Abstract
The extracellular matrix protein tenascin C (TNC) is a large glycoprotein expressed in connective tissues and stem cell niches. TNC over-expression is repeatedly observed in cancer, often at the invasive tumor front, and is associated with poor clinical outcome in several malignancies. The link between TNC expression and poor survival in cancer patients suggests a role for TNC in metastatic progression, which is responsible for the majority of cancer related deaths. Indeed, functional studies using mouse models are revealing new roles of TNC in cancer progression and underscore its important contribution to the development of metastasis. TNC has a pleiotropic role in advancing metastasis by promoting migratory and invasive cell behavior, angiogenesis and cancer cell viability under stress. TNC is an essential component of the metastatic niche and modulates stem cell signaling within the niche. This may be crucial for the fitness of disseminated cancer cells confronted with a foreign environment in secondary organs, that can exert a strong selective pressure on invading cells. TNC is a compelling example of how an extracellular matrix protein can provide a molecular context that is imperative to cancer cell fitness in metastasis.
Keywords: tenascin C, invasion, metastasis, niche, stem cell, extracellular matrix
Introduction
Metastasis is the malignant tumor growth in secondary organs, that causes profound morbidity and mortality in cancer patients. Development of overt metastasis results from a multi-step process that requires diverse cancer cell functions and includes: increased motility and invasiveness, entry and survival in blood circulation, vascular exit, resistance to selective pressures in distant organs and the growth of a secondary tumor under unfavorable conditions.1 These steps in metastatic progression are driven by genetic and epigenetic alterations in cancer cells, but also require supportive signals from the surrounding microenvironment.2,3 The tumor microenvironment, comprised of cellular and non-cellular components, provides regulatory cues that can significantly affect cancer cell behavior. Specialized microenvironment may restrict cancer cell growth, but in response to reprogramming by tumor cells, activated microenvironment can promote cancer progression.4 Indeed, metastatic cancer cells induce changes in both molecular and cellular composition of the tumor microenvironment.3 The ability of cancer cells to promote favorable changes in the microenvironment of distant organs may determine their potential to form manifest metastasis.5
The extracellular matrix (ECM) is increasingly recognized as a major player in cancer progression and metastasis, providing important regulatory cues for cellular responses.6 Functional outcome of signaling pathways is highly context dependent and can be modulated by a particular ECM composition.7 Tenascin C (TNC) is a glycoprotein of the ECM, whose intricate link to cancer has been recognized since its discovery in the mid-1980s.8,9 The TNC protein consists of several structural domains that play distinct roles in TNC function (Fig. 1A).10,11 In healthy mammals, TNC is highly expressed during embryonic development, particularly in the developing central nervous system, in migrating neural crest cells and at epithelial – mesenchymal interaction sites.10,12 In adult tissues, TNC expression is tightly regulated and generally repressed, although certain connective tissues like periosteum, ligaments, tendons and smooth muscles are positive for TNC.10,13 Interestingly, significant TNC expression is detected in stem cell niches of various tissues such as the brain, hair follicle and bone marrow and this may suggest a role in stem cell regulation.14
Figure 1.
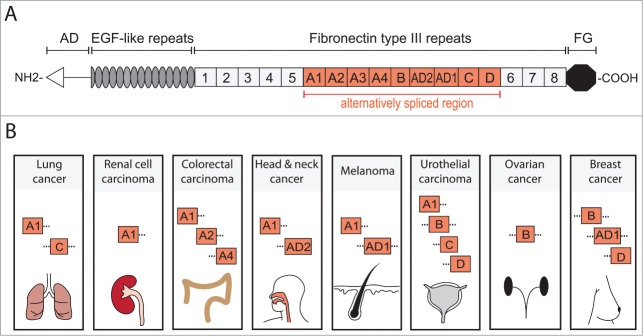
TNC structure and cancer associated domains. TNC is a multifunctional glycoprotein composed of several distinct domains. (A) Domain structure of full length human TNC protein (based on ref. 11). At the N-terminus, the assembly domain (AD) mediates the oligomerization of the protein where 2 trimers form a hexameric structure. Between the EGF-like repeats and the carboxy terminal fibrinogen globe (FG) are Fibronectin type III repeats (FNIII). In human TNC, 9 of the total 17 FNIII repeats are alternatively spliced providing the possibility of multiple different TNC isoforms. (B) Several alternatively spliced FNIII repeats have been identified in cancer. FNIII domains A1 and C are expressed in lung cancer and A1 domain in renal cell carcinoma.39,40,52 Colorectal carcinoma (CRC) expresses domains A1, A2 and A4 which are specifically enriched in CRC when compared to total TNC expression.51 Head and neck cancer exhibits A1 and AD2 domains while melanoma expresses A1 and AD1.53–56 In urothelial carcinoma, domains A1, B, C and D are present and associate with invasive cancer.57,58 FNIII domain B is expressed in ovarian cancer and is enriched compared to the short TNC isoform (lacking all alternatively spliced FNIII domains).59 Breast cancer expresses B and D domains that are associated with invasive behavior and the AD1 domain.55,60 It is important to note that these lists are not exhaustive and only include the domains that have been positively linked to a particular cancer. Information of complete isoforms is generally lacking. However, while the knowledge of different TNC isoforms expressed in cancer is still rudimentary and incomplete, the appearance of different domains in cancers may indicate a requirement for distinct aspects of TNC functions.
Although cells within epithelia are essentially negative for TNC, a striking upregulation is observed under conditions of tissue regeneration such as wound healing, inflammation or mammary gland involution.13,15 Tissue remodeling during involution of the post-lactating mammary gland is associated with immense changes in the mammary gland microenvironment, including the induction of various ECM proteins such as TNC.16 Interestingly, the matrix from an involuting mammary gland can promote tumor formation and metastasis when co-implanted with cancer cells into mice.17 The pro-tumorigenic properties of ECM components in the involuting mammary gland may explain the increased risk of breast cancer following pregnancy.18 Importantly, under tissue regeneration and repair processes, TNC expression is transient and restricted to the duration of the modeling process. TNC levels are for example greatly reduced after wound healing is completed and TNC is virtually absent in scar tissue.19 However in cancer, TNC may not follow the same organized regulation leading to sustained TNC activity. TNC has been shown to affect many aspects of cancer cell biology and modulate several steps needed to reach distant organs and form overt metastasis. In this review, we aim to discuss the current evidence on the role of TNC in cancer progression and metastasis.
Clinical association with advanced cancer
TNC expression is frequently observed in human cancer and accumulating evidence suggests that the presence of TNC in cancer tissue is not a mere passive event but has significant functional consequences.15,20 Studies on diverse types of cancer show an association between TNC and key clinical parameters, such as relapse-free or overall survival. These cancers include a diverse group of epithelial derived carcinomas, as well as melanoma and brain malignancies. Tumors of the brain rarely metastasize and will therefore not be discussed here. However, a strong association between TNC and poor clinical outcome in patients with brain tumors such as gliomas suggests that TNC may also play a significant role in the progression of these diseases (for a review see ref. 21). Several carcinomas and melanoma can carry a risk of metastasis and the link between TNC expression in these cancers and disease relapse indicates a functional TNC role in metastatic progression.
Breast cancer
Cancer of the breast is the most frequent malignancy in women with close to 1.4 million new cases diagnosed in 2008 worldwide.22 Around 10–15% of breast cancer patients develop metastasis within 3 y from diagnosis.23 A strong association has been observed between breast cancer and expression of TNC. While TNC expression is under tight control in the normal mammary gland, it is highly induced during breast cancer development.10,12 Mammary tumors in mice and human breast malignancies express TNC with a particularly strong expression in the stroma.12,24 Continuously growing evidence suggests that high TNC is associated with poor clinical outcome in breast cancer as attested by the presence of TNC in gene expression signatures that predict distant metastasis.25-27
An association has been observed between TNC expression and estrogen receptor (ER) negative tumors of the breast, which are known to be less differentiated and more aggressive than ER positive tumors.28,29 However, while this link is significant it is not exclusive or clear cut because significant heterogeneity with regards to TNC expression has been observed within ER positive tumors. Whereas no TNC is detected in well-differentiated tumor areas, TNC can be detected in invasive marginal areas.24 Generally, the invasive cases of breast carcinomas exhibit strong TNC expression.30,31 Interestingly, high TNC levels in early breast cancer, ductal carcinoma in situ (DCIS), are suggested to predict invasive behavior of cancer cells before any overt signs of invasion are apparent.32,33 Moreover, after removal of the primary tumor, high TNC expression is linked to both local and distant breast cancer recurrence, manifesting in poor overall survival.28,34,35 TNC expression is associated with poor metastasis free and overall patient survival when detected in the primary tumor or the metastatic lesions themselves.36,37
Lung cancer
Lung cancer is globally the most common cancer in men and second most common in women and causes greater number of deaths than any other malignancy.22 Less than 20% of lung cancer patients live longer than 5 years.38 High TNC expression has been observed in all major types of lung cancer.39,40 In non-small cell lung carcinoma (NSCLC), which makes up about 80–85% of lung cancer, TNC expression is greatly upregulated compared to adjacent normal lung tissue and the highest TNC expression was observed in NSCLC patients with a recurrent disease.41
Head and neck cancer
Cancer of the head and neck is a collection of cancers originating in the oral cavity, the pharynx and larynx, salivary glands and nasal cavity. Head and neck cancer arises most frequently from squamous cells within these tissues.42 High TNC expression in head and neck cancer predicts poor clinical outcome. In tumors of the salivary glands, TNC expression showed significant heterogeneity with highest expression associated with poor differentiation and high malignancy.43 In a study analyzing 158 patients with oral tongue squamous cell carcinoma, TNC was an independent prognostic factor of poor patient survival.44 In line with this, expression of TNC in laryngeal and hypopharyngeal cancers was linked to early metastatic recurrence and poor overall survival.45
Melanoma
High TNC levels are observed in cutaneous melanoma, which is the malignancy of melanocytes, a pigment producing cell type in the skin. Invasive melanoma is linked to poor clinical outcome and metastatic melanoma has a median survival of only 6–9 months.46 TNC expression has been shown to increase in and around melanoma cells as the tumor increases in thickness47 and in patient samples, TNC expression is greater in malignant melanomas and metastatic lesions compared to benign tumors.48
Colorectal cancer
Colorectal carcinoma (CRC) is a common cancer in men and women, causing worldwide over 600 thousand deaths annually.22 A link between high TNC expression and poor prognosis has been established in CRC. Studies on CRC patient samples show that stromal TNC staining is associated with advanced stage of the disease, metastatic spread and shorter patient survival.49,50 Moreover, a correlation between TNC and Annexin II, a calcium-dependent phospholipid-binding protein and a recognized binding receptor of TNC, was observed in CRC tumors and served as an independent predictor of poor prognosis.50
TNC isoforms
Numerous different isoforms expressed from the TNC gene have been identified. The isoforms are generated through alternative splicing of exons within the fibronectin type III (FNIII) repeats resulting in protein products of molecular weights between 220–320kDa. The splicing of FNIII repeats may significantly affect the function of the TNC protein and splicing specificity can be highly context dependent and may differ across various tumor entities. In line with this notion, certain TNC domains have been linked to particular cancer types (Fig. 1B), although our understanding of this is far from being complete. The isoforms containing FNIII A1, A2 and A4 domains are prominent in colorectal cancer.51 Furthermore, the A1 domain is expressed in renal cell carcinoma as well as lung cancer which in addition expresses the FNIII C domain.39,40,52 In melanoma, both A1 and the additional domain 1 (AD1) are detected while in head and neck cancer A1 and AD2 are expressed.53-56 The splicing forms containing FNIII domains A1, B, C and D are expressed in urothelial carcinoma and are increased with tumor progression.57,58 Large splice variants containing the B-domain are highly expressed in ovarian cancer and associated with malignancy.59 In breast cancer, FNIII B and D domains and AD1 are expressed.55,60 Interestingly, TNC containing AD1 domain is specifically produced by epithelial cells, primarily cancer cells, but to a lesser extent by myoepithelial cells within the tumor.61 Although most cancers express the larger isoforms of TNC, the full length may not be the most relevant to cancer progression. For example, in breast cancer cells, TNC isoforms containing B and D domains or only D domain, promote invasion more effectively than does the full length TNC.11
Regulation of TNC splicing is generally poorly understood. However, the molecular players are beginning to be identified. Certain splicing factors have been linked to generation of TNC isoforms. The RNA binding protein Sam68 and serine/arginine-rich splicing factor 6 (SRSF6) have been associated with regulation of alternative TNC splicing. Sam68 and SRSF6 promote generation of larger TNC splicing forms in neuronal stem cells and keratinocytes respectively.62,63 Interestingly, certain TNC splicing forms have been shown to be selected by changes in cellular environment. While low pH favors the short TNC splicing form, serum stimulation enriches for larger splicing forms.64,65 Secreted cytokines like interferon gamma (IFNγ) modestly increase the large TNC isoform, while tumor necrosis factor alpha (TNFα) and transforming growth factor beta (TGFβ) promote the small isoform.66,67 At the transcriptional level, paired-box protein Pax6 was shown to induce generation of larger TNC isoforms in stem/progenitor cells.68 The importance of different TNC splicing forms in metastasis, beyond a role in invasion, is not well characterized.
In addition to different splicing forms, TNC is also fragmented and remodeled by proteases. TNC is susceptible to cleavages by matrix metalloproteinases (MMPs) and serine proteases.69,70 Interestingly, the different splicing forms have distinct sensitivity to proteases. For example, the large splicing form containing alternatively spliced FNIII repeats is sensitive to MMP2 and MMP3.70 Within the FNIII repeats, MMP2 and MMP3 exclusively cleave the FNIIIA3 domain71 and therefore a splicing event that includes or excludes this domain, controls the corresponding cleavage sensitivity. MMP2 mediated cleavage leads to release of a 22-mer peptide containing the pro-adhesive site FNIIIA2 that can enhance resistance to anoikis and stimulate proliferation induced by platelet derived growth factor (PDGF).72 In lung cancer, high TNC expression is followed by significant TNC fragmentation and this may have functional implications. While healthy lung is negative for degraded TNC, squamous and adenocarcinoma show substantial TNC degradation, which is even further increased in tumors associated with lymph node metastasis.73 The link between degraded TNC and clinical prognosis is not limited to the spread to lymph nodes but may also apply to distant metastasis. A study analyzing stage 1 NSCLC patients showed a significant association between degraded TNC and poor recurrence-free and overall survival.74
TNC sources in tumors
Expression at the invasive front
In many cancers, tumor nodules exhibit heterogeneous TNC expression, where the strongest expression often being associated with the invasive front.20 Importantly, TNC heterogeneous distribution and expression at invasive fronts are also observed in metastases.36 This phenomenon may be of functional importance, since TNC positivity at the invasive front is linked to poor clinical outcome in breast cancer and predicts local and metastatic recurrence.34,75 Similarly in melanoma, TNC expression at the invasive front is associated with poor recurrence free survival.76 Mesothelioma of the lung and cholangiocarcinoma of the liver also express TNC at the invasion front where it correlates with poor overall survival.77,78
Reactive stroma
The cancer associated stroma is a prominent source of TNC.12,24 Within the stroma, activated fibroblasts (myofibroblasts) and angiogenic blood vessels are major producers of TNC. Interestingly, high levels of TNC expression can be induced in resting fibroblasts when co-cultured with cancer cells.79 TNC expression in blood vessels is low under homeostasis, however, a significant TNC increase is observed upon initiation of angiogenesis.80 The highest endothelial TNC is detected in growing tumors where several cancer entities have been shown to express substantial vascular TNC in active proliferating endothelial cells in mouse models and human tumors.52,80 Interestingly, in embryonic development the mesenchyme surrounding budding epithelia, such as the mammary gland or the hair follicle, expresses high levels of TNC.12 This may suggest a common role for TNC in epithelial-mesenchymal interactions during the formation of invading structures in normal development and cancer. TNC regulation in stroma is not fully understood, but can occur via mechanisms involving cytokines such as TGFβ, fibroblast growth factors (FGFs) and TNFα as well as mechanical strain.13,81
Production by cancer cells
TNC production is not restricted to reactive cancer stroma. In situ hybridization has revealed TNC positive cancer cells in cases of breast cancer and colon cancer.82,83 Moreover, cancer cells from oral squamous cell carcinoma have been shown to express TNC84 and in certain tumor cases, cancer cells at the invasive front have been demonstrated to be TNC positive.35,36,85 In agreement with this, various cancer cell lines, from breast cancer,11,29,86 melanoma87 and colon cancer86 show high expression of TNC. In the case of melanoma cells, TNC expression is observed even in the absence of added growth factors.87 Interestingly, stronger TNC expression was observed in melanoma cells from advanced primary tumors and metastasis, while cell lines from early melanoma tumors or normal melanocytes exhibited either weak or no TNC expression.48,87 The association between high TNC levels in cancer cells and their propensity to metastasize has also been shown in breast cancer cells, where the selection for metastatic ability to colonize the lung, in a xenograft mouse model, also selects for high TNC expression.25 How cancer cells acquire TNC expression is generally not well understood, however it is intriguing that cancer cells which do not normally express TNC can be induced to do so by co-culture with embryonic mesenchyme.88 In breast cancer, the metastasis suppressor microRNA-335 has been shown to be an important TNC regulator leading to its repression in cancer cells.26
TNC expression by cancer cells appears to correlate with poor clinical outcome for cancer patients. In situ analyses of tissue sections from breast cancer patients suggest that certain portions of primary tumors, often the tumor edge, may contain TNC positive cancer cells29,35 and this predicts exceedingly poor overall survival.35 Similarly, autocrine TNC expression in cancer cells predicts poor overall survival in colon cancer patients.89 The expression of TNC by cancer cells may have particular implications for certain stages of metastatic progression. During metastatic spread, disseminated cancer cells released by the primary tumor face a non-permissive microenvironment at distant sites to which they are poorly adapted and are therefore eliminated in great numbers. Expressing TNC in an autocrine manner could provide disseminated cancer cells with an advantage as they are confronted by a foreign environment in distant secondary organs. Indeed, high TNC expression is consistently detected in cancer cells isolated from lung pleural fluids of breast cancer patients with multiple metastases.36 Together this suggests that autocrine TNC expression, especially at the invasive tumor front where cancer cells face a high degree of selective pressure, may support metastatic progression.
Functional evidence from mouse models
Different mouse models have been used to address the functional role of TNC in metastasis. In a genetic mouse model for neuroendocrine tumors, where expression of SV40 large T antigen was targeted to the pancreas, ectopic TNC production significantly increased micrometastasis in lungs compared to controls, while TNC knockout reduced metastatic colonization.90 A second genetic mouse model was used to address TNC role in mammary tumor formation and metastasis. Development of mammary tumors was induced by targeting expression of polyomavirus middle T (PyMT) antigen to the mammary gland. In this model, TNC deficiency did not have significant effect on the growth of primary tumors or lung metastases.91 The TNC redundancy observed in this model is surprising but could be explained by compensatory mechanisms or the nature of the oncogene driving mammary tumor formation.
Recent evidence from xenograft and syngeneic mouse models strongly support a functional role for TNC in metastatic progression. TNC deficiency in human breast cancer cells significantly impairs metastasis to the lungs and to the bones in xenograft models.36 Furthermore, a study addressing the stroma as a source of TNC showed that 4T1 mammary tumor cells injected intravenously into a TNC knockout mice were less efficient in lung colonization compared to cancer cells injected into control mice.92 This is in line with the notion that both cancer cell-derived and stromal TNC play a functional role in the metastatic process. However, experiments from xenograft mouse models for melanoma suggest that there might be a distinction between the roles of autocrine and paracrine TNC production. TNC knockdown in melanoma cells inhibited their ability to colonize the lungs while it did not significantly affect the growth of subcutaneous tumors.93 Interestingly, a second xenograft study on melanoma where TNC knockout mice were crossed onto an immunocompromised background, showed that melanoma cells grew considerably slower in the TNC knockout mice compared to controls.94 Metastasis was not analyzed in this study leaving the question of a functional role for stromal derived TNC in melanoma metastasis to be answered.
The requirement for stromal versus cancer cell-derived TNC may occur at different stages during metastatic progression. When TNC deficient cancer cells are injected into TNC wild type mice, the resting stroma is not able to compensate for the TNC deficiency leading to significant reduction in lung metastatic outgrowth.36 Importantly, cancer cell derived TNC remains essential even if autocrine TNC expression is inhibited several days after metastatic colonization has begun. However, if the metastatic tumor grows to a significant size and activates the stroma, as attested by the presence of α smooth muscle actin (α-SMA) expressing myofibroblasts, the autocrine TNC expressed by cancer cells is no longer required.36 This indicates that autocrine TNC is particularly important for the early steps of metastatic colonization and suggests that cancer cells acquire an advantage by expressing TNC themselves, during the initial colonization of distant organs. These initial steps may be critical in deciding the fate of a disseminated cancer cell and therefore a delay in activation of stroma can select for autocrine TNC expression in cancer cells.
Diverse cellular roles in metastatic progression
TNC is a pleiotropic molecule and has been implicated in various cellular functions that can promote metastasis (Fig. 2). The diversity in TNC function is noteworthy and spans activities such as regulation of adhesion and migratory mechanisms, promotion of angiogenesis and cancer cell-fitness and modulation of immune responses. These cellular functions may be required at distinct stages in the life of metastatic cancer cells.
Figure 2.
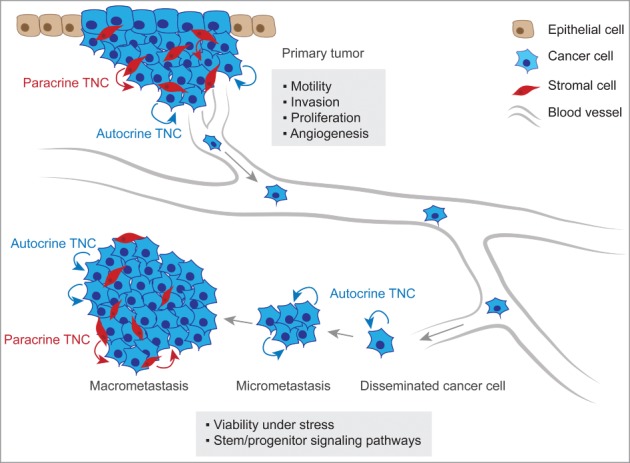
TNC function in metastatic progression. At the primary tumor site, anti-adhesive TNC properties lead to alteration of intracellular pathways in cancer cells inducing the formation of actin-rich filopodia, favoring cell motility and invasive behavior. TNC is also associated with increased cancer cell proliferation and promotes angiogenesis within the tumor. At the secondary organ site, autocrine TNC supports cancer cell viability in a microenvironment that can exert a strong selective pressure. In breast cancer, TNC engages stem/progenitor signaling i.e. the Notch and Wnt pathways thereby promoting growth of micrometastasis. The development of macrometastasis is associated with reactive stroma which becomes a significant source of TNC protein.
Modulating adhesion and motility
Regulation of cell adhesion and migration are among the best studied and characterized TNC functions. TNC has been linked to both adhesive and counter-adhesive activities.15 These functions may be determined by the context of overall ECM composition as well as the expression of particular TNC splicing forms. Anti-adhesive properties of TNC have long been recognized, as TNC inhibits the spreading on adhesive ECM substrates such as fibronectin through modulation of integrin function.95 Interestingly, different TNC splicing forms have been shown to display distinct ability to inhibit adhesion to fibronectin, with the shortest TNC isoform, lacking all variable FNIII repeats, exhibiting the most significant ability to inhibit fibronectin mediated adhesion.96 TNC binding inhibits the interaction between fibronectin and syndecan-4 (SDC4), a transmembrane heparan sulfate proteoglycan that is required for cell spreading on fibronectin in synergy with integrin α5β1.97 As a result, intracellular signaling pathways and activity of Rho family GTPases that normally are triggered by cell contact with adhesive ECM substrates are altered. Formation of actin stress fibers is inhibited via negative regulation of RhoA GTPase while Rac GTPase activity is favored. This leads to induction of actin-rich filopodia and stimulates cell motility and invasion.98 Studies have reported a functional role for TNC in promoting invasion of several cancers.11,26,99
The actin-bundling protein fascin-1 (FSCN1) is another promoter of cancer cell invasion.100 FSCN1 is an efficient inducer of filopodia and invadopodia101,102 and is regulated by Rho family GTPases such as Rac and Cdc42.100 FSCN1 is associated with cancer cell dispersion to distant sites as well as migration of disseminated cancer cells back to the primary tumor from circulation.103,104 In this regard, TNC has been shown to promote FSCN1 association with actin in membrane protrusions, suggesting that FSCN1 may be an important mediator of TNC induced invasion.105
Epithelial mesenchymal transition (EMT) is linked to major changes in cellular migratory behavior and has been suggested to be a mechanism enabling cancer-spread to distant organs.106 Epithelial properties, such as polarization and cell-cell adhesion, are lost in cells undergoing EMT while mesenchymal characteristics and increased migration capacity are acquired.107 The role of EMT is well characterized in embryonic development and in tissues undergoing regeneration and TNC has been shown to be strongly expressed during these processes.10,15 As mentioned above, TNC is expressed at epithelial mesenchymal interaction sites and this occurs particularly in budding tissue structures such as hair follicles, teeth or mammary glands during embryonic development and at the invasive front of healing wounds in adult tissues.12,15 This may suggest a role for TNC in EMT. Indeed, TNC deficiency has been shown to suppress EMT during regeneration of wounded lens of the eye.108 Moreover, evidence suggests that TNC can also promote EMT in different cancers.89,109,110 In human breast cancer samples, TNC is co-expressed with the mesenchymal marker vimentin.29 The mechanistic role of TNC in the process of EMT remains poorly defined, however, studies suggest that TNC can induce an EMT like phenotype in MCF7 breast cancer cells via the αVβ6 and αVβ1 integrins.109,110
Promoting cellular fitness under stress
Disseminated cancer cells face a microenvironment in secondary organs that may not be well suited for their growth. Indeed, studies suggest that colonization of distant sites is the most rate limiting step in the metastatic process and of the numerous cancer cells that are able to reach distant organs, only a minute fraction will progress to form an overt metastasis.111 Evidence suggests that TNC plays a significant role in these processes. TNC has been shown to promote metastatic fitness of disseminated cancer cells and facilitate colonization of secondary organs in mouse models. TNC supports the viability of breast cancer cells at the distant site by engaging the Notch and Wnt signaling pathways. TNC was shown to modulate Notch signaling by the means of the RNA binding protein musashi-1 (MSI1) promoting the expression of the Notch target genes hairy and enhancer of split-related protein 2 (HEY2) and deltex 1 (DTX1) in cancer cells.36 Furthermore, TNC induces the Wnt signaling target gene Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5), and together induction of MSI1 and LGR5 promote the initial step in metastatic colonization of the lung.36 Interestingly, in certain cancers, TNC can be induced by Wnt and Notch signaling pathways.112,113 This could suggest a positive feedback loop, but has not been addressed in breast cancer.
The ability to proliferate under sub-optimal conditions can contribute to increased cancer cell fitness in metastasis. In breast cancer, analysis of tissue sections has shown a positive correlation between high TNC expression and proliferation.114 Moreover, functional analysis using laryngeal carcinoma cell cultures has shown that ectopic TNC induces cancer cell proliferation.115 Interestingly, evidence from cell culture also suggests that TNC modulates proliferation in a context dependent manner. In non-transformed fibroblasts that rely on anchorage, TNC mediates interference between fibronectin and SDC4, inhibiting proliferation.116 However, in transformed cells the same molecular interference leads to promotion of cell proliferation.97 In addition, TNC can promote PDGF induced proliferation by directly binding to SDC4 via the alternatively spliced FNIIIA2 domain and enforcing SDC4 physical association with PDGFR and α5β1 integrin.72 The epidermal growth factor-like (EGFL) repeats within the TNC protein may also promote cell proliferation. EGFL repeats have been shown to have low but significant affinity to EGFR and are able to engage the receptor and induce proliferation.117
Developing new blood vessels
Angiogenic processes in early development and during adult tissue regeneration are strongly linked to TNC. High TNC expression is observed in the vasculature of many developing organs and studies using TNC knockout mice suggest that TNC is required for neovascularization of the embryonic lung and heart.118 In healthy adult vessels under homeostasis, TNC expression is limited, but is greatly induced upon vessel damage and is associated with vascular remodeling during dermal tissue repair. In addition, TNC is expressed during pathological angiogenesis in a wide range of diseases such as diabetes, atherosclerosis, crohn's disease, inflammatory bowel disease or cancer.118 This suggests a proangiogenic role of TNC, which may be crucial in the progression of cancer. Indeed, tumors that are formed by melanoma cells injected into TNC knockout mice show a significant decrease in vascularization suggesting that TNC is required for the process.94
TNC may impact angiogenesis via diverse mechanisms. Endothelial cells adhere to TNC via α2β1 and αVβ3 integrins119 and TNC has been suggested to promote a sprouting phenotype in cultured endothelial cells.120 TNC reduces focal adhesion and promotes migration of endothelial cells.121 In the absence of stromal derived TNC, melanoma cells secrete reduced levels of proangiogenic factors, such as VEGF, compared to control,94 but how TNC triggers the expression of VEGF is unknown. A role for Wnt signaling in the angiogenic process has also been reported. In a transgenic mouse model for neuroendocrine carcinogenesis of the pancreas, TNC was shown to induce the angiogenic switch via repression of the Wnt signaling antagonist Dickkopf-related protein 1 (DKK1).90 Together, the evidence suggests an important functional role for TNC in cancer angiogenesis. However, the extent and importance of TNC induced angiogenesis at the metastatic site remains to be experimentally addressed.
At the intersection of fibrosis and metastasis
A significant functional and molecular overlap has been observed between the processes of fibrosis and cancer. The collaboration between cancer cells undergoing EMT and fibrotic stroma promotes cancer progression and metastasis.122 Fibrosis is characterized by excess production of fibrillar collagen, hyaluronan and other ECM molecules and can occur when wound healing and tissue remodeling are deregulated. In normal wound healing, a provisional matrix is laid down that consists of fibronectin, fibrin and fibrinogen. Polymerization of fibronectin is required for the accumulation of other ECM proteins, such as Collagen I.123 In melanoma, TNC is found in fibrillar structures with pro-collagen-I during a switch to an invasive phase of the cancer.124 The provisional matrix recruits and retains fibroblasts that differentiate into myofibroblasts induced by TGFβ and mechanical tension. Eosinophils and macrophages that are attracted and retained by hyaluronan rich matrix are a major source of TGFβ.125 Normally, myofibroblasts undergo apoptosis after healing has occurred. This is dependent on induction of cytokines like interleukin 1β (IL1β) and repression of TGFβ and when this balance is skewed, for example by persistent TGFβ expression, myofibroblasts remain and continue excessive ECM production leading to fibrosis.125
A close relationship has long been recognized between TNC and myofibroblasts.82,83 Myofibroblasts are an important source of TNC,126 however, these cells have also been shown to respond to TNC under certain conditions. TNC expression precedes activation of fibroblasts during cardiac regeneration: α-SMA expressing myofibroblasts appear subsequently in TNC rich areas.127 TNC is increased in lung fibrosis induced by the chemotherapeutic bleomycin or the cytokine TGFα.126,128 Moreover, TNC expression coincides with α-SMA production when myofibroblast differentiation is induced in vitro.126 Evidence suggests that TNC plays a functional role in this process. TNC knockout mice treated with bleomycin show fewer myofibroblasts compared to control mice and are protected from lung fibrosis.129 TGFβ induced myofibroblast differentiation in vitro is also inhibited by TNC deficiency and in vivo TNC absence attenuates development of fibrosis in the liver.130
Myofibroblasts are known to enhance migration of cancer cells and an in vitro study on colon cancer cells showed that myofibroblast-derived TNC is required for this pro-invasive activity.131 Studies showed that TGFβ and PDGFβ trigger the expression of TNC by fibroblasts.129,132 Considering that metastatic cancer cells can express TNC by themselves, it can be envisaged that disseminated cancer cells at the distant site, recruit myofibroblasts which in turn facilitate the migration of the cancer cells and support the colonization of distant organs. TNC was shown to accelerate myofibroblast migration and α-SMA expression in vitro and to promote recruitment of myofibroblasts upon cardiac injury in a genetic mouse model.133
Fibrosis and cancer utilize in part the same molecular components and pathways that can lead to promotion of EMT. TGFβ and TNFα cytokines and hypoxia, that collaboratively induce an EMT phenotype, play a key role in fibrosis and cancer by reactivating developmental programs. Indeed, fibrosis has been shown to cause predisposition to cancer growth in breast and lung.134,135 Progression of fibrosis and cancer is associated with stiffer ECM caused by expression of large quantities of fibrillar collagen and collagen crosslinking enzymes of the lysyl oxidase (LOX) family.7 However, while TNC expression can be regulated by mechanical forces in fibroblasts,81 its putative contribution to tissue stiffness is likely to be indirect if it occurs. Currently, there is little evidence to suggest that TNC can form fibrils on its own. Moreover, TNC protein has been shown to exhibit significant elasticity.136 Considering this, TNC may still be involved in maintaining the fibrotic process by playing a regulatory role.
Inflammation and immunosurveillance
TNC has been linked to regulation of both innate and adaptive immunity. TNC modulates the production of several inflammatory cytokines and affects the recruitment of immune cells.15 The Toll-like receptor (TLR) family plays a fundamental role in activation of the innate immune system. TNC is an activator of TLR4, which subsequently stimulates secretion of pro-inflammatory cytokines such as IL6, IL8 or TNFα by macrophages or fibroblasts.137 Particularly in the context of chronic immune diseases, such as joint arthritis, TNC is essential for maintaining the inflammatory state.137,138 Whether TLR4 activation by TNC promotes tumor progression remains to be addressed.
In a mouse model for mammary cancer, TNC dependent changes in macrophage recruitment have been observed. While PyMT induced mammary tumors in TNC-null and control mice exhibited no difference in tumor growth, significant differences in the tumor structure and composition were identified.91 The tumor cell nests in the TNC knockout mice were surrounded by thickened ECM cords and were less infiltrated by monocytes and macrophages compared to control tumor stroma.91 This may indicate a role for TNC as a regulator of inflammatory cells in tumors.
Adaptive immunity may also be affected by TNC expression. Evidence suggests that TNC can inhibit T-lymphocyte activation in vitro.139 Moreover, in lung cancer TNC has been suggested to inhibit proliferation and IFNγ secretion of tumor-infiltrating lymphocytes.41 Within TNC FNIII repeats, 2 regions have been shown to affect T-cell behavior. FNIII1–5 block T-cell adhesion to fibronectin via α5β1 and α4β1 integrin inhibition, but are not known to affect T-cell activation.140 However, FNIIIA-D domain, particularly the A1 and A2 repeats, can inhibit T-cell activation.139
Essential component of the metastatic niche
TNC expression in normal stem cell niches
In adult tissues, stem cells are retained in specific anatomical structures or niches that nurture their self-renewal and differentiation potential. These niches maintain stem cell pools of animals throughout their lifespan. TNC expression has been detected in several adult stem cell niches.14 In the brain, expression of TNC is detected in the subventricular zone neuronal niche.141 TNC has diverse effects on neuronal stem and progenitor cell behavior and modulates proliferation, migration, neurite outgrowth and cell guidance.141,142 In addition, TNC promotes the response to basic FGF while inhibiting bone morphogenetic protein 4 (BMP4) activity and effectively maintaining oligodendrocyte progenitors.141 Together, in vitro and in vivo studies have shown that oligodendrocyte differentiation is accelerated in TNC deficient progenitors.141,142
In the bone microenvironment, TNC expression has been observed in the periosteal region and around arterial blood vessels.143 TNC is detected in the haematopoietic stem cell (HSC) niche and promotes HSC adhesion and proliferation.143,144 Haematopoietic stem progenitor cells (HSPC) adhere to TNC, possibly via α9β1 integrins.145 While under homeostasis the haematopoietic system is not significantly affected by TNC deficiency, HSC activation and stress response, as induced by the chemotherapeutic drug 5-fluorouracil, is inhibited in TNC knockout mice.146
TNC is present in the hair follicle bulge region which hosts epidermal stem cells of the skin.14 The role of TNC within the epidermal niche is still being elucidated. However, TNC has been shown to promote migration and proliferation of CD34 expressing epidermal stem cells isolated from the whisker follicle.147 Other niches, in which TNC expression has been identified, are the lymphoid niche in the thymus, the spermatogonial stem cell niche and the limbal niche in the eye. TNC expression in the lymphoid niche plays an important role for homing of lymphoid progenitors to the thymus via α9β1 integrins.148 Within the spermatogonial stem cell niche, TNC expression is observed in testicular peritubular cells which play an important role in supporting spermatogonial stem cells.149 In the limbal niches of the cornea, TNC is expressed and associates with signaling components of the Wnt and TGFβ pathways.150
Niches in cancer development and metastasis
In cancer, niches are thought to promote maintenance of malignant properties. This may be particularly important during metastatic progression due to the strong selective pressure that disseminated cancer cells face. Cancer cells must resist the negative pressure and overcome the obstacle of growing under stress inducing conditions. Cancer cells that successfully form metastasis are able to induce changes in the microenvironment that lead to generation of a metastatic niche.5,151 Here we use a broad definition of the term metastatic niche as any microenvironment that can support outgrowth of metastasis. The metastatic niche is therefore a more malleable concept compared to adult stem cell niches that are highly regulated and constrained. Moreover, the metastatic niche is not static and matures with the progression of the disease, by the continuous cross-talk and reciprocal exchange between cancer cells and the niche.151,152
Progression of the metastatic niche
The early life of disseminated cancer cells, prior to activation of the microenvironment, may be critical to the prospect of metastatic growth. Therefore, cancer cells that are self-sufficient in bringing factors supporting viability under stress, gain significant advantage on those relying on the activated stroma for equivalent factors. Autocrine TNC expression TNC may be an excellent example of this. Breast cancer cells that express their own TNC gain important advantage resulting in increased survival at early stages of colonization, before stromal fibroblasts are activated.36 Subsequently as the micrometastasis grows, reactive stroma takes over as a source of TNC and thereby maintains the viability of cancer cells.36,92 Importantly, the expression of TNC in breast cancer cells is further induced under conditions that enrich for stem cell properties, such as in spheroid cultures,36 a phenomenon that is also observed in melanoma cells.93 The expression of one's own niche components is particularly interesting as normal tissue stem cells have been shown to possess this ability. Mammary epithelial stem cells and neuronal stem cells upregulate autocrine TNC when grown in spheroid cultures.141,153
Within the metastatic niche, TNC engages and modulates stem/progenitor signaling such as the Notch and Wnt pathways.154 In breast cancer, TNC induces the Notch pathway by protecting the Notch inducer MSI1 from STAT5 mediated repression.36 This may have direct relevance to mammary gland development and maturation, since STAT5 is a prolactin responsive gene and is an inducer of terminal differentiation of mammary epithelial cells.155 The Wnt pathway is also of significant importance in maintaining stem/progenitor properties and is promoted by TNC in different cancers.36,90,156 The Wnt target gene LGR5, which is induced by TNC, is a stem cell marker in numerous tissues and promotes lung metastasis in breast cancer.36
TNC is highly expressed during metastasis initiation and as the metastatic foci develop, TNC heterogeneity is established leading to prominent TNC expression at the invasive front. The invasive front is a significant and intriguing location within tumor nodules and has been suggested to contain putative cancer stem cells associated with metastatic ability.157 Moreover, the cancer cell transition to invasiveness at the front has been linked to EMT, a process that can be associated with acquisition of stem cell properties.158 A second ECM protein observed at the invasive tumor front is periostin (POSTN), a glycoprotein expressed within the metastatic niche.159 POSTN in the niche binds Wnt ligand and presents it to cancer cells, thereby promoting metastatic colonization in a PyMT based mouse mammary tumor model.159 Interestingly, evidence suggests that TNC can bind POSTN protein directly.160 With this in mind, and the role that TNC and POSTN play in modulating Wnt signaling, it can be envisaged that these proteins play collaborative and interlinked roles within the metastatic niche.154
Resistance to cancer therapy
Metastatic spread frequently culminates in broad resistance to cancer therapy and the microenvironment is a significant contributor to reduced therapeutic efficacy.161 In addition to cellular components of the microenvironment, the ECM has surfaced as an important player in the promotion of therapy resistance. Cell viability can be promoted by anchorage to the ECM, while lack of adhesion can lead to an increase in reactive oxygen species (ROS) and cell death.162 Moreover, specific ECM composition may determine clinical outcome for cancer patients and promote resistance to cancer treatment.163
TNC expression is linked to poor clinical outcome and has been suggested to promote therapy resistance. In pancreatic cancer cells in vitro, TNC interaction with receptor Annexin A2 induces resistance to the drug gemcitabine.164 Moreover, in breast cancer cultures, TNC can abrogate G1/S arrest in cancer cells treated with the chemotherapeutic doxorubicin (Adriamycin) by downregulating p21 via the adaptor protein 14–3–3 tau.165 The 14–3–3 tau and p21 axis has also been linked to resistance to endocrine therapy such as tamoxifen treatment.165 Indeed, TNC was identified as part of a gene signature that is linked to tamoxifen resistance in patients with metastatic breast cancer.166 TNC has pleiotropic qualities and the means of mediating resistance may be distinct in different contexts. In melanoma cell cultures, TNC promotes a stem cell phenotype associated with expression of ATP-binding cassette (ABC) transporters that mediate resistance to doxorubicin treatment.93 The diverse mechanisms involved in TNC mediated resistance indicate that TNC may be an attractive target for accompanying adjuvant treatment. Further studies will be required to characterize the mechanistic details and putative synergy between targeting TNC function and standard of care cancer therapy.
Conclusions and perspectives
TNC is involved in a broad range of cellular processes including migration, invasiveness, angiogenesis and immunomodulation. In addition to its role in crucial steps along the way to secondary organs, TNC is an essential component of metastatic microenvironment and represents a key factor in promoting survival of cancer cells lodged in distant organs. Disseminated cancer cells expressing TNC by themselves are strongly favored under these conditions. Through its multiple binding sites to cell surface receptors, TNC is able to modulate cell signaling in the metastatic niche in a way that we are only beginning to understand.
The complex and versatile molecular architecture of TNC allows different context-dependent roles, some of which have been suggested to be important for cancer progression and metastasis. Due to the pleiotropic nature of TNC and context dependency, the specific role in metastasis needs further characterization. Many findings on TNC function have thus far exclusively been demonstrated in tissue culture or in a growing primary tumor and putative roles in metastasis have in many cases only been inferred from these findings. Further studies are needed in the context of metastatic disease to identify which of the many functions of TNC are the key functions in TNC promoted metastasis.
Characterization of specific TNC functions that are essential for metastasis may reveal mechanisms that can be targeted to render cancer cells more vulnerable to current therapeutic options. This includes identifying the specific TNC splicing forms, cellular receptors and activated signaling pathways, all of which could be attractive targets to disrupt the pro-metastatic activities of TNC. A comprehensive understanding of these mechanisms may be essential to determine the right treatment composition and to identify patients that could benefit from a therapy targeting TNC or TNC regulated pathways.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank M.D. Milsom, M.R. Sprick, S. Acharyya and members of the Oskarsson laboratory for helpful comments.
Funding
C.L. is supported by the Helmholtz International PhD program. T.O. is supported by the Marie Curie CIG Actions and the Dietmar Hopp Foundation.
References
- 1. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 2006; 127:679-95; PMID:17110329; http://dx.doi.org/ 10.1016/j.cell.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 2. Vanharanta S, Massague J. Origins of metastatic traits. Cancer cell 2013; 24:410-21; PMID:24135279; http://dx.doi.org/ 10.1016/j.ccr.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9:239-52; PMID:19279573; http://dx.doi.org/ 10.1038/nrc2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011; 17:320-9; PMID:21383745; http://dx.doi.org/ 10.1038/nm.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oskarsson T, Batlle E, Massague J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 2014; 14:306-21; PMID:24607405; http://dx.doi.org/ 10.1016/j.stem.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009; 326:1216-9; PMID:19965464; http://dx.doi.org/ 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011; 3:1-24; PMID:21917992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bourdon MA, Wikstrand CJ, Furthmayr H, Matthews TJ, Bigner DD. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res 1983; 43:2796-805; PMID:6342760 [PubMed] [Google Scholar]
- 9. Chiquet M, Fambrough DM. Chick myotendinous antigen. II. A novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol 1984; 98:1937-46; PMID:6202699; http://dx.doi.org/ 10.1083/jcb.98.6.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn 2000; 218:235-59; PMID:10842355; http://dx.doi.org/ 10.1002/(SICI)1097-0177(200006)218:2%3c235::AID-DVDY2%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- 11. Hancox RA, Allen MD, Holliday DL, Edwards DR, Pennington CJ, Guttery DS, Shaw JA, Walker RA, Pringle JH, Jones JL. Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res 2009; 11:R24; PMID:19405959; http://dx.doi.org/ 10.1186/bcr2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 1986; 47:131-9; PMID:2428505; http://dx.doi.org/ 10.1016/0092-8674(86)90374-0 [DOI] [PubMed] [Google Scholar]
- 13. Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta 2009; 1793:888-92; PMID:19162090; http://dx.doi.org/ 10.1016/j.bbamcr.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 14. Chiquet-Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS. Tenascins in stem cell niches. Matrix Biol 2014; 37:112-23. [DOI] [PubMed] [Google Scholar]
- 15. Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal 2009; 3:287-310; PMID:19838819; http://dx.doi.org/ 10.1007/s12079-009-0075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones PL, Boudreau N, Myers CA, Erickson HP, Bissell MJ. Tenascin-C inhibits extracellular matrix-dependent gene expression in mammary epithelial cells. Localization of active regions using recombinant tenascin fragments. J Cell Sci 1995; 108 (Pt 2):519-27; PMID:7539436 [DOI] [PubMed] [Google Scholar]
- 17. McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, Schedin P. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol 2006; 168:608-20; PMID:16436674; http://dx.doi.org/ 10.2353/ajpath.2006.050677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer 2006; 6:281-91; PMID:16557280; http://dx.doi.org/ 10.1038/nrc1839 [DOI] [PubMed] [Google Scholar]
- 19. Maseruka H, Bonshek RE, Tullo AB. Tenascin-C expression in normal, inflamed, and scarred human corneas. Br J Ophthalmol 1997; 81:677-82; PMID:9349157; http://dx.doi.org/ 10.1136/bjo.81.8.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett 2006; 244:143-63; PMID:16632194; http://dx.doi.org/ 10.1016/j.canlet.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 21. Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cell Mol Life Sci 2011; 68:3175-99; PMID:21818551; http://dx.doi.org/ 10.1007/s00018-011-0783-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855 [DOI] [PubMed] [Google Scholar]
- 23. Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005; 5:591-602; PMID:16056258; http://dx.doi.org/ 10.1038/nrc1670 [DOI] [PubMed] [Google Scholar]
- 24. Mackie EJ, Chiquet-Ehrismann R, Pearson CA, Inaguma Y, Taya K, Kawarada Y, Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci U S A 1987; 84:4621-5; PMID:2440026; http://dx.doi.org/ 10.1073/pnas.84.13.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature 2005; 436:518-24; PMID:16049480; http://dx.doi.org/ 10.1038/nature03799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massagué J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008; 451:147-52; PMID:18185580; http://dx.doi.org/ 10.1038/nature06487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gurbuz I, Ferralli J, Roloff T, Chiquet-Ehrismann R, Asparuhova MB. SAP domain-dependent Mkl1 signaling stimulates proliferation and cell migration by induction of a distinct gene set indicative of poor prognosis in breast cancer patients. Mol Cancer 2014; 13:22; PMID:24495796; http://dx.doi.org/ 10.1186/1476-4598-13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, Agnantis NJ, Pavlidis N. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer 2002; 38:2362-70; PMID:12460779; http://dx.doi.org/ 10.1016/S0959-8049(02)00210-1 [DOI] [PubMed] [Google Scholar]
- 29. Dandachi N, Hauser-Kronberger C, More E, Wiesener B, Hacker GW, Dietze O, Wirl G. Co-expression of tenascin-C and vimentin in human breast cancer cells indicates phenotypic transdifferentiation during tumour progression: correlation with histopathological parameters, hormone receptors, and oncoproteins. J Pathol 2001; 193:181-9; PMID:11180164; http://dx.doi.org/ 10.1002/1096-9896(2000)9999:9999%3c::AID-PATH752%3e3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 30. Ferguson JE, Schor AM, Howell A, Ferguson MW. Tenascin distribution in the normal human breast is altered during the menstrual cycle and in carcinoma. Differentiation 1990; 42:199-207; PMID:1692795; http://dx.doi.org/ 10.1111/j.1432-0436.1990.tb00762.x [DOI] [PubMed] [Google Scholar]
- 31. Shoji T, Kamiya T, Tsubura A, Hatano T, Sakakura T, Yamamoto M, Morii S. Immunohistochemical staining patterns of tenascin in invasive breast carcinomas. Virchows Archiv A Pathol Anat Histopathol 1992; 421:53-6; PMID:1378984; http://dx.doi.org/ 10.1007/BF01607139 [DOI] [PubMed] [Google Scholar]
- 32. Goepel C, Buchmann J, Schultka R, Koelbl H. Tenascin-A marker for the malignant potential of preinvasive breast cancers. Gynecol Oncol 2000; 79:372-8; PMID:11104607; http://dx.doi.org/ 10.1006/gyno.2000.5978 [DOI] [PubMed] [Google Scholar]
- 33. Jahkola T, Toivonen T, Nordling S, von Smitten K, Virtanen I. Expression of tenascin-C in intraductal carcinoma of human breast: relationship to invasion. Eur J Cancer 1998; 34:1687-92; PMID:9893653; http://dx.doi.org/ 10.1016/S0959-8049(98)00215-9 [DOI] [PubMed] [Google Scholar]
- 34. Jahkola T, Toivonen T, Virtanen I, von Smitten K, Nordling S, von Boguslawski K, Haglund C, Nevanlinna H, Blomqvist C. Tenascin-C expression in invasion border of early breast cancer: a predictor of local and distant recurrence. Br J Cancer 1998; 78:1507-13; PMID:9836485; http://dx.doi.org/ 10.1038/bjc.1998.714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishihara A, Yoshida T, Tamaki H, Sakakura T. Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res 1995; 1:1035-41; PMID:9816077 [PubMed] [Google Scholar]
- 36. Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 2011; 17:867-74; PMID:21706029; http://dx.doi.org/ 10.1038/nm.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suwiwat S, Ricciardelli C, Tammi R, Tammi M, Auvinen P, Kosma VM, LeBaron RG, Raymond WA, Tilley WD, Horsfall DJ. Expression of extracellular matrix components versican, chondroitin sulfate, tenascin, and hyaluronan, and their association with disease outcome in node-negative breast cancer. Clin Cancer Res 2004; 10:2491-8; PMID:15073129; http://dx.doi.org/ 10.1158/1078-0432.CCR-03-0146 [DOI] [PubMed] [Google Scholar]
- 38. Keith RL, Miller YE. Lung cancer chemoprevention: current status and future prospects. Nat Rev Clin Oncol 2013; 10:334-43; PMID:23689750; http://dx.doi.org/ 10.1038/nrclinonc.2013.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silacci M, Brack SS, Spath N, Buck A, Hillinger S, Arni S, Weder W, Zardi L, Neri D. Human monoclonal antibodies to domain C of tenascin-C selectively target solid tumors in vivo. Protein Eng Des Sel 2006; 19:471-8; http://dx.doi.org/ 10.1093/protein/gzl033 [DOI] [PubMed] [Google Scholar]
- 40. Pedretti M, Soltermann A, Arni S, Weder W, Neri D, Hillinger S. Comparative immunohistochemistry of L19 and F16 in non-small cell lung cancer and mesothelioma: two human antibodies investigated in clinical trials in patients with cancer. Lung Cancer 2009; 64:28-33; PMID:18799229; http://dx.doi.org/ 10.1016/j.lungcan.2008.07.013 [DOI] [PubMed] [Google Scholar]
- 41. Parekh K, Ramachandran S, Cooper J, Bigner D, Patterson A, Mohanakumar T. Tenascin-C, over expressed in lung cancer down regulates effector functions of tumor infiltrating lymphocytes. Lung Cancer 2005; 47:17-29; PMID:15603851; http://dx.doi.org/ 10.1016/j.lungcan.2004.05.016 [DOI] [PubMed] [Google Scholar]
- 42. Kang H, Kiess A, Chung CH. Emerging biomarkers in head and neck cancer in the era of genomics. Nat Rev Clin Oncol 2014; 12:11-26; PMID:25403939. [DOI] [PubMed] [Google Scholar]
- 43. Raitz R, Martins MD, Araujo VC. A study of the extracellular matrix in salivary gland tumors. J Oral Pathol Med 2003; 32:290-6; http://dx.doi.org/ 10.1034/j.1600-0714.2003.00019.x [DOI] [PubMed] [Google Scholar]
- 44. Wang Z, Han B, Zhang Z, Pan J, Xia H. Expression of angiopoietin-like 4 and tenascin C but not cathepsin C mRNA predicts prognosis of oral tongue squamous cell carcinoma. Biomarkers 2010; 15:39-46; PMID:19775228; http://dx.doi.org/ 10.3109/13547500903261362 [DOI] [PubMed] [Google Scholar]
- 45. Juhasz A, Bardos H, Repassy G, Adany R. Characteristic distribution patterns of tenascin in laryngeal and hypopharyngeal cancers. Laryngoscope 2000; 110:84-92; PMID:10646721; http://dx.doi.org/ 10.1097/00005537-200001000-00016 [DOI] [PubMed] [Google Scholar]
- 46. Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet 2014; 383:816-27; PMID:24054424; http://dx.doi.org/ 10.1016/S0140-6736(13)60802-8 [DOI] [PubMed] [Google Scholar]
- 47. Natali PG, Nicotra MR, Bartolazzi A, Mottolese M, Coscia N, Bigotti A, Zardi L. Expression and production of tenascin in benign and malignant lesions of melanocyte lineage. Int J Cancer 1990; 46:586-90; PMID:1698727; http://dx.doi.org/ 10.1002/ijc.2910460406 [DOI] [PubMed] [Google Scholar]
- 48. Tuominen H, Kallioinen M. Increased tenascin expression in melanocytic tumors. J Cutan Pathol 1994; 21:424-9; PMID:7532653; http://dx.doi.org/ 10.1111/j.1600-0560.1994.tb00284.x [DOI] [PubMed] [Google Scholar]
- 49. Sis B, Sagol O, Kupelioglu A, Sokmen S, Terzi C, Fuzun M, Ozer E, Bishop P. Prognostic significance of matrix metalloproteinase-2, cathepsin D, and tenascin-C expression in colorectal carcinoma. Pathol Res Pract 2004; 200:379-87; PMID:15239346; http://dx.doi.org/ 10.1016/j.prp.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 50. Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, Kamada K, Naito A, Hirao S, Nakajima Y. Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer 2001; 92:1419-26; PMID:11745218; http://dx.doi.org/ 10.1002/1097-0142(20010915)92:6%3c1419::AID-CNCR1465%3e3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- 51. Dueck M, Riedl S, Hinz U, Tandara A, Moller P, Herfarth C, Faissner A. Detection of tenascin-C isoforms in colorectal mucosa, ulcerative colitis, carcinomas and liver metastases. Int J Cancer 1999; 82:477-83; PMID:10404058; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19990812)82:4%3c477::AID-IJC2%3e3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- 52. Galler K, Junker K, Franz M, Hentschel J, Richter P, Gajda M, Göhlert A, von Eggeling F, Heller R, Giavazzi R, et al. Differential vascular expression and regulation of oncofetal tenascin-C and fibronectin variants in renal cell carcinoma (RCC): implications for an individualized angiogenesis-related targeted drug delivery. Histochem Cell Biol 2012; 137:195-204; PMID:22075565; http://dx.doi.org/ 10.1007/s00418-011-0886-z [DOI] [PubMed] [Google Scholar]
- 53. Schwager K, Villa A, Rosli C, Neri D, Rosli-Khabas M, Moser G. A comparative immunofluorescence analysis of three clinical-stage antibodies in head and neck cancer. Head Neck Oncol 2011; 3:25; PMID:21548989; http://dx.doi.org/ 10.1186/1758-3284-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mighell AJ, Thompson J, Hume WJ, Markham AF, Robinson PA. Human tenascin-C: identification of a novel type III repeat in oral cancer and of novel splice variants in normal, malignant and reactive oral mucosae. Int J Cancer 1997; 72:236-40; PMID:9219826; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19970717)72:2%3c236::AID-IJC6%3e3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 55. Derr LB, Chiquet-Ehrismann R, Gandour-Edwards R, Spence J, Tucker RP. The expression of tenascin-C with the AD1 variable repeat in embryonic tissues, cell lines and tumors in various vertebrate species. Differentiation 1997; 62:71-82; PMID:9404002; http://dx.doi.org/ 10.1046/j.1432-0436.1997.6220071.x [DOI] [PubMed] [Google Scholar]
- 56. Frey K, Fiechter M, Schwager K, Belloni B, Barysch MJ, Neri D, Dummer R. Different patterns of fibronectin and tenascin-C splice variants expression in primary and metastatic melanoma lesions. Exp Dermatol 2011; 20:685-8; PMID:21649738; http://dx.doi.org/ 10.1111/j.1600-0625.2011.01314.x [DOI] [PubMed] [Google Scholar]
- 57. Berndt A, Anger K, Richter P, Borsi L, Brack S, Silacci M, Franz M, Wunderlich H, Gajda M, Zardi L, et al. Differential expression of tenascin-C splicing domains in urothelial carcinomas of the urinary bladder. J Cancer Res Clin Oncol 2006; 132:537-46; PMID:16788848; http://dx.doi.org/ 10.1007/s00432-006-0106-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Richter P, Tost M, Franz M, Altendorf-Hofmann A, Junker K, Borsi L, Neri D, Kosmehl H, Wunderlich H, Berndt A. B and C domain containing tenascin-C: urinary markers for invasiveness of urothelial carcinoma of the urinary bladder? J Cancer Res Clin Oncol 2009; 135:1351-8; PMID:19326143; http://dx.doi.org/ 10.1007/s00432-009-0576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wilson KE, Langdon SP, Lessells AM, Miller WR. Expression of the extracellular matrix protein tenascin in malignant and benign ovarian tumours. Br J Cancer 1996; 74:999-1004; PMID:8855965; http://dx.doi.org/ 10.1038/bjc.1996.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adams M, Jones JL, Walker RA, Pringle JH, Bell SC. Changes in tenascin-C isoform expression in invasive and preinvasive breast disease. Cancer Res 2002; 62:3289-97; PMID:12036947 [PubMed] [Google Scholar]
- 61. Guttery DS, Hancox RA, Mulligan KT, Hughes S, Lambe SM, Pringle JH, Walker RA, Jones JL, Shaw JA. Association of invasion-promoting tenascin-C additional domains with breast cancers in young women. Breast Cancer Res 2010; 12:R57; PMID:20678196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moritz S, Lehmann S, Faissner A, von Holst A. An induction gene trap screen in neural stem cells reveals an instructive function of the niche and identifies the splicing regulator sam68 as a tenascin-C-regulated target gene. Stem Cells 2008; 26:2321-31; PMID:18617690; http://dx.doi.org/ 10.1634/stemcells.2007-1095 [DOI] [PubMed] [Google Scholar]
- 63. Jensen MA, Wilkinson JE, Krainer AR. Splicing factor SRSF6 promotes hyperplasia of sensitized skin. Nat Struct Mol Biol 2014; 21:189-97; PMID:24440982; http://dx.doi.org/ 10.1038/nsmb.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Borsi L, Balza E, Castellani P, Carnemolla B, Ponassi M, Querze G, Zardi L. Cell-cycle dependent alternative splicing of the tenascin primary transcript. Cell Adhes Commun 1994; 1:307-17; PMID:7521758; http://dx.doi.org/ 10.3109/15419069409097262 [DOI] [PubMed] [Google Scholar]
- 65. Borsi L, Balza E, Gaggero B, Allemanni G, Zardi L. The alternative splicing pattern of the tenascin-C pre-mRNA is controlled by the extracellular pH. J Biol Chem 1995; 270:6243-5; PMID:7534307; http://dx.doi.org/ 10.1074/jbc.270.11.6243 [DOI] [PubMed] [Google Scholar]
- 66. Latijnhouwers MA, de Jongh GJ, Bergers M, de Rooij MJ, Schalkwijk J. Expression of tenascin-C splice variants by human skin cells. Arch Dermatol Res 2000; 292:446-54; PMID:11000288; http://dx.doi.org/ 10.1007/s004030000152 [DOI] [PubMed] [Google Scholar]
- 67. Zhao Y, Young SL. TGF-β regulates expression of tenascin alternative-splicing isoforms in fetal rat lung. Am J Physiol 1995; 268:L173-80; PMID:7532367 [DOI] [PubMed] [Google Scholar]
- 68. von Holst A, Egbers U, Prochiantz A, Faissner A. Neural stem/progenitor cells express 20 tenascin C isoforms that are differentially regulated by Pax6. J Biol Chem 2007; 282:9172-81; PMID:17264084; http://dx.doi.org/ 10.1074/jbc.M608067200 [DOI] [PubMed] [Google Scholar]
- 69. Imai K, Kusakabe M, Sakakura T, Nakanishi I, Okada Y. Susceptibility of tenascin to degradation by matrix metalloproteinases and serine proteinases. FEBS Lett 1994; 352:216-8; PMID:7523186; http://dx.doi.org/ 10.1016/0014-5793(94)00960-0 [DOI] [PubMed] [Google Scholar]
- 70. Siri A, Knauper V, Veirana N, Caocci F, Murphy G, Zardi L. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem 1995; 270:8650-4; PMID:7536739; http://dx.doi.org/ 10.1074/jbc.270.15.8650 [DOI] [PubMed] [Google Scholar]
- 71. Bell SC, Pringle JH, Taylor DJ, Malak TM. Alternatively spliced tenascin-C mRNA isoforms in human fetal membranes. Mol Hum Reprod 1999; 5:1066-76; PMID:10541570; http://dx.doi.org/ 10.1093/molehr/5.11.1066 [DOI] [PubMed] [Google Scholar]
- 72. Tanaka R, Seki Y, Saito Y, Kamiya S, Fujita M, Okutsu H, Iyoda T, Takai T, Owaki T, Yajima H, et al. Tenascin-C-derived Peptide TNIIIA2 Highly Enhances Cell Survival and Platelet-derived Growth Factor (PDGF)-dependent Cell Proliferation through Potentiated and Sustained Activation of Integrin alpha5beta1. J Biol Chem 2014; 289:17699-708; PMID:24808173; http://dx.doi.org/ 10.1074/jbc.M113.546622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kusagawa H, Onoda K, Namikawa S, Yada I, Okada A, Yoshida T, Sakakura T. Expression and degeneration of tenascin-C in human lung cancers. Br J Cancer 1998; 77:98-102; PMID:9459152; http://dx.doi.org/ 10.1038/bjc.1998.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cai M, Onoda K, Takao M, Kyoko IY, Shimpo H, Yoshida T, Yada I. Degradation of tenascin-C and activity of matrix metalloproteinase-2 are associated with tumor recurrence in early stage non-small cell lung cancer. Clin Cancer Res 2002; 8:1152-6; PMID:11948127 [PubMed] [Google Scholar]
- 75. Jahkola T, Toivonen T, von Smitten K, Blomqvist C, Virtanen I. Expression of tenascin in invasion border of early breast cancer correlates with higher risk of distant metastasis. Int J Cancer 1996; 69:445-7; PMID:8980244; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19961220)69:6%3c445::AID-IJC4%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 76. Ilmonen S, Jahkola T, Turunen JP, Muhonen T, Asko-Seljavaara S. Tenascin-C in primary malignant melanoma of the skin. Histopathology 2004; 45:405-11; PMID:15469480; http://dx.doi.org/ 10.1111/j.1365-2559.2004.01976.x [DOI] [PubMed] [Google Scholar]
- 77. Kaarteenaho-Wiik R, Soini Y, Pollanen R, Paakko P, Kinnula VL. Overexpression of tenascin-C in malignant pleural mesothelioma. Histopathology 2003; 42:280-91; PMID:12605648; http://dx.doi.org/ 10.1046/j.1365-2559.2003.01568.x [DOI] [PubMed] [Google Scholar]
- 78. Aishima S, Taguchi K, Terashi T, Matsuura S, Shimada M, Tsuneyoshi M. Tenascin expression at the invasive front is associated with poor prognosis in intrahepatic cholangiocarcinoma. Mod Pathol 2003; 16:1019-27; PMID:14559985; http://dx.doi.org/ 10.1097/01.MP.0000086860.65672.73 [DOI] [PubMed] [Google Scholar]
- 79. Chiquet-Ehrismann R, Kalla P, Pearson CA. Participation of tenascin and transforming growth factor-β in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res 1989; 49:4322-5; PMID:2472877 [PubMed] [Google Scholar]
- 80. Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer cell 2007; 11:539-54; PMID:17560335; http://dx.doi.org/ 10.1016/j.ccr.2007.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta 2009; 1793:911-20; PMID:19339214; http://dx.doi.org/ 10.1016/j.bbamcr.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 82. Yoshida T, Matsumoto E, Hanamura N, Kalembeyi I, Katsuta K, Ishihara A, Sakakura T. Co-expression of tenascin and fibronectin in epithelial and stromal cells of benign lesions and ductal carcinomas in the human breast. J Pathol 1997; 182:421-8; PMID:9306963; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199708)182:4%3c421::AID-PATH886%3e3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- 83. Hanamura N, Yoshida T, Matsumoto E, Kawarada Y, Sakakura T. Expression of fibronectin and tenascin-C mRNA by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int J Cancer 1997; 73:10-5; PMID:9334802; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19970926)73:1%3c10::AID-IJC2%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 84. Hindermann W, Berndt A, Borsi L, Luo X, Hyckel P, Katenkamp D, Kosmehl H. Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J Pathol 1999; 189:475-80; PMID:10629546; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199912)189:4%3c475::AID-PATH462%3e3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 85. Mori M, Muramatsu Y, Yamada K, Shrestha P, Takai Y. Intracellular localization of tenascin in squamous cell carcinoma of oral cavity: an immunohistochemical study. Anticancer Res 1996; 16:3075-9; PMID:8920770 [PubMed] [Google Scholar]
- 86. Kawakatsu H, Shiurba R, Obara M, Hiraiwa H, Kusakabe M, Sakakura T. Human carcinoma cells synthesize and secrete tenascin in vitro. Jpn J Cancer Res 1992; 83:1073-80; PMID:1280634; http://dx.doi.org/ 10.1111/j.1349-7006.1992.tb02724.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Herlyn M, Graeven U, Speicher D, Sela BA, Bennicelli JL, Kath R, Guerry D, 4th. Characterization of tenascin secreted by human melanoma cells. Cancer Res 1991; 51:4853-8; PMID:1716515 [PubMed] [Google Scholar]
- 88. Hiraiwa N, Kida H, Sakakura T, Kusakabe M. Induction of tenascin in cancer cells by interactions with embryonic mesenchyme mediated by a diffusible factor. J Cell Sci 1993; 104 ( Pt 2):289-96; PMID:7685035 [DOI] [PubMed] [Google Scholar]
- 89. Takahashi Y, Sawada G, Kurashige J, Matsumura T, Uchi R, Ueo H, Ishibashi M, Takano Y, Akiyoshi S, Iwaya T, et al. Tumor-derived tenascin-C promotes the epithelial-mesenchymal transition in colorectal cancer cells. Anticancer Res 2013; 33:1927-34; PMID:23645740 [PubMed] [Google Scholar]
- 90. Saupe F, Schwenzer A, Jia Y, Gasser I, Spenle C, Langlois B, Kammerer M, Lefebvre O, Hlushchuk R, Rupp T, et al. Tenascin-C downregulates wnt inhibitor dickkopf-1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep 2013; 5:482-92; PMID:24139798; http://dx.doi.org/ 10.1016/j.celrep.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 91. Talts JF, Wirl G, Dictor M, Muller WJ, Fassler R. Tenascin-C modulates tumor stroma and monocyte/macrophage recruitment but not tumor growth or metastasis in a mouse strain with spontaneous mammary cancer. J Cell Sci 1999; 112 (Pt 12):1855-64; PMID:10341205 [DOI] [PubMed] [Google Scholar]
- 92. O'Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci U S A 2011; 108:16002-7; PMID:21911392; http://dx.doi.org/ 10.1073/pnas.1109493108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fukunaga-Kalabis M, Martinez G, Nguyen TK, Kim D, Santiago-Walker A, Roesch A, Herlyn M. Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene 2010; 29:6115-24; PMID:20729912; http://dx.doi.org/ 10.1038/onc.2010.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tanaka K, Hiraiwa N, Hashimoto H, Yamazaki Y, Kusakabe M. Tenascin-C regulates angiogenesis in tumor through the regulation of vascular endothelial growth factor expression. Int J Cancer 2004; 108:31-40; PMID:14618612; http://dx.doi.org/ 10.1002/ijc.11509 [DOI] [PubMed] [Google Scholar]
- 95. Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M. Tenascin interferes with fibronectin action. Cell 1988; 53:383-90; PMID:2452695; http://dx.doi.org/ 10.1016/0092-8674(88)90158-4 [DOI] [PubMed] [Google Scholar]
- 96. Chiquet-Ehrismann R, Matsuoka Y, Hofer U, Spring J, Bernasconi C, Chiquet M. Tenascin variants: differential binding to fibronectin and distinct distribution in cell cultures and tissues. Cell Regul 1991; 2:927-38; PMID:1725601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res 2001; 61:8586-94; PMID:11731446 [PubMed] [Google Scholar]
- 98. Wenk MB, Midwood KS, Schwarzbauer JE. Tenascin-C suppresses Rho activation. J Cell Biol 2000; 150:913-20; PMID:10953015; http://dx.doi.org/ 10.1083/jcb.150.4.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Grahovac J, Becker D, Wells A. Melanoma cell invasiveness is promoted at least in part by the epidermal growth factor-like repeats of tenascin-C. J Invest Dermatol 2013; 133:210-20; PMID:22951722; http://dx.doi.org/ 10.1038/jid.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol 2011; 224:289-300; PMID:21618240; http://dx.doi.org/ 10.1002/path.2894 [DOI] [PubMed] [Google Scholar]
- 101. Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, Konig I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol 2010; 20:339-45; PMID:20137952; http://dx.doi.org/ 10.1016/j.cub.2009.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol 2006; 174:863-75; PMID:16966425; http://dx.doi.org/ 10.1083/jcb.200603013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tan VY, Lewis SJ, Adams JC, Martin RM. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: a systematic review and meta-analysis. BMC Med 2013; 11:52; PMID:23442983; http://dx.doi.org/ 10.1186/1741-7015-11-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. Tumor self-seeding by circulating cancer cells. Cell 2009; 139:1315-26; PMID:20064377; http://dx.doi.org/ 10.1016/j.cell.2009.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fischer D, Tucker RP, Chiquet-Ehrismann R, Adams JC. Cell-adhesive responses to tenascin-C splice variants involve formation of fascin microspikes. Mol Biol Cell 1997; 8:2055-75; PMID:9348542; http://dx.doi.org/ 10.1091/mbc.8.10.2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011; 331:1559-64; PMID:21436443; http://dx.doi.org/ 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 107. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; http://dx.doi.org/ 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 108. Tanaka S, Sumioka T, Fujita N, Kitano A, Okada Y, Yamanaka O, Flanders KC, Miyajima M, Saika S. Suppression of injury-induced epithelial-mesenchymal transition in a mouse lens epithelium lacking tenascin-C. Mol Vis 2010; 16:1194-205; PMID:20664686 [PMC free article] [PubMed] [Google Scholar]
- 109. Katoh D, Nagaharu K, Shimojo N, Hanamura N, Yamashita M, Kozuka Y, Imanaka-Yoshida K, Yoshida T. Binding of alphavbeta1 and alphavbeta6 integrins to tenascin-C induces epithelial-mesenchymal transition-like change of breast cancer cells. Oncogenesis 2013; 2:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nagaharu K, Zhang X, Yoshida T, Katoh D, Hanamura N, Kozuka Y, Ogawa T, Shiraishi T, Imanaka-Yoshida K. Tenascin C induces epithelial-mesenchymal transition-like change accompanied by SRC activation and focal adhesion kinase phosphorylation in human breast cancer cells. Am J Pathol 2011; 178:754-63; PMID:21281808; http://dx.doi.org/ 10.1016/j.ajpath.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998; 153:865-73; http://dx.doi.org/ 10.1016/S0002-9440(10)65628-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sivasankaran B, Degen M, Ghaffari A, Hegi ME, Hamou MF, Ionescu MC, Zweifel C, Tolnay M, Wasner M, Mergenthaler S, et al. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res 2009; 69:458-65; PMID:19147558; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2610 [DOI] [PubMed] [Google Scholar]
- 113. Beiter K, Hiendlmeyer E, Brabletz T, Hlubek F, Haynl A, Knoll C, Kirchner T, Jung A. β-Catenin regulates the expression of tenascin-C in human colorectal tumors. Oncogene 2005; 24:8200-4; PMID:16091738 [DOI] [PubMed] [Google Scholar]
- 114. Tsunoda T, Inada H, Kalembeyi I, Imanaka-Yoshida K, Sakakibara M, Okada R, Katsuta K, Sakakura T, Majima Y, Yoshida T. Involvement of large tenascin-C splice variants in breast cancer progression. Am J Pathol 2003; 162:1857-67; PMID:12759243; http://dx.doi.org/ 10.1016/S0002-9440(10)64320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yoshida T, Yoshimura E, Numata H, Sakakura Y, Sakakura T. Involvement of tenascin-C in proliferation and migration of laryngeal carcinoma cells. Virchows Arch 1999; 435:496-500; PMID:10592053; http://dx.doi.org/ 10.1007/s004280050433 [DOI] [PubMed] [Google Scholar]
- 116. Orend G, Huang W, Olayioye MA, Hynes NE, Chiquet-Ehrismann R. Tenascin-C blocks cell-cycle progression of anchorage-dependent fibroblasts on fibronectin through inhibition of syndecan-4. Oncogene 2003; 22:3917-26; PMID:12813465; http://dx.doi.org/ 10.1038/sj.onc.1206618 [DOI] [PubMed] [Google Scholar]
- 117. Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol 2001; 154:459-68; PMID:11470832; http://dx.doi.org/ 10.1083/jcb.200103103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Van Obberghen-Schilling E, Tucker RP, Saupe F, Gasser I, Cseh B, Orend G. Fibronectin and tenascin-C: accomplices in vascular morphogenesis during development and tumor growth. Int J Dev Biol 2011; 55:511-25; PMID:21769776; http://dx.doi.org/ 10.1387/ijdb.103243eo [DOI] [PubMed] [Google Scholar]
- 119. Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by α 2 β 1 and α v β 3 integrins. J Cell Sci 1993; 105 (Pt 4):1001-12; PMID:7693733 [DOI] [PubMed] [Google Scholar]
- 120. Canfield AE, Schor AM. Evidence that tenascin and thrombospondin-1 modulate sprouting of endothelial cells. J Cell Sci 1995; 108 (Pt 2):797-809; PMID:7539439 [DOI] [PubMed] [Google Scholar]
- 121. Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell 1996; 7:883-92; PMID:8816995; http://dx.doi.org/ 10.1091/mbc.7.6.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 2009; 1:303-14; PMID:20049734; http://dx.doi.org/ 10.1002/emmm.200900043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 2002; 13:3546-59; PMID:12388756; http://dx.doi.org/ 10.1091/mbc.E02-01-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kaariainen E, Nummela P, Soikkeli J, Yin M, Lukk M, Jahkola T, Virolainen S, Ora A, Ukkonen E, Saksela O, et al. Switch to an invasive growth phase in melanoma is associated with tenascin-C, fibronectin, and procollagen-I forming specific channel structures for invasion. J Pathol 2006; 210:181-91; PMID:16924594; http://dx.doi.org/ 10.1002/path.2045 [DOI] [PubMed] [Google Scholar]
- 125. Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol 2011; 301:G950-5; PMID:21512158; http://dx.doi.org/ 10.1152/ajpgi.00132.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Blaauboer ME, Boeijen FR, Emson CL, Turner SM, Zandieh-Doulabi B, Hanemaaijer R, Smit TH, Stoop R, Everts V. Extracellular matrix proteins: a positive feedback loop in lung fibrosis? Matrix Biol 2014; 34:170-8; PMID:24291458; http://dx.doi.org/ 10.1016/j.matbio.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 127. Imanaka-Yoshida K, Hiroe M, Nishikawa T, Ishiyama S, Shimojo T, Ohta Y, Sakakura T, Yoshida T. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab Invest 2001; 81:1015-24; PMID:11454990; http://dx.doi.org/ 10.1038/labinvest.3780313 [DOI] [PubMed] [Google Scholar]
- 128. Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, Ikegami M, Wesselkamper SC, Davidson C, Dietsch M, et al. Genomic profile of matrix and vasculature remodeling in TGF-α induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2007; 37:309-21; PMID:17496152; http://dx.doi.org/ 10.1165/rcmb.2006-0455OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Carey WA, Taylor GD, Dean WB, Bristow JD. Tenascin-C deficiency attenuates TGF-ss-mediated fibrosis following murine lung injury. Am J Physiol Lung Cell Mol Physiol 2010; 299:L785-93; PMID:20833777; http://dx.doi.org/ 10.1152/ajplung.00385.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol 2007; 211:86-94; PMID:17121418; http://dx.doi.org/ 10.1002/path.2099 [DOI] [PubMed] [Google Scholar]
- 131. De Wever O, Nguyen QD, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J 2004; 18:1016-8; PMID:15059978 [DOI] [PubMed] [Google Scholar]
- 132. Islam MS, Kusakabe M, Horiguchi K, Iino S, Nakamura T, Iwanaga K, Hashimoto H, Matsumoto S, Murata T, Hori M, et al. PDGF and TGF-β promote tenascin-C expression in subepithelial myofibroblasts and contribute to intestinal mucosal protection in mice. Br J Pharmacol 2014; 171:375-88; PMID:24116743; http://dx.doi.org/ 10.1111/bph.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol 2005; 167:71-80; PMID:15972953; http://dx.doi.org/ 10.1016/S0002-9440(10)62954-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. New Engl J Med 1999; 340:430-6; PMID:9971867; http://dx.doi.org/ 10.1056/NEJM199902113400604 [DOI] [PubMed] [Google Scholar]
- 135. Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 1998; 157:1666-80; PMID:9603153; http://dx.doi.org/ 10.1164/ajrccm.157.5.9707141 [DOI] [PubMed] [Google Scholar]
- 136. Oberhauser AF, Marszalek PE, Erickson HP, Fernandez JM. The molecular elasticity of the extracellular matrix protein tenascin. Nat 1998; 393:181-5; PMID:9603523; http://dx.doi.org/ 10.1038/30270 [DOI] [PubMed] [Google Scholar]
- 137. Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009; 15:774-80; PMID:19561617; http://dx.doi.org/ 10.1038/nm.1987 [DOI] [PubMed] [Google Scholar]
- 138. Patel L, Sun W, Glasson SS, Morris EA, Flannery CR, Chockalingam PS. Tenascin-C induces inflammatory mediators and matrix degradation in osteoarthritic cartilage. BMC Musculoskelet Disord 2011; 12:164; PMID:21762512; http://dx.doi.org/ 10.1186/1471-2474-12-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Puente Navazo MD, Valmori D, Ruegg C. The alternatively spliced domain TnFnIII A1A2 of the extracellular matrix protein tenascin-C suppresses activation-induced T lymphocyte proliferation and cytokine production. J Immunol 2001; 167:6431-40; PMID:11714809; http://dx.doi.org/ 10.4049/jimmunol.167.11.6431 [DOI] [PubMed] [Google Scholar]
- 140. Hauzenberger D, Olivier P, Gundersen D, Ruegg C. Tenascin-C inhibits beta1 integrin-dependent T lymphocyte adhesion to fibronectin through the binding of its fnIII 1-5 repeats to fibronectin. Eur J Immunol 1999; 29:1435-47; PMID:10359097; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199905)29:05%3c1435::AID-IMMU1435%3e3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- 141. Garcion E, Halilagic A, Faissner A, ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 2004; 131:3423-32; PMID:15226258; http://dx.doi.org/ 10.1242/dev.01202 [DOI] [PubMed] [Google Scholar]
- 142. Garcion E, Faissner A, ffrench-Constant C. Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development 2001; 128:2485-96; PMID:11493565 [DOI] [PubMed] [Google Scholar]
- 143. Klein G, Beck S, Muller CA. Tenascin is a cytoadhesive extracellular matrix component of the human hematopoietic microenvironment. J Cell Biol 1993; 123:1027-35; PMID:7693718; http://dx.doi.org/ 10.1083/jcb.123.4.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Seiffert M, Beck SC, Schermutzki F, Muller CA, Erickson HP, Klein G. Mitogenic and adhesive effects of tenascin-C on human hematopoietic cells are mediated by various functional domains. Matrix Biol 1998; 17:47-63; PMID:9628252; http://dx.doi.org/ 10.1016/S0945-053X(98)90124-X [DOI] [PubMed] [Google Scholar]
- 145. Yokosaki Y, Palmer EL, Prieto AL, Crossin KL, Bourdon MA, Pytela R, Sheppard D. The integrin α 9 β 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem 1994; 269:26691-6; PMID:7523411 [PubMed] [Google Scholar]
- 146. Nakamura-Ishizu A, Okuno Y, Omatsu Y, Okabe K, Morimoto J, Uede T, Nagasawa T, Suda T, Kubota Y. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood 2012; 119:5429-37; PMID:22553313; http://dx.doi.org/ 10.1182/blood-2011-11-393645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tucker RP, Ferralli J, Schittny JC, Chiquet-Ehrismann R. Tenascin-C and tenascin-W in whisker follicle stem cell niches: possible roles in regulating stem cell proliferation and migration. J Cell Sci 2013; 126:5111-5; PMID:24101721; http://dx.doi.org/ 10.1242/jcs.134650 [DOI] [PubMed] [Google Scholar]
- 148. Ellis SL, Heazlewood SY, Williams B, Reitsma AJ, Grassinger J, Borg J, Heazlewood CK, Chidgey AP, Nilsson SK. The role of Tenascin C in the lymphoid progenitor cell niche. Exp Hematol 2013; 41:1050-61; PMID:24084079; http://dx.doi.org/ 10.1016/j.exphem.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 149. Flenkenthaler F, Windschuttl S, Frohlich T, Schwarzer JU, Mayerhofer A, Arnold GJ. Secretome analysis of testicular peritubular cells: a window into the human testicular microenvironment and the spermatogonial stem cell niche in man. J Proteome Res 2014; 13:1259-69; PMID:24422521; http://dx.doi.org/ 10.1021/pr400769z [DOI] [PubMed] [Google Scholar]
- 150. Nakatsu MN, Vartanyan L, Vu DM, Ng MY, Li X, Deng SX. Preferential biological processes in the human limbus by differential gene profiling. PloS one 2013; 8:e61833; PMID:23630617; http://dx.doi.org/ 10.1371/journal.pone.0061833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Descot A, Oskarsson T. The molecular composition of the metastatic niche. Exp Cell Res 2013; 319:1679-86; PMID:23707205 [DOI] [PubMed] [Google Scholar]
- 152. Barcellos-Hoff MH, Lyden D, Wang TC. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer 2013; 13:511-8; PMID:23760023; http://dx.doi.org/ 10.1038/nrc3536 [DOI] [PubMed] [Google Scholar]
- 153. Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17:1253-70; PMID:12756227; http://dx.doi.org/ 10.1101/gad.1061803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Oskarsson T, Massague J. Extracellular matrix players in metastatic niches. EMBO J 2012; 31:254-6; PMID:22179697; http://dx.doi.org/ 10.1038/emboj.2011.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wagner KU, Rui H. Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia 2008; 13:93-103; PMID:18228120; http://dx.doi.org/ 10.1007/s10911-008-9062-z [DOI] [PubMed] [Google Scholar]
- 156. Ruiz C, Huang W, Hegi ME, Lange K, Hamou MF, Fluri E, Oakeley EJ, Chiquet-Ehrismann R, Orend G. Growth promoting signaling by tenascin-C [corrected]. Cancer Res 2004; 64:7377-85; PMID:15492259; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1234 [DOI] [PubMed] [Google Scholar]
- 157. Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007; 1:313-23; PMID:18371365; http://dx.doi.org/ 10.1016/j.stem.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 158. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133:704-15; PMID:18485877; http://dx.doi.org/ 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2012; 481:85-9; http://dx.doi.org/ 10.1038/nature10694 [DOI] [PubMed] [Google Scholar]
- 160. Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 2010; 285:2028-39; PMID:19887451; http://dx.doi.org/ 10.1074/jbc.M109.051961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 2012; 150:165-78; PMID:22770218; http://dx.doi.org/ 10.1016/j.cell.2012.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009; 461:109-13; PMID:19693011; http://dx.doi.org/ 10.1038/nature08268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, André S, Piccart M, Campone M, Brain E, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 2009; 15:68-74; http://dx.doi.org/ 10.1038/nm.1908 [DOI] [PubMed] [Google Scholar]
- 164. Gong XG, Lv YF, Li XQ, Xu FG, Ma QY. Gemcitabine resistance induced by interaction between alternatively spliced segment of tenascin-C and annexin A2 in pancreatic cancer cells. Biol Pharm Bull 2010; 33:1261-7; PMID:20686216; http://dx.doi.org/ 10.1248/bpb.33.1261 [DOI] [PubMed] [Google Scholar]
- 165. Wang B, Liu K, Lin HY, Bellam N, Ling S, Lin WC. Fourteen-3-3Tau regulates ubiquitin-independent proteasomal degradation of p21, a novel mechanism of p21 downregulation in breast cancer. Mol Cell Biol 2010; 30:1508-27; PMID:20086099; http://dx.doi.org/ 10.1128/MCB.01335-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Helleman J, Jansen MP, Ruigrok-Ritstier K, van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn JG, Sleijfer S, Foekens JA, et al. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clin Cancer Res 2008; 14:5555-64; PMID:18765548; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0555 [DOI] [PubMed] [Google Scholar]


