural and cognitive mechanisms of altered social learning among adult women with assault-related PTSD. We assessed social learning behavior outside of an fMRI context using the trust game, a widely used neuroeconomic game that quantifies social trust based on monetary exchanges with another player. Multi-trial versions of the trust game30–33 enable the study of dynamic interactions in social dyads (e.g., characterizing how one player responds when their investments are not reciprocated). We also characterized and compared social and non-social learning mechanisms during fMRI using two-arm variants of commonly used bandit tasks34–36. In these tasks, we manipulated the reward structure of task responses and used computational modeling to probe the neural correlates of the task components of value expectation, prediction errors, and volatility (i.e. uncertainty)36–37. To isolate a hypothesized unique relationship between assault-related PTSD and neural encoding of these component mechanisms during social learning, we modeled these same components in a non-social learning task. This methodology and analytic approach enabled testing the hypothesis that assault-related PTSD is associated with altered behavioral and neural correlates of social learning. However, it is important to note that our control group included only women with no history of trauma or PTSD; accordingly, inferences cannot be derived regarding specificity of the findings for PTSD specifically (vs just assault exposure) or for assault specifically (versus general trauma exposure).
Methods
Participants and assessment
Forty adult women, aged 20–53, were enrolled in the study. Five additional women were screened, but were ineligible due to the presence of a psychotic disorder (among a woman with PTSD), a current mental health disorder (among control women), or assault exposure without a current diagnosis of PTSD. The PTSD sample was comprised of 25 adult women and the control sample was comprised of 15 women. Table 1 provides demographic and clinical characteristics of the sample. Inclusion criteria for the PTSD group were a history of directly experienced assault exposure and a current diagnosis of PTSD; exclusion criteria were the presence of psychotic disorders, a primary substance use disorder, or internal metal. Control participants were included based on female sex and age and excluded based on a history of assault exposure, mental health disorder, internal metal, or major medical disorder. All 40 women completed the trust game, but only a subset of participants (15 PTSD and 14 control) were available for the fMRI scan. The 11 women not scanned did not return for their scheduled MRI session1.
Table 1.
Clinical and demographic characteristics of the sample.
| PTSD (n=25) | Control (n=15) |
p value group difference |
|||
|---|---|---|---|---|---|
| Variable | Mean (or frequency) | SD | Mean (or frequency) | SD | |
| Age | 34.7 | 8.3 | 30.87 | 7.1 | .14 |
| Ethnicity | 64% Caucasian 32% African-American 4% Other |
- | 67% Caucasian 27% African-American 7% Other |
- | .5 |
| Education | 8% not graduate high school 28% graduate high school or GED 64% some college or more |
- | 0% not graduate high school 13% graduate high school or GED 87% some college or more |
.25 | |
| Days since last menstruation* | 15.5 | 10.0 | 12.7 | 11.95 | .48 |
| Current Job | 40% unemployed | - | 7% | .02 | |
| PCL score | 55.7 | 16.6 | 20.00 | 3.5 | <.01 |
| BDI-II score | 22.8 | 14.1 | 2.4 | 2.3 | <.01 |
| Number of total direct assaults | 6.3 | 2.6 | 0 | 0 | <.01 |
| Number of physical assaults from non-caregiver | 2.4 | 1.5 | - | - | - |
| Number of physical assaults from caregiver | 1.6 | 1.5 | - | - | - |
| Number of sexual assaults | 2.4 | 1.4 | - | - | - |
| Current Major Depressive Disorder | 44% | - | 0 | - | .01 |
| Current Generalized Anxiety Disorder | 52% | - | 0 | - | <.01 |
| Current PTSD | 100% | - | 0 | - | <.01 |
| Current Marijuana Dependence | 4% | - | 0 | - | .43 |
| Current Alcohol Dependence | 16% | - | 0 | - | .10 |
Refers to days since menstruation among women who regularly menstruate; 5 PTSD women and 1 control women either were not menstruating (e.g., menopause) or had irregular cycles (>80 days since last menstruation).
Assaultive trauma histories were characterized using the trauma assessment section of the National Women’s Survey and National Survey of Adolescents38–40, a structured interview used in prior epidemiological studies of assault exposure and mental health functioning among adult women and adolescents. Specific assaultive events were assessed with behaviorally specific dichotomous questions and included sexual assault, physical assault, and specific severe abuse from a caregiver (i.e., beaten with fists or an object to the point where bruising or bleeding occurred).
Psychological disorders were assessed with the Structured Clinical Interview for DSM-IV Disorders (SCID)41 administered by a trained clinical interviewer and supervised by a licensed clinical psychologist. Participants additionally completed the Posttraumatic Stress Checklist-Civilian Version42 and Beck Depression Inventory-II43.
Behavioral Tasks
Trust Game
The version of the trust game implemented here corresponded to previous multi-trial versions30–33. Participants were placed alone in a room and led to believe they were playing against a real person in a different room through a computer interface, when in fact the computer was actually providing the other ‘player’s’ programmed responses. During the post-experiment debriefing participants were asked whether they believed they were playing with another person, and all but one participant (a woman with PTSD) believed the deception; this individual was not included in the analyses of task performance pertaining to the trust game.
Participants completed 15 trials in which they served as the investor and the other ‘player’ was the investee. They were told that at each trial they were given a cache of $10 from which they could choose to invest any amount to the other player. Once invested, the amount would triple (e.g., an investment of $5 would turn into $15 received by the other ‘player’). The other ‘player’ could subsequently return any amount back to the participant. To motivate participants’ performance, they were told that they would receive a bonus amount of monetary compensation based on how much money they generated during this game.
The 15 trials were divided into 3 phases of computer-generated responses from the other ‘player’. During the first 5 trials, the other ‘player’ returned to the participant a random amount between 40%–60% of the amount invested. During the middle 5 trials, the other ‘player’ reduced the amount of reciprocity and only returned between 10%–30% of the amount invested. During the final 5 trials, the other ‘player’ resumed baseline levels of reciprocity by returning between 40%–60% of the amount invested. This dynamic manipulation of reciprocity from the other ‘player’ provided a context within which to examine how the participants respond to unexpected changes in social behavior (i.e., violations of expectations of trustworthiness).
fMRI social and non-social learning tasks
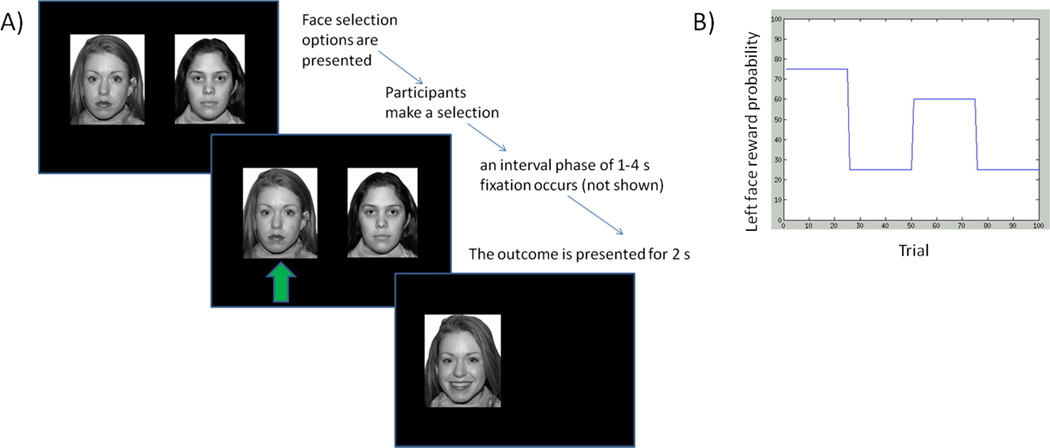
Participants completed two versions of a two-arm bandit task during fMRI. In the social version of the task (Figure 1), participants were told their goal on this task was to receive as many smiles (social reinforcer) as they could. To motivate performance, participants were told that they would receive bonus monetary compensation based upon the number of accumulated smiles. On each trial, participants viewed two faces (from the NimStim face set, always the same two faces throughout the experiment) and were told to select which face they believed was most likely to smile at them. The selected face would then smile or frown based on a predetermined probability. There were 100 total trials, divided into four epochs of 25 trials with different reward probabilities (Figure 1). For example, in the first epoch, the left face could be associated with a .75 probability of smiling and this could switch to a .4 probability in the next phase. The goal was to learn the stimulus probabilities for social reinforcement.
Figure 1.
a) Depiction of the social two-arm bandit task for fMRI. Participants were told to receive as many smiles as they could, and accordingly to choose the face on each trial they believed was most likely to smile. b) The probability of each face smiling (vs frowning) changed very 25 trials. The probabilities of the two faces were linked, such that the sum of the two probabilities always equaled 1.
The non-social version of the task was identical, except that participants were presented with two houses and asked to select which house was most likely to be open (versus locked) (see Supplemental Figure 1). To reduce learning effects carried over between tasks, we counterbalanced the order of the tasks and the reward probabilities between social and non-social tasks.
fMRI acquisition and image preprocessing
See the supplementary material for description of 3T fMRI acquisition and preprocessing.
Analyses
Trust Game Performance Data
We modeled participant behavior using the Rescorla-Wagner (RW) reinforcement learning model37, 44. This simple and frequently used model takes the form of Vt+1=Vt+ δ * α, where V refers to expected value of a chosen action, δ is a prediction error (Vt − outcomet), and α is a learning rate that ranges from 0–1. The expected value of a chosen action changes from trial to trial based upon δ, such that a positive δ (i.e., receiving more than expected) increases expected value and a negative δ (i.e., receiving less than expected) decreases expected value. The learning rate, α, controls the speed with which value expectations are updated, with higher learning rates leading to faster changes in expected value. See supplemental material for further description of the RW model.
Bandit task analyses
The two-arm bandit tasks with changing reward structures foster a more sophisticated modeling approach. Following prior research34–36, we used a modified RW model that additionally models exploration rate (i.e., selecting non-optimal choices for the sake of learning information about the task’s structure) and volatility (i.e., estimates of uncertainty about the reward probabilities). See the supplementary material for further description of model fitting. fMRI analyses focused on value expectation (θ), prediction error (δ), and volatility.
fMRI analyses
Following prior research34, 36, 45–47, we identified brain regions that scaled with the modeled computations of interest (i.e., θ, δ, and volatility) using amplitude-modulated GLMs during the first-level analyses. The three phases of the trial (decision, anticipation, outcome) are included as separate regressors, modeled as a boxcar over the length of the trial and convolved with a gamma function. We then included 3 additional amplitude-modulated regressors based on the modeled computations: anticipation × θ, outcome × δ, and outcome × volatility. We additionally included the direction of the outcome (positive vs negative) as an additional modulator of the outcome regressor (i.e., outcome × direction) to isolate the influence of the direction of the outcome (positive vs negative) from the modeled computations of interest. Accordingly, we used the absolute value of δ in analyses, given that its sign is highly collinear with the valence of the outcome. These first-level GLMs were implemented in AFNI (3dREML) using restricted maximum likelihood to account for serial correlation. A separate analysis was completed for each participant for the social and non-social versions of the task.
Given that the hypothesis under scrutiny here pertains to group differences in social learning mechanisms, it is necessary to control for possible differences in general learning mechanisms. Thus, group comparisons used voxel-wise robust regression analyses in which the voxel’s β coefficient for the computation during the social task was regressed simultaneously onto group (dummy coded for PTSD vs control) and the voxel’s corresponding β coefficient for the computation during the non-social task. This approach isolated the unique relationship between group and the voxel’s encoding of the computation during social learning specifically. To control for multiple comparisons, we achieved a corrected p < .05 by identifying significant clusters of activation with a threshold of |t| >2.467 (p < .01) and a minimum of 48 contiguous voxels, using AFNIs 3dFWHMx to estimate the amount of smoothing in these data and 3dClustSim to define the corrected cluster size given the amount of smoothing.
Results
Trust Game
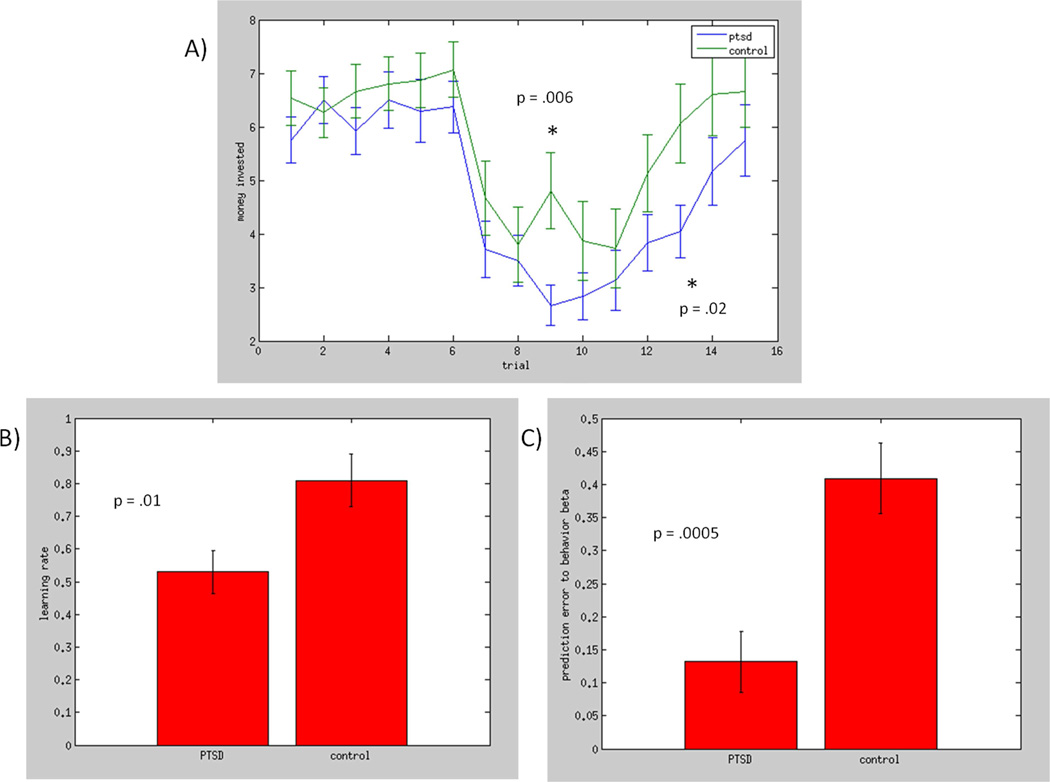
Trial-by-trial comparisons of investment behavior (Figure 2) demonstrated greater reductions in investments among the PTSD group during the second phase and slower increases in investments during the third phase. These results were followed up with analyses of the RW learning model.
Figure 2.
Results from the trust game. a) Mean trial-by-trial investments for the PTSD and control groups. The reinforcement ratio of the task was .5 (randomly within a range of .4–.6) in trials 1–5, .3 from trials 6–10, and .5 from trials 11–15. b) The PTSD group demonstrated significantly lower learning rates, derived from fitting RW models to each participant’s data. c) Modeled prediction errors on trial t were less related to subsequent investments on trial t+1 among the PTSD group.
Preliminary analyses demonstrated validity of the RW modeling approach to participant behavior during the trust game (supplemental Figure 2 and supplement material) and no differences in model fit between groups (t = 1.7, p = .099). The PTSD group demonstrated significantly lower social learning rates than the control group (Figure 1): t = 2.67, p = .01. We next tested the degree to which modeled δ on trial t impacted subsequent investment on trial t+1 (β coefficients from individual regression models) and similarly found significantly smaller β coefficients in the PTSD group: t = 3.85, p = .00052. By contrast, there were no group differences in initial expectations (Vinitial) (t = −.37, p =.71).
Neural correlates of social learning mechanisms
Between-group comparison of model fit
The learning model’s average accuracy of predicting participant’s choices was .732 (SD = .14) and .730 (SD = .15) for the social and non-social task, respectively, which is significantly better than chance (ps < .001). Model accuracy did not differ between groups for either task (ps > .14). Group comparisons of modeling parameters were not significant (see supplemental material).
Volatility
The whole-brain group comparison failed to reveal any significant clusters of neural encoding differing between the groups.
Prediction Errors
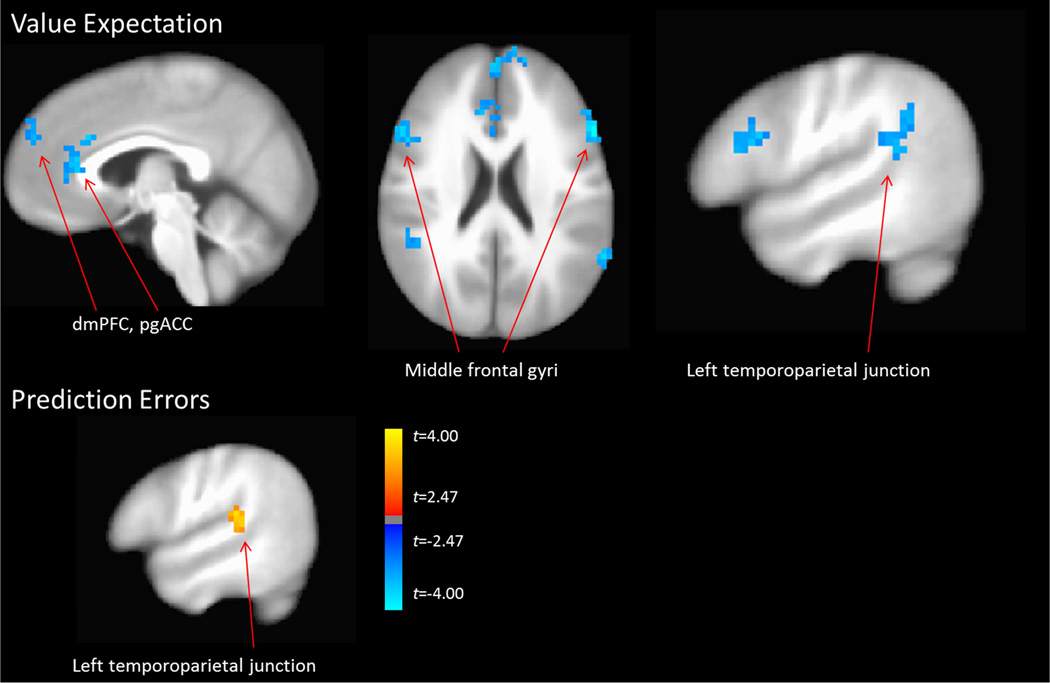
The whole-brain group comparison revealed a significant cluster in the left TPJ (Table 2; Figure 3; supplemental Figure 3), demonstrating greater encoding of social prediction errors in the PTSD compared to control group.
Table 2.
Brain regions differentially encoding the computational parameters of interest among the PTSD group in a whole-brain between-group comparison.
| Computational parameter |
Region | Cluster Size (# voxels) |
Peak t- value |
Center Mass Coordinates (MNI) |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Prediction error | Temporoparietal junction | 51 | 4.3 | −50 | −27 | 16 |
| Value Expectation | Middle frontal gyrus | 81 | −5.24 | 51 | 26 | 16 |
| Superior frontal gyrus | 73 | −3.86 | 1 | 59 | 27 | |
| Temporoparietal junction | 66 | −3.91 | −53 | −44 | 27 | |
| Parietal lobe | 61 | −4.89 | 58 | −48 | 30 | |
| pgACC/dACC | 58 | −4.07 | −5 | 31 | 20 | |
| Middle frontal gyrus | 49 | −3.94 | −51 | 21 | 22 | |
| Volatility | No significant clusters | |||||
Note. Coordinates are in MNI template space and refer to the cluster’s center of mass. The t values indicate the direction of the effect, with positive t values indicating greater encoding of the computational parameter among the PTSD group and negative t values indicating weaker encoding among the PTSD group.
Figure 3.
Top) The PTSD group demonstrated less encoding of expected social value while anticipating outcomes on the social task in the perigenual ACC, dorsomedial PFC, bilateral middle frontal gyri, and bilateral temporoparietal junction. Bottom) The PTSD group demonstrated greater prediction error encoding in the left temporoparietal junction.
Value Expectation
The whole-brain group comparison revealed significant clusters in the left and right middle frontal gyri, perigenual and dorsal ACC, dorsomedial PFC, and left and right temporoparietal junction (Table 2; Figure 3; Supplemental Figure 4 and 5).
Relationship between neural correlates of social learning mechanisms and trust game learning mechanisms
We next tested whether the learning mechanisms in the trust game were associated with any of the clusters of altered neural encoding associated with PTSD. We extracted the mean β coefficients within each cluster for each participant demonstrating a significant between-group difference and used robust regression to test for a relationship between amount of encoding in the cluster and learning mechanisms during the trust game (again controlling for the comparable degree of activity during the non-social learning task). We used FDR to control for family-wise alpha inflation.
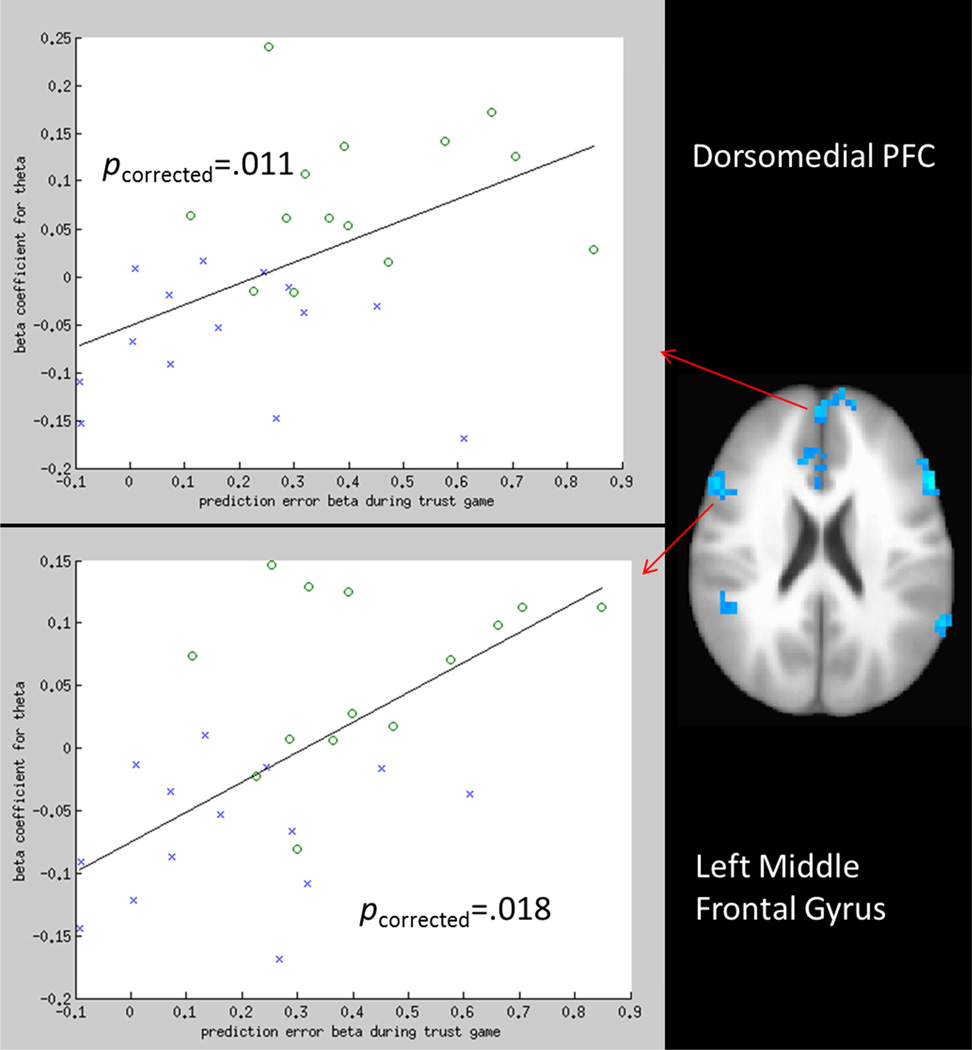
These analyses failed to reveal any clusters related to altered encoding of volatility, δ, or θ that significantly correlated with learning rate during the trust game when controlling for multiple comparisons. By contrast, the influence of δ on investment behavior during the trust game was related to dorsomedial PFC and left middle frontal gyrus encoding θ (Figure 4).
Figure 4.
The degree to which prediction errors impacted subsequent investments in the trust game were significantly correlated with encoding of positive expected value in the left middle frontal gyrus and dorsomedial PFC. Blue ‘X’s represent PTSD participants; green ‘O’s represent control participants.
Discussion
Prior to discussing results, it is again important to note that our control group was limited to women without trauma exposure or PTSD. Accordingly, inferences regarding the unique effect of PTSD or assault exposure specifically cannot be made.
Observations of participant overt investment behavior on the trust game (Figure 2) suggested that the PTSD group appeared to respond to the decrease in reinforcement with greater decreases in trust and appeared to demonstrate resistance to returning to baseline levels of trust. Concurrently, observations of the RW model parameters suggested that the PTSD group had significantly lower learning rates and that modeled prediction errors had less of an impact on subsequent observed behavior. The overt investment behavior results seem inconsistent with the hypothesis of decreased responsiveness to negative social behavior we previously observed among assault adolescents; however, the RW model parameters suggest differences in the mediating cognitive mechanisms by which the PTSD group uses social information. Lower learning rates and smaller relationships between prediction errors and subsequent behavior suggest less flexibility in updating social expectations and less use of social experiences in guiding social decision-making, respectively. Accordingly, while the present data are not consistent with broadly attenuated behavioral responses to negative social behavior in assault-related PTSD, the present data do suggest differences in how women with assault-related PTSD respond in dynamic social interaction and in how they use social information to make subsequent decisions. These observations highlight the need for future research to further define contextual and cognitive factors affecting interpersonal behavior among women with assault-related PTSD.
We also observed differences in the neural encoding of social learning mechanisms among women with assault-related PTSD. Encoding of social prediction errors in the left temporoparietal junction (TPJ) was increased in the PTSD women. Examination of the specific patterns of relationships between TPJ activity and social predictors among the PTSD and control groups (Supplemental Figure 3) demonstrated a positive relationship in the PTSD group and a negative relationship in the control group. This pattern suggests reversed TPJ encoding of social prediction errors: among the PTSD group, social prediction errors recruit greater TPJ activity; in the control group, social prediction errors actually withdraw TPJ activity relative to baseline. The TPJ is widely implicated in theory-of-mind tasks, in which individuals form representations of other’s mental states48–50, suggesting that PTSD is associated with greater mentation regarding other’s intentions during social prediction errors. The TPJ is certainly not specific to theory-of-mind and is implicated in a wide range of cognitive tasks51, thus caution must be used in linking its activation to any single cognitive process.
We observed decreased encoding of value expectation (θ) while anticipating the social outcome among the PTSD group in several regions (Table 2; Figure 3). In our previous study of social contingency learning among assaulted adolescent girls29, we observed hypoactive perigenual ACC (pgACC) responses to unexpected social outcomes. While we had speculated that these results were due to weakened encoding of social prediction errors, our current results among this sample of adults with assault-related PTSD and use of an explicit computational model suggests that hypoactivity of the pgACC is instead attributable to altered encoding of expected social expectation. It is interesting to note the direction of group differences in neural encoding of expected social value across each of the identified regions (Supplemental Figure 4 and 5). Whereas the control group demonstrated either a positive relationship (pgACC, bilateral MFG, left TPJ, dmPFC) or null relationship (right TJP) between social expectation and brain activity, the PTSD group consistently demonstrated a negative relationship between social expectation and activity in these brain regions. In the task and modeling approach used here, θ is a scalar variable ranging from 0 (negative expected value) to 1 (positive expected value). Accordingly, observations of negative relationships in the PTSD group suggests that these neural regions positively encode negative expected social value. That is, it does not appear to be the case that these regions are not encoding expected social value in PTSD; rather, they appear to be specifically encoding expectations of negative social value, whereas the normative response in these brain regions appears to be encoding positive social value.
Further, we observed that encoding of expected social value in the left middle frontal gyrus and dorsomedial PFC was significantly related to the relationship between modeled δ during the trust game and investment behavior on the subsequent trial. These relationships suggest that the altered encoding of expected social value in the brain regions may mediate decreased use of social information during social-decision making. It is interesting to consider the implications of heightened encoding of negative expected social value, and their ostensible effects on using social information during real-world social decision-making, for neurocircuitry models of PTSD, and for risk of revictimization among women assault victims. Hypoactivity of the pgACC and lateral PFC are generally consistent with exisiting neurocircuitry models1,5. Further, the concept of heightened encoding of specifically negative expected social value appears consistent with observed hypervigilance and attentional biases for threat in PTSD7. However, the observed relationships between heightened encoding of negative social value in the dmPFC and middle frontal gyrus with less use of social information during social decision-making is interesting because it suggests that there might be a social consequence of heightened encoding of negative expected outcomes. That is, it appears plausible that after a prolonged experience with heightened negative social expectations and presumed experience with false positive (i.e., expected negative social outcomes that subsequently do not happen), one might learn to ignore heightened negative social expectations during decision making (i.e., learning to ignore social danger signals). It also seems intuitive that learning to ignore heightened negative social expectations consequently results in ignoring incoming social information. Thus, the combined observations of 1) heightened neural encoding of negative social value in PTSD, and 2) relationships between encoding of negative social value and use of social information during a real-world social decision-making task are consistent with the hypothesis that observed social decision-making deficits and risk for revictimization in assault-related PTSD may be a consequence of habituating to prolonged hypervigilance. If this were true, it would suggest that a mechanism to promote the identification of danger (i.e., hypervigilance and attentional bias for threat), when chronic and unremitting, can result in learning to habituate to these danger signals and ironically increase risk for danger.
While clearly speculative, the current results advance the hypothesis that behavioral differences in social learning among women with assault-related PTSD may be mediated by altered neural encoding of expectation social value. The altered neural encoding of expected social value appears conceptually, and at least partially neuroanatomically, consistent with neurocircuitry models of PTSD, and it seems plausible, though speculative, that observed deficits in social behavior and social decision-making may be a consequence of habituating to chronic and unremitting hypervigilance for threat. If future research supports the hypothesis that deficits in social decision-making are consequences of habituating to hypervigilance, then an additional open question for future research is whether the observed deficits in social decision-making are best conceptualized as a distinct mechanism in PTSD or simply as a by-product of hypervigilance3. Parsimony within PTSD models may be optimized by conceptualizing the social deficits as consequences and focusing on hypervigilance as the primary mechanism. However, it is interesting to note that the neural mechanism canonically linked with hypervigilance, the amygdala, was not observed in the PTSD group here in this social learning context; instead, we observed altered activity of the medial and lateral PFC. These data would suggest that, at least at the neural level of analysis, the mechanisms of heightened negative social expectation are distinct from the canonical mechanism of hypervigilance for threat. Further, conceptualizing the social decision-making deficits as a by-product of hypervigilance may oversimplify this relationship and fail to explain how (the mediating mechanisms) and in whom (the moderating mechanisms) hypervigilance for threat transitions into social decision-making deficits. Finally, it also important to note that this hypothesis that social decision-making deficits are consequences of chronic and unremitting hypervigilance is preliminary and in need of further investigation.
While the current study suggests the importance of incorporating social learning mechanisms into our conceptualizations of PTSD, it is not without limitations. Of most importance, we did not have comparisons groups of assaulted women without PTSD, non-assault traumatized women without PTSD, or women with PTSD related to non-assaultive traumas. Inclusion of these additional groups would allow inferences regarding whether the observed social learning deficits specific to a PTSD diagnosis, assault exposure, or PTSD related to assault exposure. Additionally, our sample size was relatively small and limited to women, our implementation of the trust game used did not use a genuine social interaction with a conspecific, our fMRI social task did not involve an unambiguous social interaction and instead only used implied social interaction, and we implemented a previously used learning model among normative samples to explain cognitive learning mechanisms during the fMRI tasks. Finally, our PTSD group had significant comorbidity and was therefore representative of community/clinical PTSD samples; nonetheless, the degree of comorbidity limits the specificity of inferences.
Supplementary Material
Depiction of the non-social two-arm bandit task. Participants were told to receive as many unlocked houses as they could, and accordingly to choose the house on each trial they believed was most likely to be unlocked.
Demonstration of RW modeling results for the trust game. Top: The mean investment behavior across participants across trials is depicted along with the mean modeled expected return on investments. As can be seen, the modeled expected returns tracked closely with the observed investment behavior. Bottom: In our implementation of the trust game, participants received ~50% return on investment in trials 1–5, ~30% in trials 6–10, and ~50% in trials 11–15. Accordingly, if the RW model accurately reflects the cognitive decision-making model used by participants on the task, then we expect that the ratio between participant’s investments and modeled expected return on investments should converge to the actual return of investments. As depicted here, the mean ratio across participants of modeled expected return / observed investment converged towards the empirical ratio of actual returns / observed investments in each phase of the task.
Bar graphs depicting between-group differences in neural encoding of prediction errors in the left temporoparietal junction during the social and non-social bandit tasks.
Bar graphs depicting between-group differences in neural encoding of value expectation in the left middle frontal gyrus, pgACC, left temporoparietal junction, and dorsomedial PFC during the social and non-social bandit tasks.
Bar graphs depicting between-group differences in neural encoding of value expectation in the right TPJ and left middle frontal gyrus during the social and non-social bandit tasks.
Highlights.
Women with assault-related PTSD demonstrated altered learning mechanisms during the trust game
Women with assault-related PTSD demonstrated altered neural encoding during a social learning task
These results suggest a need to include social learning mechanisms in models of PTSD
Acknowledgements
We thank Cindy Mosley, Andi Ham, Jonathan Young, Shanti Tripathi, and George Andrew James for help with recruitment, analysis, and administration.
Funding sources: Portions of this work were supported through grants 1R21MH097784-01 and 1R01DA036360-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute for Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ten of the women who did not return for the fMRI scan were in the PTSD group vs one in the control group. Among women with PTSD, those who did return for the scan were significantly younger than those who did not: mean (SD) age = 31 (6) vs 40(8); p < .05
When these analyses were constrained to just the participants who also completed the fMRI task, the effect sizes of group differences remained highly similar: for learning rates, the effect size d was .87 and .74 among the full and subset samples, respectively; for δ predicting subsequent behavior, d was 1.25 and 1.26 among the full and subset samples, respectively.
We thank an anonymous reviewer for this observation.
Financial Disclosures
All authors report no financial conflicts of interest.
Contributions
Jennifer Lenow was involved in study design, interpretation, and manuscript writing. Scott Steele was involved in study design, interpretation, and manuscript writing. Sonet Smitherman was involved in study design. Clint Kilts was involved in study design and interpretation. Josh Cisler was involved in study design, analysis, interpretation, and manuscript writing. Keith Bush was involved in study design, interpretation, analysis, and manuscript writing.
References
- 1.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol. Psychiatry. 2006 Aug 15;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci. 2006 Jul;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 3.Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013 Jul;17(7):337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012 Nov;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012 Oct;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry. 2000 May 1;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 7.Cisler JM, Wolitzky-Taylor KB, Adams TG, Jr, Babson KA, Badour CL, Willems JL. The emotional Stroop task and posttraumatic stress disorder: a meta-analysis. Clin. Psychol. Rev. 2011 Jul;31(5):817–828. doi: 10.1016/j.cpr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012 Feb 15;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002 Nov;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry. 2009 Dec 15;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Admon R, Lubin G, Stern O, et al. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proc. Natl. Acad. Sci. U. S. A. 2009 Aug 18;106(33):14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. A. J. Psychiatry. 2007 Nov;164(11):1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 13.Jovanovic T, Ely T, Fani N, et al. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013 Jul-Aug;49(7):1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norrholm SD, Jovanovic T, Olin IW, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry. 2011 Mar 15;69(6):556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fani N, Tone EB, Phifer J, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 2012 Mar;42(3):533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J. Psychiatr. Res. 2008 Jun;42(7):515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol. Psychiatry. 2001 Dec 15;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 18.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2005 Mar;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 19.Moore SA, Zoellner LA, Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behav. Res. Ther. 2008 Sep;46(9):993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tull MT, Barrett HM, McMillan ES, Roemer L. A preliminary investigation of the relationship between emotion regulation difficulties and posttraumatic stress symptoms. Behav Ther. 2007 Sep;38(3):303–313. doi: 10.1016/j.beth.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Messman-Moore TL, Brown AL. Risk perception, rape, and sexual revictimization: A prospective study of college women. Psychol Women Quart. 2006 Jun;30(2):159–172. [Google Scholar]
- 22.Messman-Moore TL, Ward RM, Zerubavel N. The Role of Substance Use and Emotion Dysregulation in Predicting Risk for Incapacitated Sexual Revictimization in Women: Results of a Prospective Investigation. Psychology of Addictive Behaviors. 2013 Mar;27(1):125–132. doi: 10.1037/a0031073. [DOI] [PubMed] [Google Scholar]
- 23.Walsh K, DiLillo D, Messman-Moore TL. Lifetime Sexual Victimization and Poor Risk Perception: Does Emotion Dysregulation Account for the Links? Journal of Interpersonal Violence. 2012 Oct;27(15):3054–3071. doi: 10.1177/0886260512441081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeater EA, McFall RM, Viken RJ. The relationship between women's response effectiveness and a history of sexual victimization. J Interpers Violence. 2011 Feb;26(3):462–478. doi: 10.1177/0886260510363425. [DOI] [PubMed] [Google Scholar]
- 25.Yeater EA, Viken RJ. Factors affecting women's response choices to dating and social situations. J Interpers Violence. 2010 Aug;25(8):1411–1428. doi: 10.1177/0886260509354588. [DOI] [PubMed] [Google Scholar]
- 26.Yeater EA, Treat TA, Viken RJ, McFall RM. Cognitive processes underlying women's risk judgments: associations with sexual victimization history and rape myth acceptance. J. Consult. Clin. Psychol. 2010 Jun;78(3):375–386. doi: 10.1037/a0019297. [DOI] [PubMed] [Google Scholar]
- 27.Cisler JM, Amstadter AB, Begle AM, et al. PTSD symptoms, potentially traumatic event exposure, and binge drinking: a prospective study with a national sample of adolescents. J. Anxiety Disord. 2011 Oct;25(7):978–987. doi: 10.1016/j.janxdis.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cougle JR, Resnick H, Kilpatrick DG. A prospective examination of PTSD symptoms as risk factors for subsequent exposure to potentially traumatic events among women. J. Abnorm. Psychol. 2009 May;118(2):405–411. doi: 10.1037/a0015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenow JK, Scott Steele J, Smitherman S, Kilts CD, Cisler JM. Attenuated behavioral and brain responses to trust violations among assaulted adolescent girls. Psychiatry Res. 2014 Jul 30;223(1):1–8. doi: 10.1016/j.pscychresns.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belli SR, Rogers RD, Lau JY. Adult and adolescent social reciprocity: Experimental data from the Trust Game. J. Adolesc. 2012 Oct;35(5):1341–1349. doi: 10.1016/j.adolescence.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 31.King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008 Aug 8;321(5890):806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krueger F, McCabe K, Moll J, et al. Neural correlates of trust. Proc. Natl. Acad. Sci. U. S. A. 2007 Dec 11;104(50):20084–20089. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unoka Z, Seres I, Aspan N, Bodi N, Keri S. Trust game reveals restricted interpersonal transactions in patients with borderline personality disorder. J Pers Disord. 2009 Aug;23(4):399–409. doi: 10.1521/pedi.2009.23.4.399. [DOI] [PubMed] [Google Scholar]
- 34.Daw ND, O'Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006 Jun 15;441(7095):876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behrens TE, Hunt LT, Woolrich MW, Rushworth MF. Associative learning of social value. Nature. 2008 Nov 13;456(7219):245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat. Neurosci. 2007 Sep;10(9):1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- 37.Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 2008 Apr;11(4):389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- 38.Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. J. Consult. Clin. Psychol. 2000 Feb;68(1):19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- 39.Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J. Consult. Clin. Psychol. 2003 Aug;71(4):692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- 40.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J. Consult. Clin. Psychol. 1993 Dec;61(6):984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 41.First MB, Spitzer Robert L, Gibbon Miriam, Williams Janet BW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 42.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav. Res. Ther. 1996 Aug;34(8):669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 43.Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 44.Sutton R, Barto A. Reinforcement learning: An introduction. Cambridge, MA: The MIT Press; 1998. [Google Scholar]
- 45.Behrens TE, Hunt LT, Rushworth MF. The computation of social behavior. Science. 2009 May 29;324(5931):1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- 46.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J. Neurosci. 2008 Mar 12;28(11):2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schonberg T, Daw ND, Joel D, O'Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J. Neurosci. 2007 Nov 21;27(47):12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. J. Cogn. Neurosci. 2007 Nov;19(11):1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- 49.Vogeley K, Bussfeld P, Newen A, et al. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001 Jul;14(1 Pt 1):170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- 50.Hampton AN, Bossaerts P, O'Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc. Natl. Acad. Sci. U. S. A. 2008 May 6;105(18):6741–6746. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007 Dec;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- 52.Aupperle RL, Allard CB, Grimes EM, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Archives of General Psychiatry. 2012;69(4):360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Depiction of the non-social two-arm bandit task. Participants were told to receive as many unlocked houses as they could, and accordingly to choose the house on each trial they believed was most likely to be unlocked.
Demonstration of RW modeling results for the trust game. Top: The mean investment behavior across participants across trials is depicted along with the mean modeled expected return on investments. As can be seen, the modeled expected returns tracked closely with the observed investment behavior. Bottom: In our implementation of the trust game, participants received ~50% return on investment in trials 1–5, ~30% in trials 6–10, and ~50% in trials 11–15. Accordingly, if the RW model accurately reflects the cognitive decision-making model used by participants on the task, then we expect that the ratio between participant’s investments and modeled expected return on investments should converge to the actual return of investments. As depicted here, the mean ratio across participants of modeled expected return / observed investment converged towards the empirical ratio of actual returns / observed investments in each phase of the task.
Bar graphs depicting between-group differences in neural encoding of prediction errors in the left temporoparietal junction during the social and non-social bandit tasks.
Bar graphs depicting between-group differences in neural encoding of value expectation in the left middle frontal gyrus, pgACC, left temporoparietal junction, and dorsomedial PFC during the social and non-social bandit tasks.
Bar graphs depicting between-group differences in neural encoding of value expectation in the right TPJ and left middle frontal gyrus during the social and non-social bandit tasks.