Abstract
Tenascin-C (TNC) is highly expressed in cancer tissues. Its cellular sources are cancer and stromal cells, including fibroblasts/myofibroblasts, and also vascular cells. TNC expressed in cancer tissues dominantly contains large splice variants. Deposition of the stroma promotes the epithelial-mesenchymal transition, proliferation, and migration of cancer cells. It also facilitates the formation of cancer stroma including desmoplasia and angiogenesis. Integrin receptors that mediate the signals of TNC have also been discussed.
Keywords: cancer cell, integrins, splice variant, Stromal cell, tenascin-C
Abbreviations
- TNC
tenascin-C
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- FNIII
fibronectin type III-like
- FBG
fibrinogen-like globe
- RPTP
receptor protein-tyrosine phosphatase
- FN
fibronectin
- VN
vitronectin
- LAP
latency-associated peptide
- TGF
transforming growth factor
- MMPs
matrix metalloproteinases
- EDA
extra domain A
- OPN
osteopontin
- CAF
cancer-associated fibroblasts
- FAK
focal adhesion kinase
- PDGF
platelet-derived growth factor
- HS
heparan sulfate
- ISH
in situ hybridization
Introduction
Tenascin-C (TNC) is an extracellular matrix (ECM) glycoprotein that is highly expressed during organogenesis accompanying cell proliferation and migration, epithelial-mesenchymal transition (EMT), and interactions between the parenchyma and mesenchyme. The distribution of TNC is typically limited in adult tissues; however, the protein is re-expressed in pathological lesions undergoing tissue remodeling, such as inflammation, tissue repair, and cancers. The increased deposition of TNC has been reported in the tumor stroma of most epithelial malignancies arising, for example, in the breast, uterus (both the cervix and body), ovary, prostate, pancreas, colon, stomach, mouth, larynx, lung, urinary tract, and skin.1,2 Interactions between epithelial (cancer) and stroma cells induce the expression of TNC by both cells, thereby facilitating the remodeling of cancer tissues. Deposited TNC in the cancer stroma modulates the cell behaviors of both cell types by interactions between cells and ECM that are mediated through integrins. Integrins are a family of cell adhesion receptors binding to ECM proteins, plasma-derived proteins and cell surface adhesion molecules. All integrins are heterodimers containing an α and a β subunit. There are 18 α subunit and 8 β subunit genes in mammalian genomes, and 24 distinct α-β heterodimers have been found at the protein level. Most heterodimers are capable to bind a wide variety of ligands.3,4 Binding of integrins to extracellular ligands induces intracellular signals providing information on its location, local environment, adhesive state, and surrounding matrix. Integrins also cooperate with other cell surface receptors including growth factor- and G protein-coupled receptors, and their signals regulate biological processes such as cell proliferation and differentiation, cell shape and migration, and apoptosis/survival.5-7
The interactions involved in cancer progression as well as formation of cancer stroma mimic those in embryogenesis and repair processes after tissue injuries. Hence, we herein discussed the expression of TNC and integrins during embryonic development or tissue repair in order to make interpreting the roles in cancer tissues easier.
Integrins as TNC Receptors in Cancer
Domain structure of TNC and receptor-binding sites
Each TNC subunit (Fig. 1) is composed of a cysteine-rich N-terminus tenascin assembly domain followed by 14.5 epidermal growth factor (EGF)-like repeats, a region of up to 17 fibronectin type III-like (FNIII) repeats (9 of these, named A1/2/3/4, B, AD2, AD1, C, and D, are susceptible to alternative splicing), and a C-terminal fibrinogen-like globe (FBG). Various numbers and combinations of the alternatively spliced repeats inserted between the 5th and 6th constant repeats yield splice variants. Large TNC variants including the spliced repeats are considered to have the potential to modulate cell signaling by binding to different receptors and other ECM components.1,2 For example, annexin II binds to alternatively spliced segments with high affinity, causing the loss of focal adhesions and mitogenesis and increasing the migration of arterial endothelial cells.8,9 The extracellular domains of receptor protein-tyrosine phosphatase-ζ/β (RPTP, phosphacan) expressed by neural cells is also a receptor of these segments. The RPTP-β-dependent adhesion of glioma cells was shown to be mediated by binding to the alternatively spliced repeats of FNIII A1,2,4 of TNC,10 while an additional binding site was detected in FBG.11 The EGF receptor is also known to be a non-integrin receptor for the EGF-like repeats of TNC.12 Two heparin-binding sites and a cryptic sequence within FNIIIA2 for the binding of syndecan-4 have been reported.13-15
Figure 1.
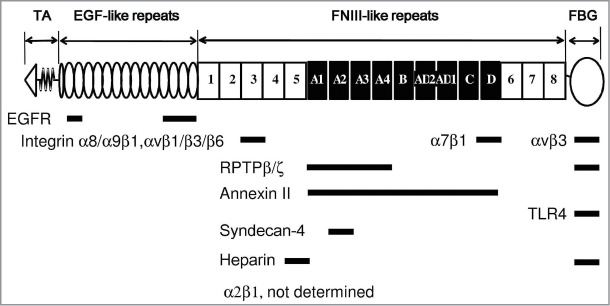
Domain structure and receptor binding sites of human tenascin-C. The N-terminal tenascin assembly (TA), epidermal growth factor (EGF)-like, fibronectin type III (FNIII)-like, and fibrinogen (FBG)-like domains are schematically depicted. The alternatively spliced FNIII repeats A1–D are shown in black. Integrins α8β1 and αvβ1/β3/β6 bound to the RGD sequence in FNIII3,95 while α9β1 did to AEIDGIEL in FNIII3.24 The binding site for α2β1 was not determined. The binding site of α7β1 was the sequence of VFDNFVLK in FNIIID.42 Integrin αvβ3 had an additional binding site in FBG.34 Receptor protein-tyrosine phosphatase-ζ/β (RPTP-ζ/β; phosphacan) expressed by neural cells was also a receptor for FNIIIA1–410 and FBG.11 The EGF receptor bound to EGF-like repeats.12 Two heparin-binding sites and a cryptic sequence within FNIIIA2 were present for the binding of syndecan-4.13-15
The integrin heterodimers of α2/7/8/9β1 and αvβ1/3/6 are known to mediate signals between cells and TNC. Of these integrins, α2/9β1 and αvβ1/3/6 are expressed in epithelial cells. Integrins α8β1 and αvβ1/3/6 are RGD-binding.4
αvβ6
Integrin αvβ6 is an epithelial-specific integrin that is a receptor for fibronectin (FN), vitronectin (VN), TNC, and the latency-associated peptide (LAP) of transforming growth factor (TGF)-β.16 The adhesion of β6-transfected SW480 cells to TNC FNIII3 recombinant fragments has already been reported.17 The expression of αvβ6 has been established in different kinds of cancers and is often associated with a poor prognosis.16,18 We recently showed that the treatment of MCF-7 cells with TGF-β1 markedly upregulated the mRNA, but not protein level of β6, whereas the protein level was markedly increased by an additional exposure to TNC, possibly due to the stabilization of αvβ6 heterodimers by binding to TNC.19 Binding to TNC induced EMT-like changes in MCF-7 cells. The upregulation of αvβ6 integrin has also been shown to cause EMT changes in colon18 and oral20 cancers. Furthermore, the increased expression of αvβ6 in oral cancer cells upregulated that of TNC and matrix metalloproteinase (MMP)-3.20 An important function of αvβ6 is the activation of TGF-β by its release from latent TGF-β complexes.16,21 Activated TGF-β may be involved in the upregulation of β6 and TNC, forming a positive feedback loop, and has also been shown to upregulate MMPs in collaboration with TNC.22
α9β1
Integrin α9β1 is a member of LDV-binding integrins.4 The heterodimer is expressed on a wide variety of cell types and interacts with many ligands, FNIII extra domain A (EDA) of FN, TNC, osteopontin (OPN), thrombospondin-1, and disintegrin and metalloproteinase domain 12.23 An attachment assay of α9-transfected SW480 cells previously revealed that the FNIII3 repeat of TNC contained the α9β1 integrin-binding sequence, AEIDGIEL, not RGD.24 Immunohistochemical analyses detected the co-localization of α9β1 and TNC at the invasive fronts of primary colorectal and gastric cancers.25 Only the basal-like subtypes of breast cancers have been reported to express α9β1, and its expression has been correlated with poorer patient outcomes.26 A recent study showed that the α9β1 integrin of the human breast cancer cell line, MDA-MB231 contributed to tumor growth, lymphatic metastasis, the recruitment of cancer-associated fibroblasts (CAFs), and host-derived production of OPN in an orthotopic xenotransplantation model.27 Endothelial cell-derived FN with EDA was also found to promote colorectal cancer metastasis by inducing EMT via an interaction with α9β1.28 TNC may contribute to cancer progression because of its co-expression in these tissues. During wound repair, the epithelial cells of oral mucosa have been shown to temporarily express α9β1 and αvβ6.29 The preferential upregulation of the α9 subunit in migrating epithelial cells and of αvβ6 integrin in the epithelium after wound closure was observed by immunohistochemistry, and was accompanied by increased TNC immunostaining. The regenerative mucosal epithelia of the nasal cavity also exhibited enhanced α9 immunolabeling and TNC mRNA expression.30
αvβ3
Integrin αvβ3 is known to be expressed in diverse cell types including epithelial cells, fibroblasts, endothelial cells, and inflammatory cells in cancer tissues, and binds highly divergent ECM proteins such as VN, FN, fibrinogen, denatured or proteolysed collagen, and TNC.31,32 Two binding sites of the integrin in TNC have been identified at the RGD sequence within the FNIII3 repeat 17,33 and in C-terminal FBG.34 However, behavioral changes induced in αvβ3-expressing cells by the TNC treatment have not yet been explored in detail. When plated on FNIII3 fragments of TNC, the spreading and proliferation of β3-trasnfected SW480 cells were enhanced and accompanied by the phosphorylation of focal adhesion kinase (FAK) and paxillin.17 We also demonstrated that TNC-coated substrates promoted the platelet-derived growth factor (PDGF)-BB-induced proliferation and migration of smooth muscle cells due to amplified crosstalk signaling between integrin αvβ3 and PDGF receptor-β by SRC and FAK in focal adhesion complexes.35 Therefore, the proliferation and migration of various cells expressing αvβ3 in cancer tissues are likely to be influenced by TNC, possibly in conjunction with growth factor receptors.
αvβ1
Integrin αvβ1 is a receptor for FN, VN, and OPN. Human melanoma, breast cancer, squamous cell carcinoma, and neuroblastoma as well as embryonic kidney cells have been shown to express this integrin.36 We recently identified TNC as a ligand for αvβ1 integrin by a binding assay of an MCF-7 lysate to TNC-conjugated beads, and their binding was found to induce EMT-like changes in MCF-7 cells.19 However, information on the functions of αvβ1 integrin in cancer tissues is currently limited.
α2β1
Integrin α2β1 is expressed on epithelial cells, endothelial cells, fibroblasts, and cells of haematopoietic origin, and functions as a receptor for native collagens as well as other ECM and non-ECM proteins, such as laminin, decorin, E-cadherin, MMP-1, and endorepellin.37 TNC is also considered to be a ligand for this integrin. Anti-α2 and -β1 neutralizing antibodies were found to abolish the promoting effects of TNC on glioma cell migration.38 Human endothelial cells can attach and spread on TNC by α2β1 and αvβ3 integrins. The affinity purification of an endothelial cell extract on a TNC column followed by immunoprecipitation with monoclonal antibodies identified both α2β1 and αvβ3 integrins as TNC receptors.39 However, the lack of binding by α2β1 and αvβ3 integrins on osteosarcoma MG63 to TNC in an adhesion assay has also been reported. In our analyses to identify the proteins binding to TNC-conjugated beads, the subunits of αv, β1, and β6, but not α2 could be detected in the MCF-7 lysate.19 The binding of α2β1 to TNC may be regulated in a cell type-specific manner. The immunolocalization of α2 subunits in cultured cells and cancer tissues was previously observed in intercellular contacts between epithelial cells.19,40 We showed that α2β1 did not appear to be involved in TNC-induced EMT-like changes in MCF-7 cells, whereas the integrin α2β1 was responsible for collagen-I-mediated EMT in pancreatic cancer cells.40 Integrin α2β1 may also be involved in angiogenesis in cancer tissues.39
α7β1
Integrin α7β1 was originally identified as a laminin receptor. Its expression is relatively restricted to striated and cardiac muscle and certain endothelial and neural cell types including melanoma.41 This integrin is known to bind to the amino acid sequence, VFDNFVLK, within the FNIIID of TNC, thereby promoting the extension of neuronal processes.42 Mutations have recently been detected in the integrin α7 gene in human cancer samples, such as prostate cancers and gliomas typically expressing TNC. These mutations have been associated with the shorter survival of patients with these cancers, suggesting suppressive effects of α7β1 on tumor progression.43 The relationship between these mutations and TNC has not yet been elucidated in cancer tissues.
α8β1
Integrin α8β1 is a receptor for FN, VN, the RGD site of TNC fragments, and OPN, and is expressed in neuronal and mesenchymal cells, including vascular and visceral smooth muscle cells, alveolar myofibroblasts, and glomerular mesangial cells.44 Using the recombinant fragments of TNC and α8-transfected 293 cells in adhesion assays, the α8β1-binding domain was determined to be an RGD-containing FNIII3 repeat.45 However, a subsequent study using α8-expressing K562 cells found that full length TNC did not serve as a ligand, implying that the RGD site in native TNC is a cryptic binding site.46 The co-expression of the α8 subunit with tenascin-W has been reported in murine mammary tumors.47 Mammary tumor cells expressing α8 subunits show haptotactic migration to tenascin-W, but not TNC. CAFs, a repertoire of myofibroblasts, may also express α8 subunits.
α5β1 and syndecan-4
Although α5β1 integrin does not directly bind to TNC, TNC modulates the co-receptor functions of syndecan-4 in FN-induced α5β1 integrin signaling. The heparan sulfate (HS) side chains of syndecans were found to bind to TNC.48 TNC blocked tumor cell adhesion to FN by binding to FNIII13 of FN instead of syndecan-4. This binding impaired the co-receptor function of syndecan-4 in integrin signaling, followed by tumor cell proliferation.49 When fibroblasts were plated on a mixed FN/TNC substratum, cell spreading and adhesion signaling were less than on FN alone, as determined by delayed FAK phosphorylation, and cell proliferation was also arrested. The overexpression of syndecan-4 and addition of a recombinant peptide of FNIII13 restored adhesion, FAK phosphorylation, and cell proliferation, indicating the requirement of syndecan-4/integrin co-signaling.50 The binding of TNC to syndecan-4 has also been reported to modulate the integrin-dependent contraction of fibrin-FN gels by inhibiting the activation of RhoA and FAK.51 The synthetic peptide TNIIIA2 or matricryptic site of the FNIIIA2 of TNC exposed by MMP-2 processing was bound to syndecan-4 via its HS chains, inducing conformational changes and functional activation in β1 integrin, and stimulated β1 integrin-mediated cell adhesion.15 Furthermore, a TNIIIA2 treatment of fibroblasts induced a lateral association between PDGF receptor β and the molecular complex of activated β1 integrin and syndecan-4 in the membrane microdomains enriched with cholesterol/caveolin-1, resulting in the prolonged activation of PDGF receptor β followed by cell proliferation. 52
Cellular Sources of TNC in Cancer Tissues
In situ hybridization studies using antisense TNC probes have clearly demonstrated the expression of TNC mRNA in both cancer and stromal cells. In breast cancers, cancer cells in intraductal components or large nests are frequently positive for TNC mRNA, with strong immunolabeling being observed around these ducts and nests.53,54 Scirrhus carcinoma is characterized by invading small cancer nests and desmoplastic stroma with activated fibroblasts as well as larger amounts of collagen fibers. In this histology, stromal cells have often been positive for TNC mRNA, while the cancer cells rarely expressed TNC. However, the relatively small number of cases of fully invasive cancer with the strong cytoplasmic immunostaining of cancer cells has been associated with poorer patient outcomes,55 indicating markedly high TNC expression levels. The expression of TNC by epithelial and stromal cells has also been reported in developing mammary glands in the mouse.56 TNC mRNA was detected in the epithelial cells of mammary buds sprouting from the epidermis on the 14th and 15th day of gestation, and in the mesenchymal cells at the epithelial-mesenchymal border of the growing bud from the 14th to the 17th day. Epithelial cells of the lactating mammary gland were also found to produce TNC, which was secreted in breast milk.56,57 In adenocarcinomas of the colon58 (Fig. 2) and prostate,59 epithelial and stromal cells both expressed TNC mRNA.
Figure 2.
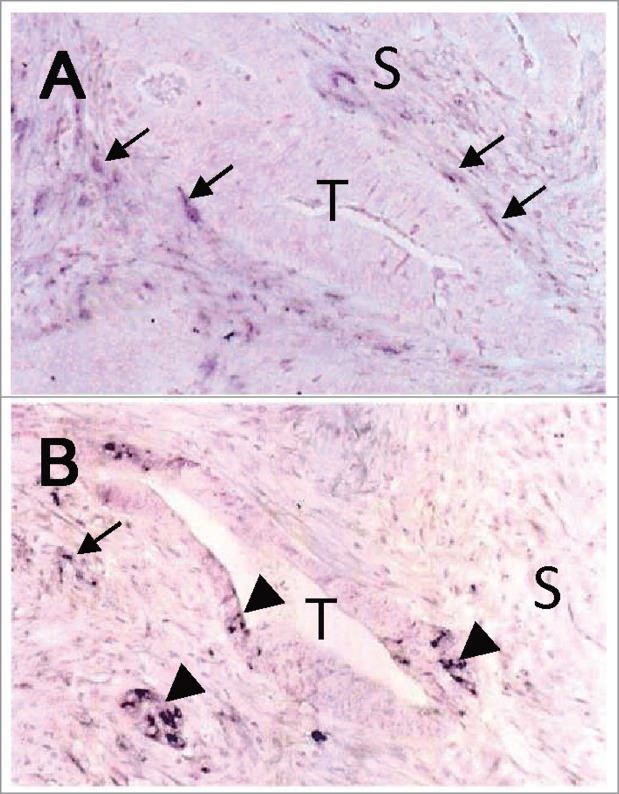
Expression of TNC mRNA in colon cancer labeled by in situ hybridization. (A) Spindle-shaped fibroblasts in the cancer stroma (S), especially those adjacent to the tumor glands (T), were positive for TNC mRNA (arrows). (B) Cancer cells were also positive (arrow heads) in some cases. Nuclei were lightly counterstained with nuclear fast red. The images were taken from a sample used in our previous study.58
Positive TNC mRNA signals have been reported in squamous cell carcinomas arising in the oral mucosa,60,61 pharynx,62 and larynx.63 Positive cancer cells have frequently been detected in the peripheral zone of large cancer nests and in invading nests adjacent to the stroma. In the uterine cervix, the basal keratinocytes of intraepithelial neoplasms were shown to be positive for TNC mRNA, even in pre-malignancy.64 In squamous cell carcinomas, TNC immunohistochemistry has often exhibited positive cytoplasmic staining in cancer cells at the invasion fronts and in the basal zone of non-invasive lesions.61 Previous studies showed that TNC mRNA was also expressed by cells at the migrating fronts of regenerative epithelia during skin wound healing65,66 and after nasal mucosa injury,30 emphasizing a close relationship with cell migration. The epithelial expression of TNC is reactivated in regenerating epithelia, which is not specific to malignant transformation. This expression pattern may be related to the intraductal and intraepithelial expansion of cancer cells migrating along basement membranes.
Fibroblasts are also a major source of TNC deposited widely in the stroma of various cancers. TNC mRNA-positive fibroblasts have been detected in close proximity to cancer nests and displayed positive immunostaining for α-smooth muscle actin,58,61 denoting myofibroblasts and/or CAFs. In addition to the expression of TNC by mammary glands in the murine embryo described above, mesenchymal cells surrounding terminal end buds, which invade the mammary fat pad during puberty, have been shown to synthesize TNC.56
This stromal expression of TNC appears to reflect epithelial-mesenchymal interactions. In co-cultures of cancer cells and fibroblasts, breast cancer MCF-7 cells upregulated the expression of TNC in fibroblasts,67,68 whereas embryonic fibroblasts induced the synthesis of TNC in cancer cells typically not producing TNC.69 The expression of TNC by epithelial cells and fibroblasts is likely to be controlled by their reciprocal interactions in the microenvironment consisting of paracrine factors such as ECM proteins and cytokines. Furthermore, inflammatory cells that infiltrate the stroma may be responsible for upregulating the expression of TNC in both cancer and stromal cells. Endothelial cells and vascular pericytes as well as smooth muscle cells of the vessel walls express TNC.58,70
Splice Variants of TNC Expressed in Cancer
Large splice variants of TNC are preferentially expressed in cancer tissues. Immunohistochemistry and ISH analyses using antibodies against the epitopes or probes of antisense sequences of the spliced domains revealed the preferential expression of the large TNC variants in cancer cells and stromal fibroblasts in cancer tissues.53,71-73 In breast cancers, immunostaining using the antibody specific to domain B (αIIIB) showed periductal stromal staining in the ductal components and intense staining in the peritumoral stroma of invasive carcinomas. ISH demonstrated that variants containing FNIIIB were expressed by stromal fibroblasts in invasive cancers and both periductal fibroblasts and myoepithelial cells in intraductal carcinoma.53 We also reported that FNIIIB-containing variants were preferentially expressed at the microinvasion sites of intraductal components (Fig. 3) and in stroma at the fronts of invasive ductal cancers using a monoclonal antibody (4C8MS) against the FNIIIB domain.72 Immunostaining of large variants containing the A1/4 repeats was previously observed in basal epithelial cells and granulation tissue during wound healing in the oral mucosa.74 Variants including the A1 and D domains were also found to be expressed in the diseased cornea.75
Figure 3.
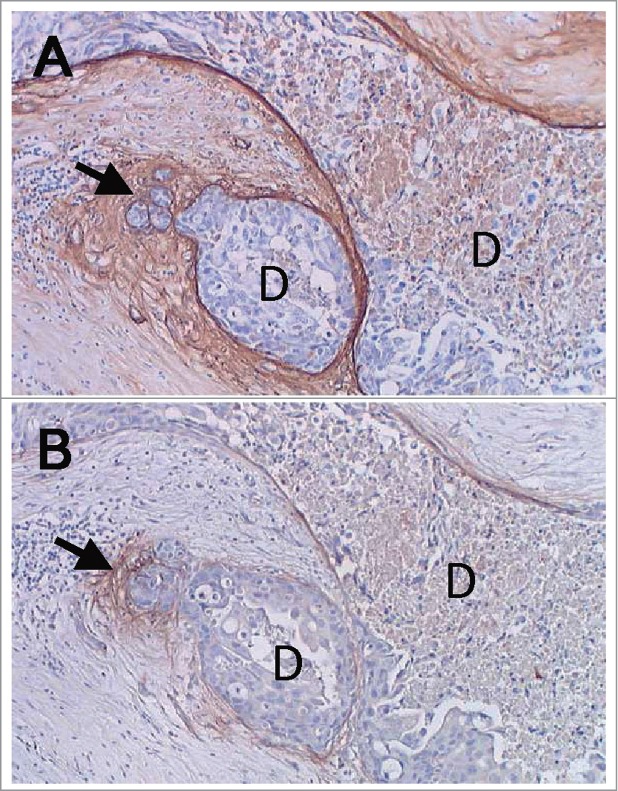
Expression of large splice variants of TNC including FNIIIB in breast cancer tissues. (A) Immunostaining of an antibody against all TNC variants was localized around the intraductal components (D) and in a site with cancer microinvasion (arrow), also showing the weak cytoplasmic staining of cancer cells. (B) Immunostaining of an antibody specific to FNIIIB showed almost exclusive staining at the site of microinvasion (arrow). Nuclei were lightly counterstained with hematoxylin. The images were taken from a sample used in our previous study.72
We previously demonstrated that the recombinant protein including alternatively spliced segments enhanced mitogenesis and the migration of breast cancer cells72 and cardiac fibroblasts76 derived from TNC-null mice. Several cell surface receptors, annexin II, and syndecan-4 are known to bind these segments. In clinicopathological studies, the co-overexpression of annexin II and stromal TNC was identified as an independent factor of poor prognosis in patients with colorectal cancers, and has been suggested to be related to progression and metastatic spread.77 In pancreatic cancer, the expression of annexin II and stromal TNC increased in progression from low-grade intraepithelial neoplasia to pancreatic cancer.78 However, their functional implications in cancer progression are poorly defined. As described above, syndecan-4 binding to a cryptic sequence of the FNIIIA2 domain after MMP-2 processing, but not to intact TNC, was reported to modulate cell adhesion, survival, and proliferation in fibroblasts by activating α5β1 integrin.15,52 Another important property of these segments is their susceptibility to MMPs. Large variants were previously shown to be more easily degraded by MMPs than the smallest variant without the spliced repeats.79 Further studies are needed in order to explore the additional functions of large splice variants and find novel receptors for the segments.
The large TNC variants produced in the cancer stroma may enter the circulation, thereby elevating TNC levels in the sera of patients. Several studies identified a positive correlation between serum levels of TNC containing the FNIIIC domain and tumor aggressiveness.80,81 Urinary levels of TNC with FNIIIB significantly increased in parallel with tumor progression in urinary bladder carcinoma, suggesting its potential as a urine surveillance marker.82
Roles of TNC in Cancer
Epithelial-mesenchymal transition
EMT is the process by which polarized epithelial cells are converted into mesenchymal cells during embryogenesis and in diseased tissues.83 EMT events occur in cancer tissues at the beginning of cancer cell invasion, during which carcinoma cells lose their epithelial polarity and intercellular connections, allowing them to escape the surrounding epithelium. In embryonic development, EMT events, including gastrulation and formation of the neural crest, endocardial cushion, and secondary palate, have been closely associated with the expression of TNC.84 A previous study described morphological changes in MCF-7 breast cancer cells, their partial detachment from the substratum and loss of cell-cell contracts, following the addition of TNC to the culture medium.67 Our immunohistochemical analysis of invasive ductal breast cancers showed that the deposition of TNC was more frequently observed in stroma with scattered cancer cells than with large cancer cell clusters. The addition of TNC to the medium of MCF-7 cells caused EMT-like changes that are the delocalization of E-cadherin and β-catenin from cell-cell contacts, associated with the phosphorylation of focal adhesion kinase (FAK) by SRC.84 These phenotypic changes were enhanced by the combined treatment with TGF-β1; however, this was not complete EMT. Tenascin-X, another member of the tenascin family, was also found to induce EMT in normal murine mammary gland (NMuMG) epithelial cells through the FBG-mediated activation of latent TGF-β elicited by integrin α11β1, a tenascin-X receptor.85
Cell proliferation
Cells that are positive for the proliferating cell marker, MIB-1, are often co-localized with TNC staining in cancer tissues.1 The ratio of the proliferating population was shown to be significantly higher in large TNC variant-positive areas in breast cancer.72 Previous studies reported that the proliferation of cancer cell lines was stimulated by the addition of TNC at a low serum concentration.49,63,72,78 As a possible mechanism, the binding of TNC to the syndecan-4-binding sites of FN were suggested to interfere with the growth inhibitory signal of integrin α5β1.49 The alterations induced in signals from integrin complexes triggered by binding to TNC are also plausible. Recombinant fragments containing alternatively spliced repeats have been shown to have proliferative effects on cancer cells.72 The deposition of TNC has been associated with epithelial proliferation in regenerating epithelia.29,30 TNC is known to sustain or inhibit cell proliferation in fibroblasts,50,76 but enhances that in smooth muscle cells,35 implicating its positive effects on the proliferation of cancer-associated fibroblasts. TNC has also been shown to function as a growth stimulant in endothelial cells.86
Cell migration
TNC was found to promote the migratory activities of various cell types, including epithelial (cancer) cells, fibroblasts, smooth muscle cells and endothelial cells, in the wound closure assay and chemotactic transwell migration assay.1,35,63,72,76,84,86 TNC, as well as other matricellular proteins, is known to modulate cell adhesion and shifts from stable focal adhesion with stress fibers to intermediate adherence.87 The intermediate state of adhesion is favorable for cell migration. Figure 4 shows the modulatory effects of TNC on haptotaxis (Fig. 4A) and chemotaxis (Fig. 4B) in the breast cancer cell line, MDA-MB-231. TNC accelerated cell migration when coated on the upper surfaces, whereas migration across membranes was inhibited when the coating was on the lower surfaces, resulting in no haptotactic activity. In contrast, FN exhibits haptotactic activity. These findings indicate that TNC yielded a dynamic adhesion state for cancer cell migration; however, this adhesion was not sufficiently stable or force-generating for haptotactic migration. Interestingly, TNC was a strong inducer of haptotaxis in human neural stem cells.88 The neural cells may employ different receptors from the cancer cells for migration.
Figure 4.
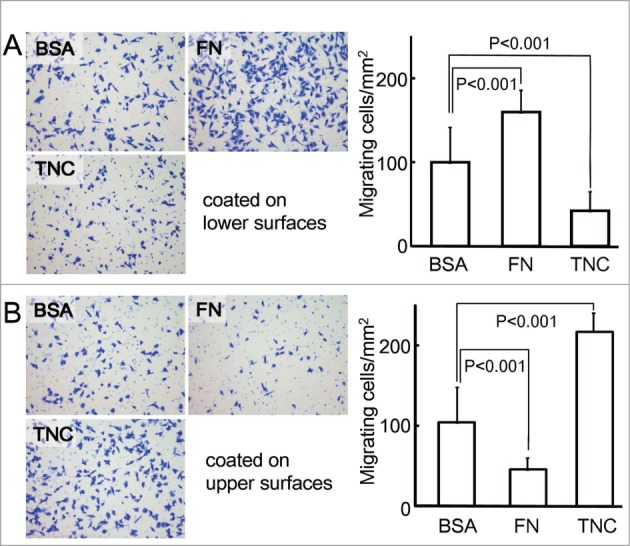
Transwell migration of the breast cancer cell line, MDA-MB-231 on various substrates coated on the lower (A) or upper surfaces (B) of culture insert membranes. The lower or upper surfaces of the membranes (8-μm pore size) of 12-well plates were coated with 50 μl of 10 μg/ml bovine serum albumin (BSA), fibronectin (FN), and TNC overnight at 4°C, followed by blocking with medium containing 0.2% BSA for 1 h at 37°C and rinsing 3 times with medium. The lower chambers were filled with medium supplemented with 2% fetal bovine serum and 0.2% BSA, and the inserts were placed into the wells. The cells (3×10 4 cells/well) suspended in serum-free medium with 0.2% BSA were poured into the upper chambers, and allowed to migrate for 4 h. After being scraped from the upper surfaces, the cells migrating to the lower surfaces were stained with 0.1% crystal violet solution, and counted in 3 optical fields per one membrane under a microscope. Experiments were performed in triplicate. The migration of cells to the lower surfaces was significantly enhanced on FN-coated lower surfaces, but not on TNC-coated surfaces, indicating the haptotactic activity of FN. When the upper surfaces were treated with TNC, the migration of cells was significantly faster to the lower surfaces, possibly due to locomotive adhesion to TNC substrates in order to facilitate chemotaxis.
Previous studies reported that a recombinant fragment containing alternatively spliced repeats enhanced the migration of cancer cells,72 while the constant FNIII repeats and fibrinogen domain promoted fibroblast migration,76 suggesting the involvement of integrin αvβ3 in fibroblast migration. The migration of smooth muscle cells and endothelial cells is also known to be promoted by TNC.35,86
Other roles of TNC in cancer
In comparisons between wild type and TNC-null mice, several studies demonstrated that TNC enhanced inflammatory responses.89,90 TNC has been shown to activate toll-like receptor-4 signaling in fibroblasts and macrophages in rheumatoid arthritis models,91 resulting in the upregulated synthesis of pro-inflammatory cytokines. Enhanced inflammation in the cancer stroma may augment macrophage recruitment and the secretion of tumor promoting- and inflammatory cytokines by macrophages and fibroblasts. A previous study also demonstrated that TNC promoted the transdifferentiation of fibroblasts to myofibroblasts.76 These findings indicated that the upregulation of TNC in the cancer microenvironment facilitated the stromal formation characteristic of cancer tissue, thereby contributing to tumor progression.92 Furthermore, the secretion of TNC by stromal fibroblasts93 as well as breast cancer cells94 may support the metastatic colonization of cancer cells in the lung.
Conclusion
The relationship between the expression of TNC in cancer tissues and patient outcomes was controversial in earlier clinicopathological studies on TNC. However, the expression of TNC in most tumors is now considered to be associated with the worse prognoses of cancer patients, reflecting cancer aggressiveness and the higher activities of tissue remodeling in the cancer stroma. Many recent studies on the roles of TNC in tumor growth, EMT, migration, metastasis, angiogenesis, and stromal inflammation have consolidated the findings of clinicopathological studies. We herein introduced the expression patterns of TNC in developing tissues as well as those undergoing repair, and found them to be similar to those of cancer tissues. The expression of TNC in the latter tissues was high, but spatiotemporally regulated, whereas its expression in cancer tissues was often markedly high and continued for a long period of time. The aberrant expression of TNC clearly promotes cancer progression and stromal remodeling. In addition, studies that focus on the TNC receptors expressed in cancer tissues are warranted in the future.
Disclosure of Potential Conflicts of Interest
TY receives royalties on TNC antibodies from the Immuno-Biological Lab, Japan. The remaining authors declare no conflict of interest.
Funding
TA is supported by the program for undergraduate research training of Mie University School of Medicine. This study was also supported by a grant (#24390087) to TY from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett 2006; 244:143-63; PMID:16632194; http://dx.doi.org/ 10.1016/j.canlet.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 2. Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cell Mol Life Sci 2011; 68:3175-99; PMID:21818551; http://dx.doi.org/ 10.1007/s00018-011-0783-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673-87; PMID:12297042; http://dx.doi.org/ 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- 4. Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci 2006; 119:3901-3; PMID:16988024; http://dx.doi.org/ 10.1242/jcs.03098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci 2009; 122:159-63; PMID:19118207; http://dx.doi.org/ 10.1242/jcs.018093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol 2002; 4:E83-90; PMID:11944041; http://dx.doi.org/ 10.1038/ncb0402-e83 [DOI] [PubMed] [Google Scholar]
- 7. Ganguly KK, Pal S, Moulik S, Chatterjee A. Integrins and metastasis. Cell Adh Migr 2013; 7:251-61; PMID:23563505; http://dx.doi.org/ 10.4161/cam.23840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung CY, Erickson HP. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J Cell Biol 1994; 126:539-48; ; PMID:7518469; http://dx.doi.org/ 10.1083/jcb.126.2.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell 1996; 7:883-92; PMID:8816995; http://dx.doi.org/ 10.1091/mbc.7.6.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adamsky K, Schilling J, Garwood J, Faissner A, Peles E. Glial tumor cell adhesion is mediated by binding of the FNIII domain of receptor protein tyrosine phosphatase β (RPTPbeta) to tenascin C. Oncogene 2001; 20:609-18; PMID:11313993; http://dx.doi.org/ 10.1038/sj.onc.1204119 [DOI] [PubMed] [Google Scholar]
- 11. Milev P, Fischer D, Häring M, Schulthess T, Margolis RK, Chiquet-Ehrismann R, Margolis RU. The fibrinogen-like globe of tenascin-C mediates its interactions with neurocan and phosphacan/protein-tyrosine phosphatase-zeta/β. J Biol Chem 1997; 272:15501-9; PMID:9182584; http://dx.doi.org/ 10.1074/jbc.272.24.15501 [DOI] [PubMed] [Google Scholar]
- 12. Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol 2001; 154:459-68; PMID:11470832; http://dx.doi.org/ 10.1083/jcb.200103103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer D, Chiquet-Ehrismann R, Bernasconi C, Chiquet M. A single heparin binding region within the fibrinogen-like domain is functional in chick tenascin-C. J Biol Chem 1995; 270:3378-84; PMID:7531705; http://dx.doi.org/ 10.1074/jbc.270.27.16315 [DOI] [PubMed] [Google Scholar]
- 14. Weber P, Zimmermann DR, Winterhalter KH, Vaughan L. Tenascin-C binds heparin by its fibronectin type III domain five. J Biol Chem 1995; 270:4619-23; PMID:7533163; http://dx.doi.org/ 10.1074/jbc.270.9.4619 [DOI] [PubMed] [Google Scholar]
- 15. Saito Y, Imazeki H, Miura S, Yoshimura T, Okutsu H, Harada Y, Ohwaki T, Nagao O, Kamiya S, Hayashi R, et al. A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4. J Biol Chem 2007; 282:34929-37; PMID:17901052; http://dx.doi.org/ 10.1074/jbc.M705608200 [DOI] [PubMed] [Google Scholar]
- 16. Bandyopadhyay A, Raghavan S. Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets 2009; 10:645-52; PMID:19601768; http://dx.doi.org/ 10.2174/138945009788680374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yokosaki Y, Monis H, Chen J, Sheppard D. Differential effects of the integrins alpha9beta1, alphavbeta3, and alphavbeta6 on cell proliferative responses to tenascin. Roles of the β subunit extracellular and cytoplasmic domains. J Biol Chem 1996; 271:24144-50; PMID:8798654; http://dx.doi.org/ 10.1074/jbc.271.39.24144 [DOI] [PubMed] [Google Scholar]
- 18. Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, Sheppard D, Oettgen P, Mercurio AM. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest 2005; 115:339-47; PMID:15668738; http://dx.doi.org/ 10.1172/JCI200523183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katoh D, Nagaharu K, Shimojo N, Hanamura N, Yamashita M, Kozuka Y, Imanaka-Yoshida K, Yoshida T. Binding of αvβ1 and αvβ6 integrins to tenascin-C induces epithelial-mesenchymal transition-like change of breast cancer cells. Oncogenesis 2013; 2:e65; PMID:23958855; http://dx.doi.org/ 10.1038/oncsis.2013.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramos DM, Dang D, Sadler S. The role of the integrin α v beta6 in regulating the epithelial to mesenchymal transition in oral cancer. Anticancer Res 2009; 29:125-30; PMID:19331141 [PubMed] [Google Scholar]
- 21. Sheppard D. Integrin-mediated activation of latent transforming growth factor β. Cancer Metastasis Rev 2005; 24:395-402; PMID:16258727; http://dx.doi.org/ 10.1007/s10555-005-5131-6 [DOI] [PubMed] [Google Scholar]
- 22. Kalembeyi I, Inada H, Nishiura R, Imanaka-Yoshida K, Sakakura T, Yoshida T. Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: Direct and synergistic effects with transforming growth factor β 1. Int J Cancer 2003; 105:53-60; PMID:12672030; http://dx.doi.org/ 10.1002/ijc.11037 [DOI] [PubMed] [Google Scholar]
- 23. Høye AM, Couchman JR, Wewer UM, Fukami K, Yoneda A. The newcomer in the integrin family: integrin α9 in biology and cancer. Adv Biol Regul 2012; 52:326-39; PMID:22781746; http://dx.doi.org/ 10.1016/j.jbior.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 24. Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, Shigeto N, Chen J, Sheppard D. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. J Biol Chem 1998; 273:11423-8; PMID:9565552; http://dx.doi.org/ 10.1074/jbc.273.19.11423 [DOI] [PubMed] [Google Scholar]
- 25. Gulubova M, Vlaykova T. Immunohistochemical assessment of fibronectin and tenascin and their integrin receptors alpha5beta1 and alpha9beta1 in gastric and colorectal cancers with lymph node and liver metastases. Acta Histochem 2006; 108:25-35; PMID:16430945; http://dx.doi.org/ 10.1016/j.acthis.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 26. Allen MD, Vaziri R, Green M, Chelala C, Brentnall AR, Dreger S, Vallath S, Nitch-Smith H, Hayward J, Carpenter R, et al. Clinical and functional significance of α9β1 integrin expression in breast cancer: a novel cell-surface marker of the basal phenotype that promotes tumour cell invasion. J Pathol 2011; 223:646-58; PMID:21341269; http://dx.doi.org/ 10.1002/path.2833 [DOI] [PubMed] [Google Scholar]
- 27. Ota D, Kanayama M, Matsui Y, Ito K, Maeda N, Kutomi G, Hirata K, Torigoe T, Sato N, Takaoka A, et al. Tumor-α9β1 integrin-mediated signaling induces breast cancer growth and lymphatic metastasis via the recruitment of cancer-associated fibroblasts. J Mol Med (Berl) 2014; 92(12):1271-81; PMID:25099519 [DOI] [PubMed] [Google Scholar]
- 28. Ou J, Peng Y, Deng J, Miao H, Zhou J, Zha L, Zhou R, Yu L, Shi H, Liang H. Endothelial cell-derived fibronectin extra domain A promotes colorectal cancer metastasis via inducing epithelial-mesenchymal transition. Carcinogenesis 2014; 35:1661-70; PMID:24743511; http://dx.doi.org/ 10.1093/carcin/bgu090 [DOI] [PubMed] [Google Scholar]
- 29. Häkkinen L, Hildebrand HC, Berndt A, Kosmehl H, Larjava H. Immunolocalization of tenascin-C, alpha9 integrin subunit, and alphavbeta6 integrin during wound healing in human oral mucosa. J Histochem Cytochem 2000; 48:985-98; http://dx.doi.org/ 10.1177/002215540004800712 [DOI] [PubMed] [Google Scholar]
- 30. Yoshimura E, Majima A, Sakakura Y, Sakakura T, Yoshida T. Expression of tenascin-C and the integrin α 9 subunit in regeneration of rat nasal mucosa after chemical injury: involvement in migration and proliferation of epithelial cells. Histochem Cell Biol 1999; 111:259-64; PMID:10219625; http://dx.doi.org/ 10.1007/s004180050356 [DOI] [PubMed] [Google Scholar]
- 31. Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer 2004; 90:561-5; PMID:14760364; http://dx.doi.org/ 10.1038/sj.bjc.6601576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weis SM, Cheresh DA. αV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med 2011; 1:a006478; PMID:22229119; http://dx.doi.org/ 10.1101/cshperspect.a006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prieto AL, Edelman GM, Crossin KL. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc Natl Acad Sci U S A 1993; 90:10154-8; PMID:7694284; http://dx.doi.org/ 10.1073/pnas.90.21.10154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yokoyama K, Erickson HP, Ikeda Y, Takada Y. Identification of amino acid sequences in fibrinogen gamma -chain and tenascin C C-terminal domains critical for binding to integrin α vbeta 3. J Biol Chem 2000; 275:16891-8; PMID:10747940; http://dx.doi.org/ 10.1074/jbc.M000610200 [DOI] [PubMed] [Google Scholar]
- 35. Ishigaki T, Imanaka-Yoshida K, Shimojo N, Matsushima S, Taki W, Yoshida T. Tenascin-C Enhances Crosstalk Signaling of Integrin α v β 3/PDGFR-β Complex by SRC Recruitment Promoting PDGF-Induced Proliferation and Migration in Smooth Muscle Cells. J Cell Physiol 2011; 226:2617-24; PMID:21792920; http://dx.doi.org/ 10.1002/jcp.22614 [DOI] [PubMed] [Google Scholar]
- 36. Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA, Nemerow GR. Integrin α(v)beta1 is an adenovirus coreceptor. J Virol 2001; 75:5405-9; PMID:11333925; http://dx.doi.org/ 10.1128/JVI.75.11.5405-5409.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mercurio AM. Lessons from the alpha2 integrin knockout mouse. Am J Pathol 2002; 161:3-6; PMID:12107082; http://dx.doi.org/ 10.1016/S0002-9440(10)64149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deryugina EI, Bourdon MA. Tenascin mediates human glioma cell migration and modulates cell migration on fibronectin. J Cell Sci 1996; 109 ( Pt 3):643-52; PMID:8907709 [DOI] [PubMed] [Google Scholar]
- 39. Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by α 2 β 1 and α v β 3 integrins. J Cell Sci 1993; 105 ( Pt 4):1001-12; PMID:7693733 [DOI] [PubMed] [Google Scholar]
- 40. Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol 2008; 180:1277-89; PMID:18362184; http://dx.doi.org/ 10.1083/jcb.200708137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stipp CS. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med 2010; 12:e3; PMID:20078909; http://dx.doi.org/ 10.1017/S1462399409001355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mercado ML, Nur-e-Kamal A, Liu HY, Gross SR, Movahed R, Meiners S. Neurite outgrowth by the alternatively spliced region of human tenascin-C is mediated by neuronal alpha7beta1 integrin. J Neurosci 2004; 24:238-47; PMID:14715956; http://dx.doi.org/ 10.1523/JNEUROSCI.4519-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ren B, Yu YP, Tseng GC, Wu C, Chen K, Rao UN, Nelson J, Michalopoulos GK, Luo JH. Analysis of integrin alpha7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J Natl Cancer Inst 2007; 99:868-80; PMID:17551147; http://dx.doi.org/ 10.1093/jnci/djk199 [DOI] [PubMed] [Google Scholar]
- 44. Hartner A, Dötsch J. Lessons in congenital and acquired renal disease from alpha8 integrin mutant mice. Pediatr Nephrol 2002; 17:882-8; PMID:12432427; http://dx.doi.org/ 10.1007/s00467-002-0950-y [DOI] [PubMed] [Google Scholar]
- 45. Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin α 8 β 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J Biol Chem 1995; 270:23196-202; PMID:7559467; http://dx.doi.org/ 10.1074/jbc.270.39.23196 [DOI] [PubMed] [Google Scholar]
- 46. Denda S, Müller U, Crossin KL, Erickson HP, Reichardt LF. Utilization of a soluble integrin-alkaline phosphatase chimera to characterize integrin α 8 β 1 receptor interactions with tenascin: murine α 8 β 1 binds to the RGD site in tenascin-C fragments, but not to native tenascin-C. Biochemistry 1998; 37:5464-74; PMID:9548928; http://dx.doi.org/ 10.1021/bi9727489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scherberich A, Tucker RP, Degen M, Brown-Luedi M, Andres AC, Chiquet-Ehrismann R. Tenascin-W is found in malignant mammary tumors, promotes alpha8 integrin-dependent motility and requires p38MAPK activity for BMP-2 and TNF-α induced expression in vitro. Oncogene 2005; 24:1525-32; PMID:15592496; http://dx.doi.org/ 10.1038/sj.onc.1208342 [DOI] [PubMed] [Google Scholar]
- 48. Salmivirta M, Elenius K, Vainio S, Hofer U, Chiquet-Ehrismann R, Thesleff I, Jalkanen M. Syndecan from embryonic tooth mesenchyme binds tenascin. J Biol Chem 1991; 266:7733-9; PMID:1708391 [PubMed] [Google Scholar]
- 49. Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res 2001; 61:8586-94; PMID:11731446 [PubMed] [Google Scholar]
- 50. Orend G, Huang W, Olayioye MA, Hynes NE, Chiquet-Ehrismann R. Tenascin-C blocks cell-cycle progression of anchorage-dependent fibroblasts on fibronectin through inhibition of syndecan-4. Oncogene 2003; 22:3917-26; PMID:12813465; http://dx.doi.org/ 10.1038/sj.onc.1206618 [DOI] [PubMed] [Google Scholar]
- 51. Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell 2004; 15:5670-7; PMID:15483051; http://dx.doi.org/ 10.1091/mbc.E04-08-0759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tanaka R, Seki Y, Saito Y, Kamiya S, Fujita M, Okutsu H, Iyoda T, Takai T, Owaki T, Yajima H, et al. Tenascin-C-derived peptide TNIIIA2 highly enhances cell survival and platelet-derived growth factor (PDGF)-dependent cell proliferation through potentiated and sustained activation of integrin α5β1. J Biol Chem 2014; 289:17699-708; PMID:24808173; http://dx.doi.org/ 10.1074/jbc.M113.546622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Adams M, Jones JL, Walker RA, Pringle JH, Bell SC. Changes in tenascin-C isoform expression in invasive and preinvasive breast disease. Cancer Res 2002; 62:3289-97; PMID:12036947 [PubMed] [Google Scholar]
- 54. Yoshida T, Matsumoto EI, Hanamura N, Kalembeyi I, Katsuta K, Ishihara A, Sakakura T. Co-expression of tenascin and fibronectin in epithelial and stromal cells of benign lesions and ductal carcinomas in the human breast. J Pathol 1997; 182:421-8; PMID:9306963; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199708)182:4%3c421::AID-PATH886%3e3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- 55. Ishihara A, Yoshida T, Tamaki H, Sakakura T. Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res 1995; 1:1035-41; PMID:9816077 [PubMed] [Google Scholar]
- 56. Kalembeyi I, Yoshida T, Iriyama K, Sakakura T. Analysis of tenascin mRNA expression in the murine mammary gland from embryogenesis to carcinogenesis: an in situ hybridization study. Int J Dev Biol 1997; 41:569-73; PMID:9303344 [PubMed] [Google Scholar]
- 57. Fouda GG, Jaeger FH, Amos JD, Ho C, Kunz EL, Anasti K, Stamper LW, Liebl BE, Barbas KH, Ohashi T, et al. Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk. Proc Natl Acad Sci U S A 2013; 110:18220-5; PMID:24145401; http://dx.doi.org/ 10.1073/pnas.1307336110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hanamura N, Yoshida T, Matsumoto E, Kawarada Y, Sakakura T. Expression of fibronectin and tenascin-C mRNA by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int J Cancer 1997; 73:10-5; PMID:9334802; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19970926)73:1%3c10::AID-IJC2%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 59. Katenkamp K, Berndt A, Hindermann W, Wunderlich H, Haas KM, Borsi L, Zardi L, Kosmehl H. mRNA expression and protein distribution of the unspliced tenascin-C isoform in prostatic adenocarcinoma. J Pathol 2004; 203:771-9; PMID:15221936; http://dx.doi.org/ 10.1002/path.1589 [DOI] [PubMed] [Google Scholar]
- 60. Metwaly H, Maruyama S, Yamazaki M, Tsuneki M, Abe T, Jen KY, Cheng J, Saku T. Parenchymal-stromal switching for extracellular matrix production on invasion of oral squamous cell carcinoma. Hum Pathol 2012; 43:1973-81; PMID:22575259; http://dx.doi.org/ 10.1016/j.humpath.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 61. Hindermann W, Berndt A, Borsi L, Luo XM, Hyckel P, Katenkamp D, Kosmehl H. Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J Pathol 1999; 189:475-80; PMID:10629546; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199912)189:4%3c475::AID-PATH462%3e3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 62. Herold-Mende C, Andl T, Laemmler F, Reisser C, Eichhorn S. Expression and localization profile of tenascin in squamous cell carcinomas of the head and neck. HNO 1999; 47:723-9 [DOI] [PubMed] [Google Scholar]
- 63. Yoshida T, Yoshimura E, Numata H, Sakakura Y, Sakakura T. Involvement of tenascin-C in proliferation and migration of laryngeal carcinoma cells. Virchows Arch 1999; 435:496-500; PMID:10592053; http://dx.doi.org/ 10.1007/s004280050433 [DOI] [PubMed] [Google Scholar]
- 64. Pollanen R, Soini Y, Vuopala S, Laara E, Lehto VP. Tenascin in human papillomavirus associated lesions of the uterine cervix. J Clin Pathol 1996; 49:521-3; PMID:8763275; http://dx.doi.org/ 10.1136/jcp.49.6.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Latijnhouwers M, Bergers M, Ponec M, Dijkman H, Andriessen M, Schalkwijk J. Human epidermal keratinocytes are a source of tenascin-C during wound healing. J Invest Dermatol 1997; 108:776-83; PMID:9129232; http://dx.doi.org/ 10.1111/1523-1747.ep12292170 [DOI] [PubMed] [Google Scholar]
- 66. Aukhil I, Sahlberg C, Thesleff I. Basal layer of epithelium expresses tenascin mRNA during healing of incisional skin wounds. J Periodontal Res 1996; 31:105-12; PMID:8708937; http://dx.doi.org/ 10.1111/j.1600-0765.1996.tb00471.x [DOI] [PubMed] [Google Scholar]
- 67. Chiquet-Ehrismann R, Kalla P, Pearson CA. Participation of tenascin and transforming growth factor-β in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res 1989; 49:4322-5; PMID:2472877 [PubMed] [Google Scholar]
- 68. Martinez-Outschoorn U, Pavlides S, Whitaker-Menezes D, Daumer K, Milliman J, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation Implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle 2010; 9:2423-33; PMID:20562526; http://dx.doi.org/ 10.4161/cc.9.12.12048 [DOI] [PubMed] [Google Scholar]
- 69. Hiraiwa N, Kida H, Sakakura T, Kusakabe M. Induction of tenascin in cancer-cells by interactions with embryonic mesenchyme mediated by a diffusible factor. J Cell Sci 1993; 104:289-96; PMID:7685035 [DOI] [PubMed] [Google Scholar]
- 70. Zagzag D, Friedlander DR, Dosik J, Chikramane S, Chan W, Greco MA, Allen JC, Dorovini-Zis K, Grumet M, et al. Tenascin-C expression by angiogenic vessels in human astrocytomas and by human brain endothelial cells in vitro. Cancer Res 1996; 56:182-9; PMID:8548761 [PubMed] [Google Scholar]
- 71. Borsi L, Carnemolla B, Nicolò G, Spina B, Tanara G, Zardi L. Expression of different tenascin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer 1992; 52:688-92; PMID:1385335; http://dx.doi.org/ 10.1002/ijc.2910520504 [DOI] [PubMed] [Google Scholar]
- 72. Tsunoda T, Inada H, Kalembeyi I, Imanaka-Yoshida K, Sakakibara M, Okada R, Katsuta K, Sakakura T, Majima Y, Yoshida T. Involvement of large tenascin-C splice variants in breast cancer progression. Amer J Pathol 2003; 162:1857-67; http://dx.doi.org/ 10.1016/S0002-9440(10)64320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Berndt A, Anger K, Richter P, Borsi L, Brack S, Silacci M, Franz M, Wunderlich H, Gajda M, Zardi L, et al. Differential expression of tenascin-C splicing domains in urothelial carcinomas of the urinary bladder. J Cancer Res Clin Oncol 2006; 132:537-46; PMID:16788848; http://dx.doi.org/ 10.1007/s00432-006-0106-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Latijnhouwers MA, de Jongh GJ, Bergers M, de Rooij MJ, Schalkwijk J. Expression of tenascin-C splice variants by human skin cells. Arch Dermatol Res 2000; 292:446-54; PMID:11000288; http://dx.doi.org/ 10.1007/s004030000152 [DOI] [PubMed] [Google Scholar]
- 75. Ljubimov AV, Saghizadeh M, Spirin KS, Khin HL, Lewin SL, Zardi L, Bourdon MA, Kenney MC. Expression of tenascin-C splice variants in normal and bullous keratopathy human corneas. Invest Ophthalmol Vis Sci 1998; 39:1135-42; PMID:9620072 [PubMed] [Google Scholar]
- 76. Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Amer J Pathol 2005; 167:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, Kamada K, Naito A, Hirao S, Nakajima Y. Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer 2001; 92:1419-26; PMID:11745218 [DOI] [PubMed] [Google Scholar]
- 78. Paron I, Berchtold S, Vörös J, Shamarla M, Erkan M, Höfler H, Esposito I. Tenascin-C enhances pancreatic cancer cell growth and motility and affects cell adhesion through activation of the integrin pathway. PLoS One 2011; 6:e21684; PMID:21747918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Siri A, Knauper V, Veirana N, Caocci F, Murphy G, Zardi L. Different susceptibility of small and large human tenascin-c isoforms to degradation by matrix metalloproteinases. J Biol Chem 1995; 270:8650-4; PMID:7536739 [DOI] [PubMed] [Google Scholar]
- 80. Ishiwata T, Takahashi K, Shimanuki Y, Ohashi R, Cui R, Takahashi F, Shimizu K, Miura K, Fukuchi Y. Serum tenascin-C as a potential predictive marker of angiogenesis in non-small cell lung cancer. Anticancer Res 2005; 25:489-95; PMID:15816617 [PubMed] [Google Scholar]
- 81. Takeda A, Otani Y, Iseki H, Takeuchi H, Aikawa K, Tabuchi S, Shinozuka N, Saeki T, Okazaki Y, Koyama I. Clinical significance of large tenascin-C spliced variant as a potential biomarker for colorectal cancer. World J Surg 2007; 31:388-94; PMID:17219282 [DOI] [PubMed] [Google Scholar]
- 82. Gecks T, Junker K, Franz M, Richter P, Walther M, Voigt A, Neri D, Kosmehl H, Wunderlich H, Kiehntopf M, et al. B domain containing Tenascin-C: A new urine marker for surveillance of patients with urothelial carcinoma of the urinary bladder? Clin Chim Acta 2011; 412:1931-6 [DOI] [PubMed] [Google Scholar]
- 83. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376 [DOI] [PubMed] [Google Scholar]
- 84. Nagaharu K, Zhang X, Yoshida T, Katoh D, Hanamura N, Kozuka Y, Ogawa T, Shiraishi T, Imanaka-Yoshida K. Tenascin C induces epithelial-mesenchymal transition-like change accompanied by SRC activation and focal adhesion kinase phosphorylation in human breast cancer cells. Am J Pathol 2011; 178:754-63; PMID:21281808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alcaraz LB, Exposito JY, Chuvin N, Pommier RM, Cluzel C, Martel S, Sentis S, Bartholin L, Lethias C, Valcourt U. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-β. J Cell Biol 2014; 205:409-28; PMID:24821840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Castellon R, Caballero S, Hamdi HK, Atilano SR, Aoki AM, Tarnuzzer RW, Kenney MC, Grant MB, Ljubimov AV. Effects of tenascin-C on normal and diabetic retinal endothelial cells in culture. Invest Ophthalmol Vis Sci 2002; 43:2758-66; PMID:12147613 [PubMed] [Google Scholar]
- 87. Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest 2001; 107:785-90; PMID:11285293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ziu M, Schmidt NO, Cargioli TG, Aboody KS, Black PM, Carroll RS. Glioma-produced extracellular matrix influences brain tumor tropism of human neural stem cells. J Neurooncol 2006; 79:125-33; PMID:16598423 [DOI] [PubMed] [Google Scholar]
- 89. Nakahara H, Gabazza EC, Fujimoto H, Nishii Y, D'Alessandro-Gabazza CN, Bruno NE, Takagi T, Hayashi T, Maruyama J, Maruyama K, et al. Deficiency of tenascin C attenuates allergen-induced bronchial asthma in the mouse. Eur J Immunol 2006; 36:3334-45; PMID:17125141 [DOI] [PubMed] [Google Scholar]
- 90. El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol 2007; 211:86-94; PMID:17121418 [DOI] [PubMed] [Google Scholar]
- 91. Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009; 15:774-80; PMID:19561617; http://dx.doi.org/ 10.1038/nm.1987 [DOI] [PubMed] [Google Scholar]
- 92. Rudnick JA, Kuperwasser C. Stromal biomarkers in breast cancer development and progression. Clin Exp Metastasis 2012; 29:663-72; PMID:22684404; http://dx.doi.org/ 10.1007/s10585-012-9499-8 [DOI] [PubMed] [Google Scholar]
- 93. O'Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci U S A 2011; 108:16002-7; PMID:21911392; http://dx.doi.org/ 10.1073/pnas.1109493108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 2011; 17:867-74; PMID:21706029; http://dx.doi.org/ 10.1038/nm.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]


