Abstract
Light-emitting diode therapy (LEDT) applied over the leg, gluteus and lower-back muscles of mice using a LED cluster (630 nm and 850 nm, 80 mW/cm2, 7.2 J/cm2) increased muscle performance (repetitive climbing of a ladder carrying a water-filled tube attached to the tail), ATP and mitochondrial metabolism; oxidative stress and proliferative myocyte markers in mice subjected to acute and progressive strength training. Six bi-daily training sessions LEDT-After and LEDT-Before-After regimens more than doubled muscle performance and increased ATP more than tenfold. The effectiveness of LEDT on improving muscle performance and recovery suggest applicability for high performance sports and in training programs.
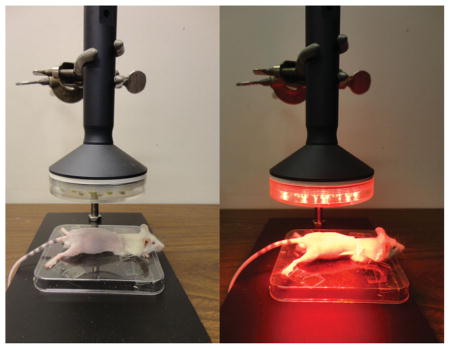
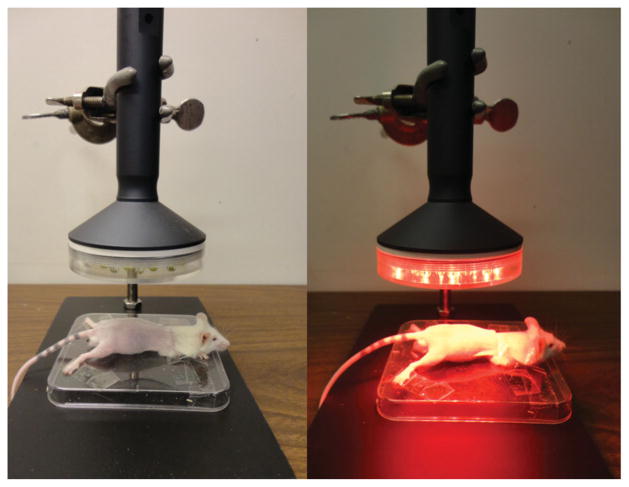
Positioning of the mice and light-emitting diode therapy (LEDT) applied on mouse legs, gluteus and lower-back muscles without contact.
Keywords: LLLT, LEDT, muscle performance, muscle recovery, ATP
1. Introduction
Low-level laser (light) therapy has several applications in medicine such as treatment of pain [1, 2], tendinopathies [3] and acceleration of tissue repair [2, 4]. Since the 1960s when the first laser (Light Amplification by Stimulated Emission of Radiation) devices were constructed, many applications of this therapy and its mechanisms of action have been investigated around the world [5].
Light therapy can be delivered by different light sources such as diode lasers or light emitting diodes (LEDs). These light sources differ in monochromaticity and coherence, since diode lasers are coherent with a tiny spectral bandwidth and less divergence of the light beams compared to the light emitted by LEDs [5]. The spectral regions generally used for light therapy range between red (600 nm) to near infrared (1,000 nm) with total power in range of 1 mW–500 mW and power density (irradiance) in the range of range 1 mW–5 W/cm2 [5]. These lasers and LEDs are considered to produce equivalent effects on the tissue if the dose of light delivered/applied is in accordance with the possible biphasic dose-response previously reported [5–7]. The light-tissue interaction depends on light absorption by specific structures in the cells that are known as chromophores [8–11].
Recently light therapy using lasers and LEDs has been used to increase muscle performance in exercises involving strength [12] or fatigue resistance [13–15]; and light therapy may have a role to play in preparing athletes competing in high performance sports. Recent reviews have reported positive effects of light therapy on muscle performance, highlighting protection from exercise-induced muscle damage [16]; an increased number of repetitions in maximum exertion tests [17]; increased workload, torque and muscle fatigue resistance in training programs; as well as an overview of the main possible mechanisms of action of the light therapy on muscle tissue [18].
Several biological factors govern success or optimum performance in sports that involve high-intensity exercise, or alternatively involve endurance exercise, that both require muscle adaptation during pre-competition training programs. Among these factors are the depletion of the energy supply for muscle contraction which comprises adenosine tri-phosphate (ATP) and glycogen; accumulation of possibly deleterious metabolites from energy metabolism such as lactate, adenosine diphosphate (ADP), adenosine monophosphate (AMP), ions Ca2+ and H+; production of reactive oxygen species (ROS) [19–22]; and the recovery process from microlesions or muscle damage [23]. Light therapy seems to be able to benefit all these “limitations” since its mechanism of action involves the improvement of mitochondrial metabolism and increased ATP synthesis [24, 25] owing to increased activity of cytochrome c oxidase (COX) in the electron transport chain (ETC) [9, 25, 26]; reduction of reactive oxygen species (ROS) or improvement of oxidative stress defense [27, 28]; and can stimulate faster muscle repair due to an increased proliferation and differentiation of muscle cells [29].
Experimental and clinical trials with different methodologies have reported the benefits of light therapy on muscle performance when applied before [15, 30, 31] or after exercise [12, 13, 32]. However there is no consensus about the best time regimen for use of light therapy [18]. The best wavelength (red or infrared) to stimulate muscle cells and increase muscle performance is also unclear.
In the current study we used an experimental model of mice exercising on a ladder similar to that reported in a previous study [33], in order to simulate a clinical strength training program that would allow us to identify which light therapy regimen would be better to increase muscle performance. Four different regimens of light therapy were applied to the mouse leg, gluteus and lower-back muscles during a training program: sham; before; before-after; and after each training session. Light therapy was delivered from LEDs (LEDT) with two simultaneous wavelengths (red and infrared). Assessment of muscle performance (load, number of repetitions, muscle work and power), markers of cellular energy and metabolism (ATP, glycogen and COX), oxidative stress markers (protein carbonyls, glutathione, catalase activity, lipid peroxidation, protein thiols) and muscle cell proliferation (BrdU – 5-bromo-2′-deoxyuridine) and adult myonuclei (DAPI – 4′,6-diamidino-2-phenylindole) were carried out.
2. Materials and methods
2.1 Animals
This study was performed with 8 week-old male Balb/c mice, weighing on average 22.22 g (SEM 0.24), housed at five mice per cage and kept on a 12 hour light 12 hour dark cycle. The 22 animals were provided by Charles River Inc and were provided with water and fed ad libitum at the animal facility of Massachusetts General Hospital. All procedures were approved by the IACUC of Massachusetts General Hospital (protocol #2014N000055) and met the guidelines of the National Institutes of Health.
2.2 Experimental groups
Twenty-two animals were randomly allocated into 4 exercise groups with 5 animals in each group, and 2 animals were allocated into an “absolute” control group:
LEDT-Sham group: animals were treated with sham LEDT (LEDT device in placebo mode) over both legs, gluteus and lower-back muscles 5 minutes before each training session on ladder.
LEDT-Before: animals were treated with real LEDT over both legs, gluteus and lower-back muscles 5 minutes before each training session on ladder.
LEDT-Before-After: animals were treated with real LEDT over both legs, gluteus and lower-back muscles 5 minutes before and 5 minutes after each training session on ladder.
LEDT-After: animals were treated with real LEDT over both legs, gluteus and lower-back muscles 5 minutes after each training session on ladder.
Control: animals were not subjected to any LEDT or exercise or muscle performance assessment.
2.3 Ladder
An inclined ladder (80°) with dimensions of 100 cm × 9 cm (length and width, respectively) with bars spaced at 0.5 cm intervals was used in this study as reported in a previous study [33] (Figure 1).
Figure 1.
Ladder. Inclined ladder (80°) with 100 cm × 9 cm (length and width, respectively) used for the training program and muscle performance assessments. Falcon tube filled with water and attached to the mouse tail.
2.4 Load
A Falcon tube (50 ml) was filled with measured volumes of water and weighed using a precise scale. The target load was achieved adding or removing water from the tube and then this tube was attached to the mouse tail using adhesive tape (Figure 1). All loads were calculated in grams.
2.5 Procedures
The schedule of the various exercise procedures is described in Table 1.
Table 1.
Schedule for exercise procedures.
| Day | Procedure | # repetitions | Load |
|---|---|---|---|
| Day 1 | Familiarization | 4 × 10 = 40 | zero |
| Day 2 | 3RM baseline | 3 | Starting at 2 × BWa |
| Day 3 | Training 1 | 5 × 10 = 50 | 0.8 × 3RMb |
| Day 5 | Training 2 | 5 × 10 = 50 | 0.9 × 3RM |
| Day 7 | Training 3 | 5 × 10 = 50 | 1.0 × 3RM |
| Day 9 | Training 4 | 5 × 10 = 50 | 1.1 × 3RM |
| Day 11 | Training 5 | 5 × 10 = 50 | 1.2 × 3RM |
| Day 13 | Training 6 | 5 × 10 = 50 | 1.3 × 3RM |
| Day 14 | 3RM final | 3 | Starting at 3 × BW |
body weight
average load carried during 3RM baseline measurement
2.5.1 Familiarization with ladder-climbing
All experimental groups, except Control group, were familiarized with climbing the ladder one day before the start of muscle performance assessment and training. The familiarization procedure was 4 sets of 10 climbs on the ladder (repetitions) with rest periods of 2 minutes between individual sets. No load was attached to the mouse tail during this procedure.
2.5.2 Three repetitions maximum load (3RM)
This test was the first evaluation of muscle performance and was set as the average of the maximum load carried by each animal during 3 consecutive full climbs of the inclined ladder (3RM). Slight pressure with tweezers was applied on mouse tail if the animal stopped during a climb. The test was stopped when mice were not able to climb or lost their grip on the ladder due to failure of concentric muscle contraction. The first attempt included a load corresponding to 200% of the individual mouse body weight. A maximum of 3 climb attempts was applied. If a mouse finished the climb the load was increased by 10% for the next climb, while if the mouse failed to finish a climb, the load was decreased by 10% for the next climb. The 3RM evaluation was performed twice; the first time was 24 h after familiarization procedure (baseline) and the second time was 24 h after the last training session (final).
2.5.3 Acute strength training protocol
After 24 h from initial 3RM baseline assessment, all experimental groups, except Control, were subjected to 6 training sessions carried out on alternate days (every 48 h). Each training session consisted of 5 sets of 10 repetitions (climbs) on the ladder with a rest period of 2 minutes between each set. If the animal could not complete a set or failed during a climb, the distance climbed (in cm) was measured and the rest period was started immediately. During some repetitions, a slight pressure on the mouse tail was performed with tweezers to stimulate the animal to climb and complete the exercise. If after three applications of gentle pressures the mouse could not resume climbing, and stopped or lost its grip on the ladder, the set of repetitions was stopped and the rest interval was started.
The number of repetitions in each set was measured as well as the time spent to complete the exercise. These data were used to calculate the muscle work and muscle power in each training session. The load of each training session was progressively increased and calculated as percentages of the 3RM (in grams) measured at baseline as follows: first training (80%), second training (90%), third training (100%), fourth training (110%), fifth training (120%) and sixth training (130%).
2.5.4 Light-emitting diode therapy (LEDT)
A non-commercial cluster of 40 LEDs (20 red –630 ± 10 nm; 20 infrared – 850 ± 20 nm) with diameter of 76 mm was used in this study. A complete description of the LEDT parameters is presented in Table 2. The optical power reaching the surface of the mouse skin was measured with an optical energy meter PM100D Thorlabs® fitted with a sensor S142C (area of 1.13 cm2). All mice (except mice in Control) were shaved and fixed on a plastic plate using adhesive tapes. Afterwards, in accordance with experimental group, these animals were treated with LEDT over both legs, gluteus and lower-back muscles at a distance of 45 mm (without contact) (Figure 2). Irradiation lasted 90 s per session with fixed parameters as described in Table 1. LEDT placebo had no energy (0 J) and no power (0 mW) applied over the targeted muscles. The light dose was based on the possible biphasic dose response reported previously [5, 6]. Moreover, dual wavelengths were chosen to function at the same time in this study based on specificities of the chromophores in the cells and therefore optimizing the effects of the light therapy (LEDT) by a double band of absorption [8–11].
Table 2.
Optical parameters of light-emitting diode therapy (LEDT). LEDT-Sham group received a placebo therapy (device switched off) with the same time of treatment.
| Number of LEDs (cluster): 40 (20 infrared-IR and 20 red-RED) |
| Wavelength: 850 ± 20 nm (IR) and 630 ± 10 nm (RED) |
| Pulse frequency: continuous |
| Optical output of each LED: 50 mW (IR) and 25 mW (RED) |
| Optical output (cluster): 1,000 mW (IR) and 500 mW (RED) |
| LED cluster size: 45 cm2 |
| Power density (at skin surface): 80 mW/cm2 |
| Treatment time: 90 s |
| Energy density applied (at skin surface): 7.2 J/cm2 |
| Application mode: without contact |
| Distance from mice or power meter: 45 mm |
Figure 2.
LEDT. Positioning of the mice and light-emitting diode therapy (LEDT) applied on mouse legs, gluteus and lower-back muscles without contact.
2.5.5 Anesthesia, surgical and sacrifice procedures
Anesthesia
5 h after the final 3RM test, all trained and control mice were anesthetized using Ketamine and Xylazine at a proportion of 80 mg/kg of Ketamine and 12 mg/kg of Xylazine.
Surgery
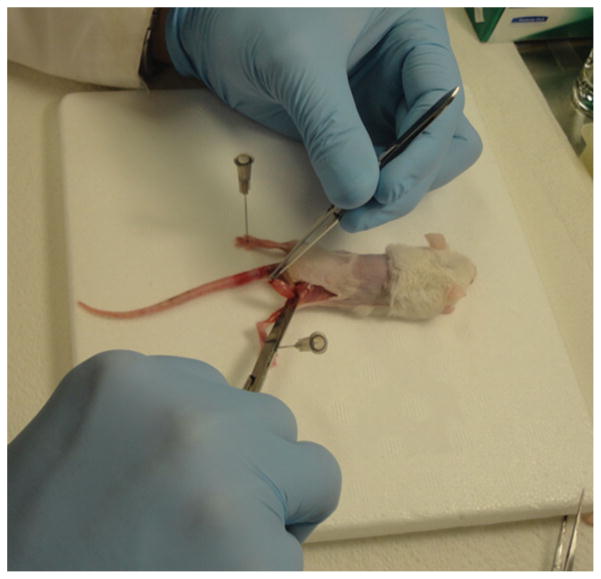
After the anesthesia procedure, gastrocnemius and quadriceps femoris muscles were excised bilaterally (Figure 3). Gastrocnemius plus soleus muscles from one leg were excised, separated surgically and immediately frozen in liquid nitrogen. Both muscles were stored at −80 °C until analysis of ATP in gastrocnemius performed exactly 7 days after the surgery. Both quadriceps femoris muscles were excised, frozen in liquid nitrogen and stored at −80 °C until analysis of glycogen contents and oxidative stress markers. Gastrocnemius muscle from the second leg was excised, separated surgically from the soleus and fixed in 10% buffered formalin (Fisher Scientific, SF100–20) during 72 hours for paraffin embedding and immunofluorescence staining.
Figure 3.
Surgery. After anesthesia, mice were subjected to surgical procedures for excision of gastrocnemius plus soleus and quadriceps femoris muscles. The gastrocnemius and soleus muscles were separated surgically before analysis.
Sacrifice
Finally, animals were sacrificed by cervical dislocation at the end of the surgery while they were still under anesthesia.
2.6 Outcomes
2.6.1 Muscle performance
The 3RM test was the first evaluation for muscle performance. This test measured the maximum load (in grams) carried by each animal during 3 consecutive full climbs on the inclined ladder.
During each training session the load, number of repetitions (rep), distance climbed and time spent to complete each repetition were recorded. These data were used to calculate muscle work and power.
Although the ladder had a total length of 100 cm available the maximum distance available to climb was set at 70 cm in order to avoid the load touching the floor. Thereby the muscle work was calculated as follows:
where “m” is mass of the load (grams converted to kilogram) in each training session plus mouse body mass (values converted to kilogram); “g” is acceleration due to gravity and “h” is the distance climbed (converted to meters). Results were obtained in Joules (J) and presented as average ± standard error of mean (SEM) for each group at each training session.
Muscle power was calculated from results of muscle work (J) and time spent (s) to perform all repetitions of each set at all training sessions as follows:
where “J” is Joule and represents the muscle work performed and “s” is time in seconds. Result were obtained in milliwatts (mW) and presented as average ± standard error of mean (SEM) per each group at each training session.
2.6.2 Muscular ATP
The gastrocnemius muscle from one leg of each animal was used for analysis of muscular ATP. Muscle samples were thawed in ice for 5 min, homogenized at a proportion of 3–4 mg of tissue to 500 μl of 10% perchloric acid (HClO4) following procedures previously published [34]. Afterwards, an aliquot of 10 μl of the muscle homogenate plus 40 μl of CellTiter Glo Luminescent Cell Viability Assay mix (Promega), totaling 50 μl, were placed in the well microplate (Costar™ 96-Well White Clear-Bottom Plates). Luminescence signals were measured in a SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA) with integration time of 5 s to increase low signals [34]. A standard curve was prepared using ATP standard (Sigma) according to manufacturer’s guidelines and then ATP concentration was calculated in nanomol (nmol) per milligram (mg) of protein. An aliquot of muscle homogenate was used to quantify the total protein by Quanti-Pro™ BCA Assay kit (Sigma-Aldrich) following manufacturer’s guidelines.
2.6.3 Muscular glycogen
Quadriceps femoris muscles were thawed in ice for 30 min and muscular glycogen was measured in 50 mg of quadriceps femoris tissue homogenized with 6 N NaOH at a proportion of 50 mg/ml. A standard curve was prepared using absolute ethanol (100%), K2SO4 (10%), phenol (4.1%) and 1 mM of glucose (2%) according to Dubois et al. [35]. Optical density was read at 480 nm in spectrophotometer (Evolution™ 300 UV-Vis, software VISPRO – Thermo Scientific). Data were normalized per mg of muscle tissue.
2.6.4 Oxidative stress markers
Protein carbonyl
Quadriceps femoris muscles were homogenized in deionized water (dH2O) at a proportion of 10 mg/200 μl. Protein carbonyl content was quantified using Protein Carbonyl Content Assay kit (Biovision) with the colorimetric method and following manufacturer’s guidelines. All results were normalized per total protein quantified by Quanti-Pro™ BCA Assay kit (Sigma-Aldrich) following manufacturer’s guidelines.
Glutathione
Quadriceps femoris muscles were homogenized in 100 mM ice cold phosphate buffer (pH = 7.4) at a proportion of 10 mg/250 μl. Phosphate buffer was prepared with dibasic (Na2HPO4) and monobasic (NaH2PO4) sodium phosphate at equal proportions. Total and oxidized glutathione analysis was carried out with Glutathione Colorimetric Assay kit (ARBOR Assays) following manufacturer’s guidelines. In addition, all results were normalized per total protein of the samples using QuantiPro™ BCA Assay kit (Sigma-Aldrich) following manufacturer’s guidelines.
Catalase activity
Quadriceps femoris muscles were homogenized in cold assay buffer provided in a Catalase Activity Assay kit (Biovision) at a proportion of 50 mg/100 μl. This analysis used the colorimetric method and followed manufacture’s guidelines.
Lipid peroxidation using TBARS (Thiobarbituric Acid Reactive Substances)
Quadriceps femoris muscles were homogenized with RIPA Buffer (Sigma-Aldrich) at a proportion of 25 mg/250 μl. Next, TBARS Colorimetric Assay kit (Cayman Chemical) was used following manufacturer’s guidelines.
Protein Thiols
Quadriceps femoris muscles were homogenized in ice cold 100 mM phosphate buffer at a proportion of 10 mg/250 μl. Next, a Fluorescent Protein Thiol Detectiont kit (ARBOR Assays) was used following manufacturer’s guidelines. In addition, all results were normalized per total protein quantified by QuantiPro™ BCA Assay kit (Sigma-Aldrich) following manufacturer’s guidelines.
2.6.5 Immunofluorescence analyses
5-bromo-2′-deoxyuridine (BrdU)
BrdU reagent (Sigma-Aldrich) was diluted in saline solution (PBS) at a concentration of 10 mg/ml. Next, during the last 8 days of the experiment all animals (including Control group) received a single daily intra peritoneal injection (50 mg/kg) of BrdU. Mice were anesthetized and submitted to surgical procedures described previously. Gastrocnemius muscles were embedded in paraffin, cut in axial slices of 5 μm thickness from the muscle belly region by a microtome and mounted on slides for immunohistochemical procedures. Briefly, slides were deparaffinized with graded ethanol and then passed through antigen retrieval solution in a water bath pre-heated at 98 °C for 30 min. Afterwards slides were washed and incubated for 15 min at room temperature with 0.1% Triton X-100 TBS for cell membrane permeabilization, washed again and incubated for 30 min in protein blocking solution consisting of 3% BSA (Bovine Serum Albumin – Sigma) and 10% goat serum in TBS. Next, slides were immunostained with sheep anti-BrdU (Ab1893 – Abcam, Cambridge, MA) at 1: 50 working concentration and selected anti-sheep (Alexa Fluor® 647 – Invitrogen) fluorescent secondary antibody matched to the primary antibody to stain at 1: 200 working concentration. Finally, slides were cover-slipped with mounting media containing DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen). Cells positively stained for BrdU were imaged using confocal microscope (Olympus America Inc. Center Valley, PA, USA) from three random fields. BrdU and DAPI staining were quantified using software Image J (NIH, Bethesda, MD).
Cytochrome c oxidase subunit IV (COX IV)
Gastrocnemius muscles were subjected to the same procedures described for BrdU staining. Slides were immunostained with rabbit anti-COX IV (Cell Signaling Technology®) at 1: 500 working concentration and selected anti-rabbit (Alexa Fluor® 680 – Invitrogen) secondary antibody matched with primary antibody to stain at 1: 200 working concentration. Cells positively stained for COX IV were imaged using confocal microscopy as above and then the red channel of the exported images was changed to yellow.
2.6.6 Statistical analysis
Shapiro-Wilk’s W test verified the normal distribution of the data. All experimental groups subjected to training protocols were compared at each training session for number of repetitions, muscle work and muscle power using one-way analysis of variance (ANOVA) and Tukey HSD post-hoc test. The load of 3RM among these same groups was compared by Two-way ANOVA with repeated measures (baseline versus final) and Tukey HSD post-hoc test. For muscular ATP, glycogen, oxidative stress markers and immunofluorescence stains, all experimental groups were compared by one-way ANOVA and Tukey’s HSD post-hoc test. Significance was set at p < 0.05.
3. Results
3.1 Muscle performance
3RM
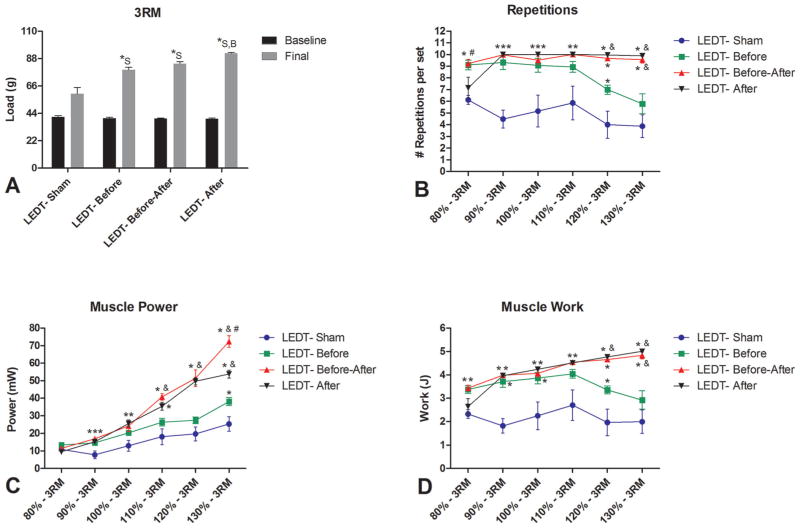
The final load 3RM was significantly higher (p < 0.05) in all experimental groups at the end of the experiment period compared to baseline. The final load of LEDT-After (92.28 g, SEM 0.82) was higher than LEDT-Sham (59.58 g, SEM 5.28; p < 0.001) and LEDT-Before (78.98 g, SEM 1.96; p = 0.020). In addition, LEDT-Sham had a significantly lower final load (p < 0.001) compared to LEDT-Before as well as LEDT-Before/After (83.91 g, SEM 1.49) (Figure 4A).
Figure 4.
Muscle performance (n = 5 animals per group). (A) Baseline and Final test of 3 repetitions maximum (3RM) measuring the total load carried by mice during this test. * statistical significance (p < 0.05) comparing the final 3RM load between groups. (B) Number of repetitions or climbs performed by each group treated with different regimens of LEDT during the progressive training program. (C) Muscle power developed by each group treated with different regimens of LEDT during the progressive training program. (D) Muscle work developed by each group treated with different regimens of LEDT during the progressive training program. * statistical significance (p < 0.05) compared to LEDT-Sham. # statistical significance (p < 0.05) compared to LEDT-After. & statistical significance (p < 0.05) compared to LEDT-Before. Abbreviations: LEDT = light-emitting diode therapy; LEDT-Sham (Sham – S) = LEDT placebo (LEDT device in placebo mode) on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before (Before – B) = LEDT applied on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before-After (Before-After – A–B) = LEDT applied on muscles immediately before (5 minutes) and immediately after (5 minutes) each training session on ladder; LEDT-After (After – A) = LEDT applied on muscles immediately after (5 minutes) each training session on ladder. The load of 3RM at baseline versus final was analyzed by Two-way analysis of variance (ANOVA) with repeated measures. Number of repetitions, muscle work and power were analyzed by One-way ANOVA.
Number of repetitions
There were significantly differences (p < 0.05) between all groups in each training session (Figure 4B). At 80% of 3RM (first session): animals in LEDT-Before and LEDT-Before-After groups performed more repetitions compared to animals in LEDT-Sham and LEDT-After (p < 0.01) groups. At 90% of 3RM (second session): animals in LED-Sham group performed fewer repetitions than animals in LEDT-Before, LEDT-Before-After and LEDT-After groups (p < 0.001). At 100% of 3RM (third session): animals in LEDT-Sham group performed fewer repetitions compared to animals in LEDT-Before (p = 0.014), LED-Before-After (p = 0.010) and LEDT-After (p = 0.002) groups. At 110% of 3RM (fourth session): animals in LEDT-Sham group performed fewer repetitions than animals in LEDT-Before-After (p = 0.013) and LEDT-After (p = 0.009) groups. At 120% of 3RM (fifth session): animals in LEDT-After group performed more repetitions than animals in LEDT-Before (p = 0.022) and LEDT-Sham (p < 0.001) groups. In addition, animals in LEDT-Sham performed fewer repetitions than animals in LEDT-Before (p = 0.022), LEDT-Before-After and LEDT-After (p < 0.001) groups. At 130% of 3RM (sixth session): animals in LEDT-Before-After and LEDT-After groups performed more repetitions than animals in LEDT-Sham (p < 0.001) and LEDT-Before (p < 0.01) groups.
Muscle Power
At 80% of 3RM there were no significant differences among all groups (p > 0.05). At 90% of 3RM: animals in LEDT-Sham group had lower muscle power compared to animals in LEDT-Before, LEDT-Before-After and LEDT-After (p < 0.01) groups. At 100% of 3RM: animals in LEDT-Sham group had lower muscle power than animals in LEDT-Before-After (p = 0.025) and LEDT-After (p = 0.007) groups. At 110% of 3RM: animals in LEDT-Before-After group developed more muscle power than animals in LEDT-Sham (p < 0.001) and LEDT-Before (p = 0.013) groups. In addition, animals in LEDT-After group had more muscle power than animals in LEDT-Sham (p = 0.002) group. At 120% of 3RM: animals in LEDT-Before-After and LEDT-After groups developed more muscle power than animals in LEDT-Sham and LEDT-Before (p < 0.001) groups. At 130% of 3RM: animals in LEDT-Before-After group developed more muscle power than animals in LEDT-Sham and LEDT-Before (p < 0.001) as well as LEDT-After (p = 0.001) groups. In addition, animals in LEDT-After group had more muscle power than animals in LEDT-Sham (p < 0.001) and LEDT-Before (p = 0.004) groups. Finally, animals in LEDT-Before group had major muscle power than animals in LEDT-Sham (p = 0.020) group (Figure 4C).
Muscle Work
Similar to results presented in Figure 4B, at 80% of 3RM only animals in LEDT-Before and LEDT-Before-After groups performed more muscle work compared to LEDT-Sham (p < 0.05) group (Figure 4D). At 90% of 3RM: animals in LEDT-Sham group performed less muscle work than animals in LEDT-Before, LEDT-Before-After and LEDT-After (p < 0.001) groups. These results were similar at 100% of 3RM (p < 0.001). At 110% of 3RM: animals in LEDT-Sham group had lower muscle work compared to animals in LEDT-Before-After (p = 0.015) and LEDT-After (p = 0.011) groups. At 120% of 3RM: animals in LEDT-Sham group performed lower muscle work compared to animals in LEDT-Before (p = 0.027) and LEDT-Before-After and LEDT-After (p < 0.001) groups. In addition, animals in LEDT-After group performed more muscle work than animals in LEDT-Before (p = 0.026) group. At 130% of 3RM: animals in LEDT-Before-After and LEDT-After groups performed more muscle work than animals in LEDT-Sham (p < 0.001) and LEDT-Before (p < 0.01) groups (Figure 4D).
3.2 Muscle ATP content
Animals in LEDT-After group had significantly (p < 0.001) more ATP concentration (1,367.64 nmol/mg protein, SEM 105.30) compared to animals in LEDT-Sham (15.85 nmol/mg protein, SEM 5.14), LEDT-Before (81.00 nmol/mg protein, SEM 10.11), LEDT-Before-After (687.62 nmol/mg protein, SEM 11.76) and Control (17.53 nmol/mg protein, SEM 7.47) groups. In addition, animals in LEDT-Before-After group had also major contents of ATP compared to animals in LEDT-Before, LEDT-Sham and Control (p < 0.001) groups (Figure 5A).
Figure 5.
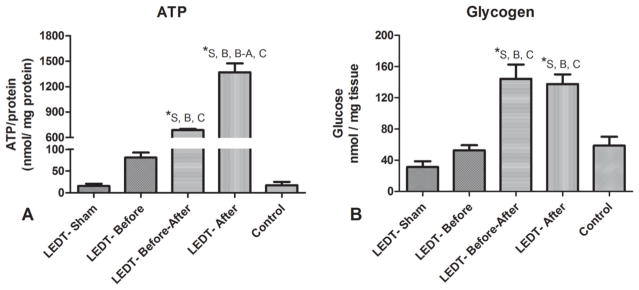
Muscular ATP and glycogen contents (n = 5 animals per group). (A) Adenosine triphosphate (ATP) contents in gastrocnemius muscle after the training program. (B) Glycogen contents in quadriceps femoris muscles after the training program. * statistical significance (p < 0.05). Abbreviations: LEDT = light-emitting diode therapy; LEDT-Sham (Sham – S) = LEDT placebo (LEDT device in placebo mode) on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before (Before – B) = LEDT applied on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before-After (Before-After – A–B) = LEDT applied on muscles immediately before (5 minutes) and immediately after (5 minutes) each training session on ladder; LEDT-After (After – A) = LEDT applied on muscles immediately after (5 minutes) each training session on ladder. Control (C) = no exercise or muscle performance assessment. Comparisons among all groups were conducted using One-way analysis of variance (ANOVA).
3.3 Muscle glycogen content
Animals in LEDT-After (137.76 nmol/mg tissue, SEM 11.40) and LEDT-Before-After (144.44 nmol/mg tissue, SEM 16.23) groups had significantly higher concentrations of glycogen in quadriceps femoris muscles (p < 0.001) compared to animals in LEDT-Sham (31.36 nmol/mg tissue, SEM 7.45), LEDT-Before (52.76 nmol/mg tissue, SEM 6.53) and Control (58.78 nmol/mg tissue, SEM 7.17) groups (Figure 5B).
3.4 Oxidative stress markers
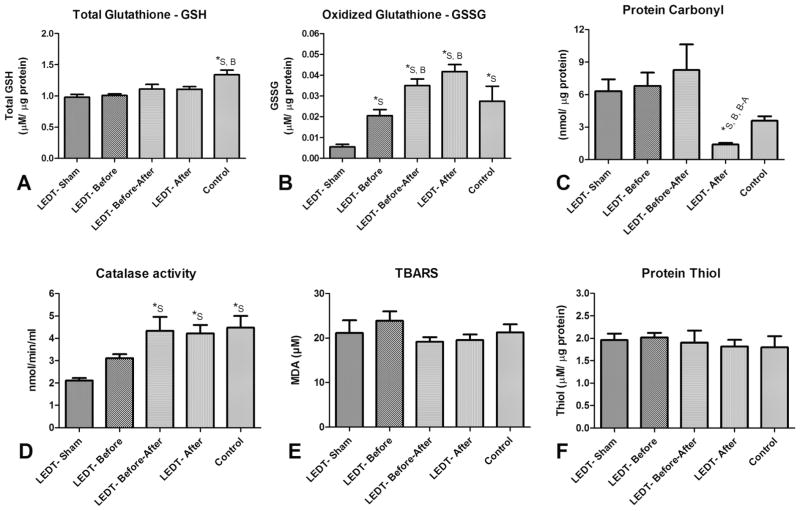
Total glutathione
Animals in Control group (1.33 μM/μg protein, SEM 0.11) had a significantly higher concentration of total glutathione compared to animals in LEDT-Sham (0.097 μM/μg protein, SEM 0.046; p = 0.005) and LEDT-Before (1.00 μM/μg protein, SEM 0.02; p = 0.010) groups (Figure 6A).
Figure 6.
Oxidative stress markers (n = 5 animals per group) in quadriceps femoris muscles. (A) Total Glutathione (reduced glutathione – GSH). (B) Oxidized Glutathione (GSSG). (C) Protein Carbonyl. (D) Catalase activity. (E) Lipid peroxidation using TBARS (Thiobarbituric Acid Reactive Substances). (F) Protein Thiol. * statistical significance (p < 0.05). Abbreviations: LEDT = light-emitting diode therapy; LEDT-Sham (Sham – S) = LEDT placebo (LEDT device in placebo mode) on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before (Before – B) = LEDT applied on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before-After (Before-After – A–B) = LEDT applied on muscles immediately before (5 minutes) and immediately after (5 minutes) each training session on ladder; LEDT-After (After – A) = LEDT applied on muscles immediately after (5 minutes) each training session on ladder. Control (C) = no exercise or muscle performance assessment. Comparisons among all groups were conducted using One-way analysis of variance (ANOVA).
Oxidized glutathione
Animals in LEDT-Sham group (0.005 μM/μg protein, SEM 0.001) had significantly minor concentration of glutathione oxidized compared to animals in LEDT-Before (0.20 μM/μg protein, SEM 0.002; p = 0.015), LEDT-Before-After (0.035 μM/μg protein, SEM 0.003; p < 0.001), LEDT-After (0.041 μM/μg protein, SEM 0.003; p < 0.001) and Control (0.027 μM/μg protein, SEM 0.007; p = 0.006) groups. In addition, animals in LEDT-Before group had significantly minor concentration of oxidized glutathione compared to animals in LEDT-After (p < 0.001) and LEDT-Before-After (p = 0.024) groups (Figure 6B).
Protein carbonyl
Animals in LEDT-After group (1.40 nmol/μg protein, SEM 0.15) had significantly lower concentrations of protein carbonyls compared to animals in LEDT-Sham (6.31 nmol/μg protein, SEM 1.09; p = 0.030), LEDT-Before (6.81 nmol/μg protein, SEM 1.21; p = 0.040) and LEDT-Before-After (8.27 nmol/μg protein, SEM 2.35; p = 0.008) groups (Figure 6C).
Catalase activity
Animals in LEDT-Sham group (2.11 nmol/min/ml, SEM 0.10) had significantly lower catalase activity (p < 0.01) compared to animals in LEDT-Before-After (4.33 nmol/min/ml, SEM 0.62), LEDT-After (4.22 nmol/min/ml, SEM 0.37) and Control (4.47 nmol/min/ml, SEM 0.52) groups (Figure 6D).
Lipid peroxidation using TBARS
There were no significant differences between any of the groups (p > 0.05) assessed. Animals in Control group had a concentration of 21.29 μM (SEM 1.13); animals in LEDT-Sham had 21.12 μM (SEM 2.86); animals in LEDT-Before had 23.87 μM (SEM 1.13); animals in LEDT-Before-After had 19.19 μM (SEM 1.01) and animals in LEDT-After had 19.55 μM (SEM 1.24) (Figure 6E).
Protein Thiols
There were no significant differences between any of the groups (p > 0.05) assessed. Animals in Control had 1.79 μM/μg protein (SEM 0.16); animals in LEDT-Sham had 1.96 μM/μg protein (SEM 0.14); animals in LEDT-Before had 2.02 μM/μg protein (SEM 0.10); animals in LEDT-Before-After had 1.90 μM/μg protein (SEM 0.26) and animals in LEDT-After had 1.81 μM/μg protein (SEM 0.15) (Figure 6F).
3.5 Immunofluorescence analyses
BrdU staining
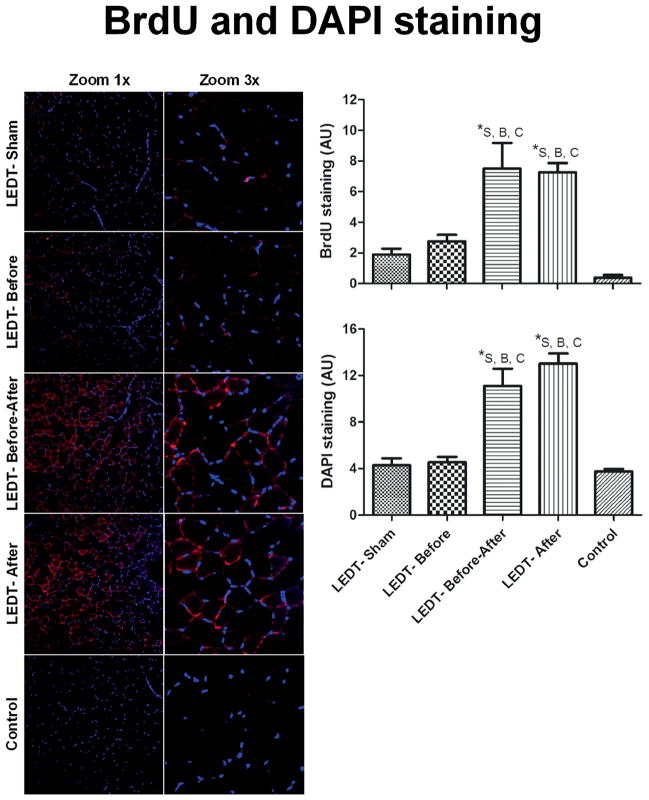
Animals in LEDT-Before-After (7.49 AU, SEM 1.68) and LEDT-After (7.26 AU, SEM 0.59) groups had significantly (p < 0.001) higher BrdU staining compared to animals in LEDT-Sham (1.87 AU, SEM 0.37), LEDT-Before (2.75 AU, SEM 0.42) and Control (0.33 AU, SEM 0.21) groups. AU is arbitrary unit measured with Image J software (Figure 7).
Figure 7.
Muscle cells in proliferative state and adult myonuclei in gastrocnemius muscle (n = 5 animals per each trained group; n = 2 animals in control group). BrdU (5-bromo-2′-deoxyuridine) immunofluorescence stained positive muscles cells in proliferative state. All the red staining indicates newly formed myonuclei. Purple dots mean the merge of red (new myonuclei in formation) and blue (adult myonuclei). DAPI (4′,6-diamidino-2-phenylindole) stained adult myonuclei or already formed myonuclei. Images were acquired with confocal microscopy (Olympus America Inc. Center Valley, PA, USA) at a magnification of 20× with zoom of 1× and 3×. BrdU and DAPI staining were quantified using software Image J (NIH, Bethesda, MD). * statistical significance (p < 0.05). Abbreviations: LEDT = light-emitting diode therapy; LEDT-Sham (Sham – S) = LEDT placebo (LEDT device in placebo mode) on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before (Before – B) = LEDT applied on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before-After (Before-After – A–B) = LEDT applied on muscles immediately before (5 minutes) and immediately after (5 minutes) each training session on ladder; LEDT-After (After – A) = LEDT applied on muscles immediately after (5 minutes) each training session on ladder. Control (C) = not submitted to any exercise or muscle performance assessment. AU = arbitrary units. Comparisons among all groups were conducted using One-way analysis of variance (ANOVA).
DAPI staining
Animals in LEDT-After (12.85 AU, SEM 0.87) and LEDT-Before-After (11.10 AU, SEM 1.48) groups had significantly (p < 0.001) higher DAPI staining compared to animals in LEDT-Sham (4.27 AU, SEM 0.83), LEDT-Before (4.54 AU, SEM 0.46) and Control (3.75 AU, SEM 0.45) groups (Figure 7).
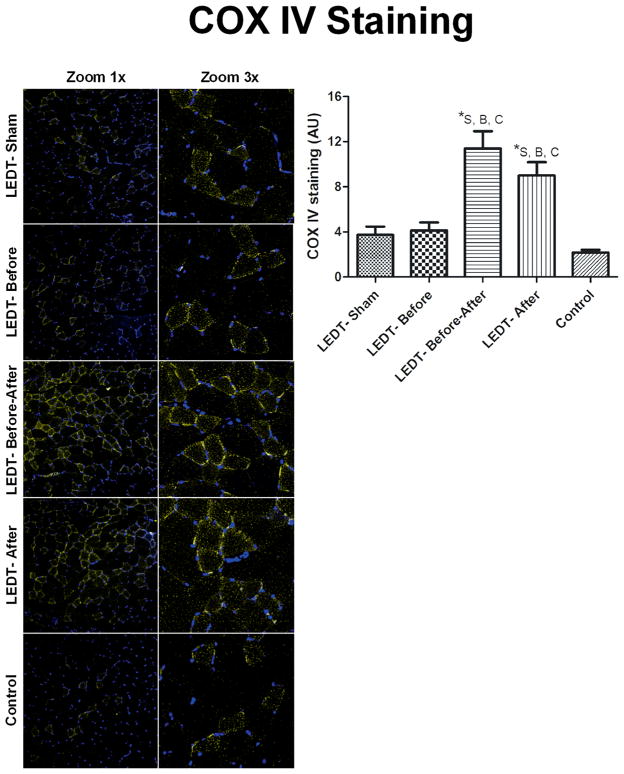
COX IV staining
Animals in LEDT-Before-After (11.39 AU, SEM 1.54) group had significantly higher (p < 0.001) COX IV staining compared to animals in LEDT-Sham (3.74 AU, SEM 0.72), LEDT-Before (4.12 AU, SEM 0.71) and Control (2.18 AU, SEM 0.22) groups. Animals in LEDT-After (9.00 AU, SEM 1.17) group had also significantly (p < 0.01) more COX IV stain compared to animals in LEDT-Sham, LEDT-Before and Control groups (Figure 8).
Figure 8.
Mitochondrial metabolism in gastrocnemius muscle (n = 5 animals per each trained group; n = 2 animals in control group). Cytochrome c oxidase subunit IV (COX IV) immunofluorescence stained in yellow for positive muscles cells with mitochondrial metabolism. Images were acquired with confocal microscopy (Olympus America Inc. Center Valley, PA, USA) at a magnification of 20× with zoom of 1× and 3×. COX IV was quantified using software Image J (NIH, Bethesda, MD). * statistical significance (p < 0.05). Abbreviations: LEDT = light-emitting diode therapy; LEDT-Sham (Sham – S) = LEDT placebo (LEDT device in placebo mode) on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before (Before – B) = LEDT applied on muscles immediately before (5 minutes) each training session on ladder; LEDT-Before-After (Before-After – A–B) = LEDT applied on muscles immediately before (5 minutes) and immediately after (5 minutes) each training session on ladder; LEDT-After (After – A) = LEDT applied on muscles immediately after (5 minutes) each training session on ladder. Control (C) = not submitted to any exercise or muscle performance assessment. AU = arbitrary units. Comparisons among all groups were conducted using One-way analysis of variance (ANOVA).
4. Discussion
Although previous studies have reported on effects of light therapy on muscle performance in protocols involving acute exercise [16–18], as well as during training programs [12, 13], to our knowledge this is the first study investigating what is the best time to use this intervention in a training program by comparing LED therapy as sham, before, before and after or after each training session.
The literature contains a number of reports showing an increased number of repetitions and better protective effect against exercise-induced muscle damage when light therapy is applied on muscles immediately before a bout of intense exercise [15–17, 30, 31]. In accordance with these previous studies, our results in the first session at 80% of 3RM (LEDT before exercise) showed a significantly increased number of repetitions and muscle work (~38%) performed in both groups treated with LEDT before this training session. However, these beneficial effects were not seen in muscle power.
On the other hand, the protective effect of light therapy against exercise-induced muscle damage measured by the decrease in creatine kinase (CK) activity in blood [15–17, 30, 31], could argue against the development of better performance, considering that microlesions or microdamage from exercise are thought to be essential for improvement in muscle tissue structure and metabolism [23]. Our results for muscle performance clearly confirm this hypothesis.
Both groups treated with LEDT after each training session (LEDT-Before-After and LEDT-After) developed significantly more muscle power, repetitions and muscle work than LEDT-Before at the fourth and fifth training session, respectively, as well as from the second training session compared to LEDT-Sham. Moreover, only the LEDT-After group had a higher final load of 3RM compared to LEDT-Sham and LEDT-Before (see Figure 4).
Several hypotheses have been proposed in the literature to explain the effects of low-level laser (light) therapy on muscles [18]. The first one is the stimulation of cytochrome c oxidase (protein expression and/or enzyme activity) that consequently can improve mitochondrial electric transport and increase ATP synthesis [9, 25, 26]. Our results for cytochrome c oxidase subunit IV (COX IV) staining in gastrocnemius muscle strongly suggest increased mitochondrial activity mainly when LEDT is applied after each training session. COX IV staining in LEDT-Before-After and LEDT-After groups was approximately 2.5-fold higher than in LEDT-Before, LEDT-Sham or Control, confirming the hypothesis of increased mitochondrial activity and suggesting more ATP synthesis by aerobic metabolism.
Confirming hypothesis, the ATP content in gastrocnemius muscle of LEDT-After group surprisingly increased significantly around 80-fold compared to Control, as well as 85-fold compared to LEDT-Sham, 16-fold compared to LEDT-Before and 2-fold compared to LEDT-Before-After groups. These results strongly reinforce two ideas previously reported in the literature about low-level laser (light) therapy and muscle performance: (1) light therapy can increase ATP synthesis in cells [18, 24, 25], resulting in more energy that includes creatine phosphate re-synthesis [12] and leads to improved muscle cell metabolism and consequently better performance in terms of: strength, power, repetitions, work, fatigue resistance and faster recovery post-exercise [16, 18]; and (2) light therapy produces better responses when cells or tissues have been subjected to a metabolic or mechanical stress [36, 37].
The current study also evaluated the content of another important source of energy supply for muscles, i.e. muscle glycogen [19]. LEDT-After and LEDT-Before-After had very similar values for glycogen content, increasing around 4.5-fold compared to LEDT-Sham, around 2.6-fold compared to LEDT-Before, and around 2.4-fold compared to Control. These results suggest that muscular glycogen content can be restored quickly and further increased when light therapy is applied after exercise, suggesting effects of LEDT on glucose metabolism in all steps of glycolysis, as well as possibly increasing glucose transporters in the cells (glucose transporter type 4). In summary, our results suggest that ATP and glycogen can be increased, restored quickly and become more available to muscle cells at each training session if LEDT is applied after a bout of exercise.
The literature has also reported the importance of the balance between the production and reduction of reactive oxygen species (ROS), known as oxidative stress, to achieve better muscle performance as well as to prevent cell damage after intense exercise [20]. In this context, the effect of LEDT to protect against exercise-muscle damage has suggested a modulation of ROS [27, 28] produced naturally during exercise [18, 20] and for this reason we investigated several oxidative stress markers in the current study: protein carbonyls, glutathione (total and oxidized), catalase, lipid peroxidation using TBARS (contents of malondialdehyde – MDA) and protein thiols. Protein carbonyl content was significantly decreased only in LEDT-After group, promoting an effective protection against protein oxidation and injury as well as suggesting better protein function [20–22]. Protein carbonyls were investigated in the current study because they are formed quickly after a bout of oxidative stress and are stable for hours or days, being a specific and reliable oxidative stress marker [22]. To our knowledge this is the first study measuring protein carbonyls as a marker of oxidative stress of muscles exposed to LEDT.
Confirming previous hypotheses regarding oxidative stress balance, the content of total glutathione (GSH) was significantly moved towards oxidized (GSSH) in LEDT-Before animals compared to animals in LEDT-Sham; LEDT-Before-After animals compared to LEDT-Sham and LEDT-Before as well as in LEDT-After animals compared to LEDT-Sham and LEDT-Before. These results may have occurred due to the increased activity or up-regulation of glutathione peroxidase (GPX) enzyme activity [18] responsible for oxidizing GSH to GSSH during the process of reduction of hydrogen peroxide (H2O2) to water [20–22]. Moreover, the muscular GSH content was significantly higher in Control group animals compared to LEDT-Sham and LEDT-Before, but without statistical significance compared to both groups treated with LEDT after each training session. These results suggest that LEDT after exercises minimize oxidative stress similarly to control or normal levels. Catalase activity in LEDT-After, LEDT-Before-After and Control groups was significantly higher compared to LEDT-Sham, corroborating the idea of improved oxidative stress defense by LEDT [18].
We assessed other oxidative stress markers including the level of lipid peroxidation using TBARS (malondialdehyde – MDA content) and protein thiols. During oxidative stress, MDA is formed from the attack of free radicals on the polyunsaturated fatty acids present in the cell membrane, which might lead to impairment of cellular control of ion gradients and Ca2+ transport [20, 21]. A decreased level of protein thiols has been used as an alternative oxidative stress marker when ROS production is chronic i.e., lasting for weeks and months [20–22]. However, the MDA content values were very similar and without statistical differences between all the groups in this study contrasting with previous studies [27, 28], as well as no difference in protein thiol content. These results with protein carbonyls, glutathione and catalase activity suggests there was oxidative stress produced during the exercise protocol but insignificant cell membrane damage and no prolonged periods (weeks and months) of oxidative stress.
In addition to availability of muscle energy and lower oxidative stress, muscle performance in training programs depends also on how fast the recovery process from microdamage or microlesions occurs [23]. After microdamage or microlesions in mucle tissue, occurs activation of satellite cells and addition of new myonuclei in muscle cells are responsible for allowing an increased protein synthesis to encourage muscle hypertrophy and tissue repair [29, 38]. Regarding these very important issues, the current study also evaluated the formation of new myonuclei by incorporation of BrdU into the DNA showing more myocytes in the mitosis phase (proliferation) [29], as well as assessing the staining of already formed (or adult) myonuclei by DAPI. Our results confirmed an increase in BrdU and DAPI staining when light therapy was applied, mainly after each training session, suggesting an increase in muscle cell proliferation possibly by activation of satellite cells [12, 18, 29]. This result implies a rapid synthesis of new muscle proteins to repair muscle damage acquired from intense exercise [18], as well as more myonuclei per muscle cell leading to an improved myonuclear domain [38].
Low-level laser (light) therapy exhibits a biphasic dose response which is responsible for promoting beneficial effects if the dose of light is above a threshold needed to stimulate the biological processes, but the dose of light also needs to be below an upper threshold above which tissue inhibition occurs [5, 6]. The current study utilized a dose based on this possible biphasic dose response [5, 6] and in accordance with previous studies in experimental models [30, 31]. Moreover, previous studies have generally used red or near infrared wavelengths singly, but the use of both wavelengths simultaneously may have added benefits. With this perspective in mind, red and near infrared wavelengths emitted by LEDs were used in this study to optimize the absorption through a double absorption band [8–11] by the chromophores located in the cells, especially in the mitochondrial electric transport chain to produce more ATP [24, 25].
This study has shown a clear improvement in muscle performance, energy metabolism, oxidative stress defense and repair/proliferation with different regimens of LEDT applied to muscles in conjunction with a training regimen. The main lessons to be learned are: (a) if the purpose is to improve muscle performance before only one bout of acute exercise, such as in single athletic competitions, the LEDT should be used before the exercise; (b) if the purpose is to develop the best enduring improvement muscle performance then one should adopt an appropriate training program for the specific sport in question combined with LEDT over the entire target muscle groups [12] after each training session [12, 13, 18]. Our results could easily be applied to humans, where LEDT could be used to accelerate muscle recovery after injuries, as well as improve muscle performance in exercise [12, 13, 15].
Acknowledgments
Cleber Ferraresi would like to thank FAPESP for his Ph.D. scholarships (numbers 2010/07194-7 and 2012/05919-0). MR Hamblin was supported by US NIH grant R01AI050875. We acknowledge Andrea L. Brissette for administrative assistance and Jenny Zhao for assistance with the core laboratory of histopathology, microscopy and digital imaging of the Wellman Center for Photomedicine, Massachusetts General Hospital.
Footnotes
Competing Interests The authors declare no conflict of interest.
Author biographies Please see Supporting Information online.
References
- 1.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Lancet. 2009;374:1897–1908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- 2.Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. Photomed Laser Surg. 2004;22:323–329. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- 3.Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD. Photomed Laser Surg. 2010;28:3–16. doi: 10.1089/pho.2008.2470. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Avci P, Sadasivam M, Chandran R, Parizotto N, Vecchio D, de Melo WC, Dai T, Chiang LY, Hamblin MR. Biotechnol Adv. 2012 doi: 10.1016/j.biotechadv.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YY, Chen AC, Carroll JD, Hamblin MR. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YY, Sharma SK, Carroll J, Hamblin MR. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enwemeka CS. Photomed Laser Surg. 2005;23:159–160. doi: 10.1089/pho.2005.23.159. [DOI] [PubMed] [Google Scholar]
- 8.Karu TI, Kolyakov SF. Photomed Laser Surg. 2005;23:355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 9.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Photomed Laser Surg. 2008;26:593–599. doi: 10.1089/pho.2008.2246. [DOI] [PubMed] [Google Scholar]
- 10.Karu TI, Pyatibrat LV, Afanasyeva NI. Photochem Photobiol. 2004;80:366–372. doi: 10.1562/2004-03-25-RA-123. [DOI] [PubMed] [Google Scholar]
- 11.Karu TI. IUBMB Life. 2010;62:607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 12.Ferraresi C, de Brito Oliveira T, de Oliveira Zafalon L, de Menezes Reiff RB, Baldissera V, de Andrade Perez SE, Matheucci E, Junior, Parizotto NA. Lasers Med Sci. 2011;26:349–358. doi: 10.1007/s10103-010-0855-0. [DOI] [PubMed] [Google Scholar]
- 13.Vieira WH, Ferraresi C, Perez SE, Baldissera V, Parizotto NA. Lasers Med Sci. 2012;27:497–504. doi: 10.1007/s10103-011-0984-0. [DOI] [PubMed] [Google Scholar]
- 14.Baroni BM, Leal EC, Junior, Geremia JM, Diefenthaeler F, Vaz MA. Photomed Laser Surg. 2010;28:653–658. doi: 10.1089/pho.2009.2688. [DOI] [PubMed] [Google Scholar]
- 15.Leal EC, Junior, Lopes-Martins RA, Frigo L, De Marchi T, Rossi RP, de Godoi V, Tomazoni SS, Silva DP, Basso M, Filho PL, de Valls Corsetti F, Iversen VV, Bjordal JM. J Orthop Sports Phys Ther. 2010;40:524–532. doi: 10.2519/jospt.2010.3294. [DOI] [PubMed] [Google Scholar]
- 16.Borsa PA, Larkin KA, True JM. J Athl Train. 2013;48:57–67. doi: 10.4085/1062-6050-48.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leal EC, Junior, Vanin AA, Miranda EF, de Carvalho PD, Dal Corso SS, Bjordal JM. Lasers Med Sci. 2013 doi: 10.1007/s10103-013-1465-4. [DOI] [PubMed] [Google Scholar]
- 18.Ferraresi C, Hamblin MR, Parizotto NA. Photonics Lasers Med. 2012;1:267–286. doi: 10.1515/plm-2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen DG, Lamb GD, Westerblad H. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 20.Powers SK, Jackson MJ. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finaud J, Lac G, Filaire E. Sports Med. 2006;36:327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee AK, Mandal A, Chanda D, Chakraborti S. Mol Cell Biochem. 2003;253:307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- 23.Folland JP, Williams AG. Sports Med. 2007;37:145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 24.Karu T, Pyatibrat L, Kalendo G. J Photochem Photobiol B. 1995;27:219–223. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 25.Karu T. J Photochem Photobiol B. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 26.Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R. J Photochem Photobiol B. 2009;95:89–92. doi: 10.1016/j.jphotobiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Silveira PC, Silva LA, Freitas TP, Latini A, Pinho RA. Lasers Med Sci. 2011;26:125–131. doi: 10.1007/s10103-010-0839-0. [DOI] [PubMed] [Google Scholar]
- 28.Luo L, Sun Z, Zhang L, Li X, Dong Y, Liu TC. Lasers Med Sci. 2013;28:725–734. doi: 10.1007/s10103-012-1133-0. [DOI] [PubMed] [Google Scholar]
- 29.Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T. Exp Physiol. 2009;94:1005–1015. doi: 10.1113/expphysiol.2009.047738. [DOI] [PubMed] [Google Scholar]
- 30.Lopes-Martins RA, Marcos RL, Leonardo PS, Prianti AC, Jr, Muscara MN, Aimbire F, Frigo L, Iversen VV, Bjordal JM. J Appl Physiol (1985) 2006;101:283–288. doi: 10.1152/japplphysiol.01318.2005. [DOI] [PubMed] [Google Scholar]
- 31.Santos LA, Marcos RL, Tomazoni SS, Vanin AA, Antonialli FC, Grandinetti VD, Albuquerque-Pontes GM, de Paiva PR, Lopes-Martins RA, de Carvalho PD, Bjordal JM, Leal EC., Junior Lasers Med Sci. 2014 doi: 10.1007/s10103-014-1560-1. [DOI] [PubMed] [Google Scholar]
- 32.Corazza AV, Paolillo FR, Groppo FC, Bagnato VS, Caria PH. Lasers Med Sci. 2013;28:1467–1474. doi: 10.1007/s10103-012-1251-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Barton ER, Sweeney HL, Farrar RP. J Appl Physiol (1985) 2004;96:1097–1104. doi: 10.1152/japplphysiol.00479.2003. [DOI] [PubMed] [Google Scholar]
- 34.Khan HA. J Biosci. 2003;28:379–382. doi: 10.1007/BF02705114. [DOI] [PubMed] [Google Scholar]
- 35.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 36.Karu T. Photomed Laser Surg. 2013;31:189–191. doi: 10.1089/pho.2013.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer AP, Oron U, Kajander EO, Mester AR. J Proteome Res. 2002;1:475. doi: 10.1021/pr0255396. [DOI] [PubMed] [Google Scholar]
- 38.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]