Abstract
Background:
β-Cell dysfunction is a core defect in T2DM, and chronic, sustained hyperglycemia has been implicated in progressive β-cell failure, ie, glucotoxicity. The aim of the present study was to examine the effect of lowering the plasma glucose concentration with dapagliflozin, a glucosuric agent, on β-cell function in T2DM individuals.
Research Design and Methods:
Twenty-four subjects with T2DM received dapagliflozin (n = 16) or placebo (n = 8) for 2 weeks, and a 75-g oral glucose tolerance test (OGTT) and insulin clamp were performed before and after treatment. Plasma glucose, insulin, and C-peptide concentrations were measured during the OGTT.
Results:
Dapagliflozin significantly lowered both the fasting and 2-hour plasma glucose concentrations and the incremental area under the plasma glucose concentration curve (ΔG0–120) during OGTT by −33 ± 5 mg/dL, −73 ± 9 mg/dL, and −60 ± 12 mg/dL · min, respectively, compared to −13 ± 9, −33 ± 13, and −18 ± 9 reductions in placebo-treated subjects (both P < .01). The incremental area under the plasma C-peptide concentration curve tended to increase in dapagliflozin-treated subjects, whereas it did not change in placebo-treated subjects. Thus, ΔC-Pep0–120/ΔG0–120 increased significantly in dapagliflozin-treated subjects, whereas it did not change in placebo-treated subjects (0.019 ± 0.005 vs 0.002 ± 0.006; P < .01). Dapagliflozin significantly improved whole-body insulin sensitivity (insulin clamp). Thus, β-cell function, measured as ΔC-Pep0–120/ ΔG0–120 ÷ insulin resistance, increased by 2-fold (P < .01) in dapagliflozin-treated vs placebo-treated subjects.
Conclusion:
Lowering the plasma glucose concentration with dapagliflozin markedly improves β-cell function, providing strong support in man for the glucotoxic effect of hyperglycemia on β-cell function.
β-Cell failure is a core defect in type 2 diabetes mellitus (T2DM) and is the major factor responsible for the development and progression of hyperglycemia (1). Multiple factors including advancing age, genes, insulin resistance, β-cell incretin resistance, incretin deficiency, islet-associated amylin polypeptide, lipotoxicity, and others (1) have been implicated in the development of β-cell failure in T2DM. Chronic elevation of the plasma glucose concentration also impairs insulin secretion, ie, glucose toxicity (2), although proof of the glucotoxicity hypothesis in man is yet to be established conclusively (3). The toxic effect of chronic hyperglycemia on β-cell function was demonstrated in experimental animals more than 60 years ago (4). Chronic (>4 d) elevation of plasma glucose concentration to 29 mm in cats and dogs completely obliterated the β-cell response to a glucose stimulus (5–7). Moreover, the severity of the β-cell defect and the time required for recovery of β-cell function after correction of the hyperglycemia were directly related to the level of hyperglycemia produced (5–7). Using the hyperglycemic clamp technique, Rossetti et al (2) demonstrated that even a small (16 mg/dL) persistent increase in the plasma glucose concentration impairs both first- and second-phase insulin secretion in partially pancreatectomized diabetic rats. Furthermore, correction of the hyperglycemia with phlorin restored glucose-stimulated insulin secretion to normal (8). Although the glucotoxic effect of hyperglycemia is well established in in vitro and in vivo studies in experimental animals, conclusive evidence for the detrimental effect of chronic hyperglycemia on β-cell function in T2DM has yet to be provided. In normal glucose-tolerant individuals, a modest elevation in daylong plasma glucose concentration for 24 hours caused a 24% decrease in β-cell function (9). Conversely, lowering the plasma glucose concentration with insulin therapy in T2DM patients significantly improved insulin secretion (10, 11). Although insulin is very effective in lowering the plasma glucose concentration in T2DM, it has many other metabolic effects that also could lead to an improvement in β-cell function. For example, insulin is a powerful inhibitor of lipolysis and markedly lowers the plasma free fatty acid (FFA) concentration (12), which could lead to improved β-cell function (13).
To examine the glucotoxicity hypothesis in man, we used dapagliflozin, a potent and specific sodium-glucose cotransporter 2 (SGLT2) inhibitor (14), to lower the plasma glucose concentration and examined the effect of this intervention on β-cell function. Because the primary effect of dapagliflozin is on the kidney to inhibit renal glucose reabsorption and produce glucosuria, this provides a novel approach to examine the glucotoxicity hypothesis with regard to the development of β-cell failure in T2DM individuals.
Subjects and Methods
Subjects
Twenty-four T2DM males treated with metformin (n = 17) or metformin plus sulfonylurea (n = 7) participated in the study. The mean glycated hemoglobin (HbA1c) was 8.5 ± 0.4 (range, 7.0–11.0%). Other than having diabetes, subjects were in general good health as determined by medical history, physical examination, screening lab tests, urinalysis, and electrocardiogram. Table 1 summarizes the clinical characteristics of the study participants. Body weight was stable (±1.36 kg) in all subjects for ≥3 months before the study, and no subject participated in any excessively heavy exercise program. No subjects were taking any medications (other than metformin or sulfonylurea) known to affect glucose metabolism. The study protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio, and all subjects gave their written voluntary consent before participation.
Table 1.
Clinical and Metabolic Characteristics of the Study Participants at Baseline and at 14 Days
| Dapagliflozin |
Placebo |
|||
|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | |
| Age, y | 51 ± 2 | 55 ± 2 | ||
| Weight, kg | 99.5 ± 4.4 | 98.3 ± 4.4 | 96.5 ± 5.4 | 95.5 ± 5.4 |
| BMI, kg/m2 | 32.3 ± 1.3 | 31.9 ± 1.3 | 33.1 ± 1.7 | 32.7 ± 1.7 |
| SBP, mm Hg | 133 ± 3 | 127 ± 2 | 135 ± 3 | 131 ± 4 |
| HbA1c, % | 8.4 ± 0.3 | 8.6 ± 0.4 | ||
| Serum creatinine, mg/dL | 0.99 ± 0.05 | 1.03 ± 0.05 | 0.87 ± 0.04 | 0.87 ± 0.04 |
| Fasting FFA, mEq/L | 0.49 ± 0.03 | 0.47 ± 0.03 | 0.44 ± 0.03 | 0.41 ± 0.03 |
| FPG, mg/dL | 182 ± 8 | 149 ± 8a,b | 184 ± 19 | 171 ± 9 |
| 2-h PG, mg/dL | 342 ± 12 | 269 ± 14a,b | 334 ± 16 | 301 ± 16 |
| ΔG0–120, ng/mL · h | 257 ± 12 | 197 ± 16a,b | 220 ± 22 | 203 ± 18 |
| Fasting C-Pep, ng/mL | 4.3 ± 0.4 | 4.7 ± 0.4 | 5.9 ± 0.8 | 5.7 ± 0.6 |
| 2-h C-Pep, ng/mL | 9.1 ± 1.1 | 9.9 ± 0.8 | 10.3 ± 1.3 | 10.0 ± 0.9 |
| ΔC-Pep0–120, ng/mL · h | 5.72 ± 0.94 | 6.81 ± 0.8 | 5.53 ± 0.7 | 5.22 ± 0.9 |
| (ΔC-Pep/ΔG)0–120 | 0.022 ± 0.003 | 0.041 ± 0.006a,b | 0.026 ± 0.006 | 0.028 ± 0.006 |
| TGD, mg/kg · min | 4.5 ± 0.5 | 5.2 ± 0.6a,b | 4.3 ± 0.8 | 4.4 ± 0.6 |
| (ΔC-Pep0–120/ΔG)0–120 ÷ IR | 0.12 ± 0.04 | 0.26 ± 0.05a,b | 0.14 ± 0.04 | 0.17 ± 0.03 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; 2-h PG, 2-h plasma glucose; C-Pep, C-peptide. Data are expressed as mean ± SEM.
P < .05 vs baseline.
P < .05 vs placebo.
Research design
At baseline, all subjects received a 75-g oral glucose tolerance test (OGTT) at 8 am after a 10-hour overnight fast. Plasma glucose, insulin, and C-peptide were measured at −30, −15, and 0 minutes, and every 30 minutes after glucose ingestion. On a separate day, subjects received a 4-hour euglycemic hyperinsulinemic clamp with 3-3H-glucose infusion to quantitate whole-body insulin-mediated glucose disposal and endogenous glucose production (EGP) (see following description). After completing the baseline studies, subjects received dapagliflozin, 10 mg, or placebo in a randomized double-blind fashion (2:1 randomization) for 14 days. On days 13 and 14, the OGTT and euglycemic hyperinsulinemic clamp were repeated.
Euglycemic insulin clamp
Subjects fasted after 10 pm, and at 6 am the following day a catheter was placed into an antecubital vein for the infusion of all test substances. A second catheter was inserted retrogradely into a vein on the dorsum of the hand, and the hand was placed in a thermoregulated box heated to 70°C. At 6 am, a prime (25 μCi × fasting plasma glucose [FPG]/100)-continuous (0.25 μCi/min) infusion of 3-3H-glucose (DuPont NEN Life Science Products) was started as described above and continued for 7 hours. After a 3-hour basal tracer equilibration period (9 am), subjects received a prime-continuous insulin infusion (80 mU/m2 · min) for 240 minutes (15). During the insulin infusion, plasma glucose concentration was measured every 5 minutes. After the start of insulin, no glucose was infused until the plasma glucose concentration declined to 100 mg/dL, at which level it was maintained with a coefficient of variation <5% by the adjustment of a variable glucose infusion based on the negative feedback principle (15). Plasma samples were collected every 15–30 minutes from 0 to 180 minutes after the start of insulin and every 5–10 minutes from 180 to 240 minutes for the determination of plasma glucose and insulin concentrations and tritiated glucose specific activity. Urine was collected from 0–240 minutes, and urinary volume and glucose concentration were measured. Urinary glucose loss was subtracted from the total rate of glucose disposal to determine insulin-mediated tissue glucose uptake.
Analytical techniques
Plasma glucose was measured by the glucose oxidase reaction (Glucose Oxidase Analyzer; Analox). Plasma insulin and glucagon concentrations were measured by RIA (Linco Research). Plasma 3-3H-glucose radioactivity was measured in Somogyi precipitates.
Calculations and statistical analysis
Under steady-state postabsorptive conditions, the basal rate of endogenous glucose appearance (Ra) equals the 3-3H-glucose infusion rate divided by steady-state plasma tritiated glucose specific activity. During the insulin clamp, nonsteady conditions for 3-3H-glucose specific activity prevail; the rate of glucose appearance (Ra) was calculated with Steele's equation (16). The rate of residual EGP during the insulin clamp was calculated by subtracting the exogenous glucose infusion rate from the tracer-derived Ra. The insulin-stimulated rate of total body glucose disposal (TGD) was calculated by adding the rate of residual EGP to the exogenous glucose infusion rate and was used as the insulin sensitivity index.
The incremental area under the plasma glucose, insulin, and C-peptide concentration curves was calculated with the trapezoid rule. Insulin secretion was measured as the ratio between the incremental area under the plasma C-peptide concentration to the incremental area under the plasma glucose concentration during the OGTT (ΔC-Pep0–120/ΔG0–120). β-Cell function was measured as the insulin secretion/insulin resistance or disposition index, where insulin resistance (IR) was the inverse of TGD measured with the euglycemic insulin clamp (ΔC-Pep0–120/ΔG0–120 ÷ IR). This index is widely used for the assessment of β-cell health (17–22). β-Cell sensitivity to glucose was calculated using the Mari model as previously described (23, 24).
Values are expressed as mean ± SEM. The change from baseline in TGD, EGP, ΔC-Pep0–120/ΔG0–120, and ΔC-Pep0–120/ΔG0–120 ÷ IR in dapagliflozin-treated and placebo-treated groups was compared with ANOVA. Rates of TGD, EGP, ΔC-Pep/ΔG, and ΔC-Pep/ΔG ÷ IR after dapagliflozin were compared to those before dapagliflozin with paired t test. Statistical significance was set at alpha <0.05.
Results
Table 1 presents the clinical characteristics of the study participants before and after 2 weeks of treatment. Subjects were matched in age, sex, and body mass index. There was a small, statistically insignificant decrease in body weight at 2 weeks, which was similar in both groups. No significant difference was observed in lipid profile.
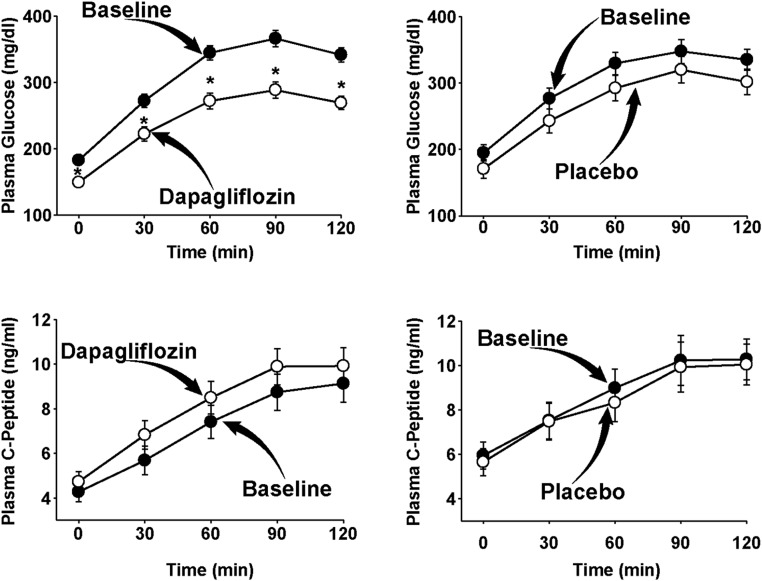
The plasma glucose concentration during the OGTT in dapagliflozin-treated and placebo-treated individuals is shown in Figure 1, A and B. Dapagliflozin caused a significant decrease in both the fasting (−33 ± 5 mg/dL) and 2-hour (−73 ± 9 mg/dL) plasma glucose concentrations (both P < .001 vs baseline) during the OGTT, whereas no significant change was observed in placebo-treated individuals (−13 ± 9, P = not significant [NS]; and −33 ± 13, P = NS, respectively). The decrease from baseline in both the FPG and 2-hour plasma glucose concentration was significantly greater (P < .01) in dapagliflozin-treated subjects compared to placebo-treated subjects. Similarly, the incremental area under the plasma glucose concentration curve (ΔG0–120) decreased significantly in dapagliflozin-treated compared to placebo-treated subjects (−60 ± 12 vs −18 ± 9; P < .01).
Figure 1.
Plasma glucose and C-peptide concentrations during the OGTT in dapagliflozin-treated and placebo-treated T2DM patients at baseline and after 2 weeks of treatment. *, P < .05.
Figure 1, C and D, depicts the plasma C-peptide concentration during the OGTT. The incremental area under the plasma C-peptide concentration curve in subjects treated with placebo decreased slightly but not significantly (−0.31 ± 0.6; P = NS), whereas the incremental area under the plasma C-peptide concentration curve in dapagliflozin-treated subjects increased slightly but not significantly (+1.1 ± 0.8; P = NS) at 2 weeks. The ratio between the incremental area under the plasma C-peptide curve to the plasma glucose concentration curve (ΔC-Pep0–120 AUC/ΔG0–120 AUC) increased significantly in dapagliflozin-treated (0.022 ± 0.003 to 0.041 ± 0.006; P < .05 vs baseline and placebo) compared to placebo-treated (0.026 ± 0.006 to 0.028 ± 0.006; P = NS) subjects (Table 1).
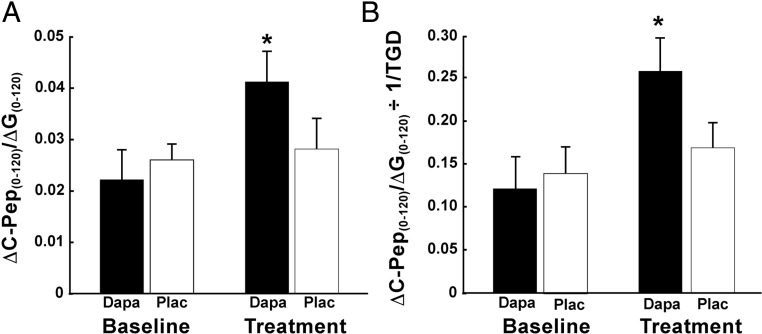
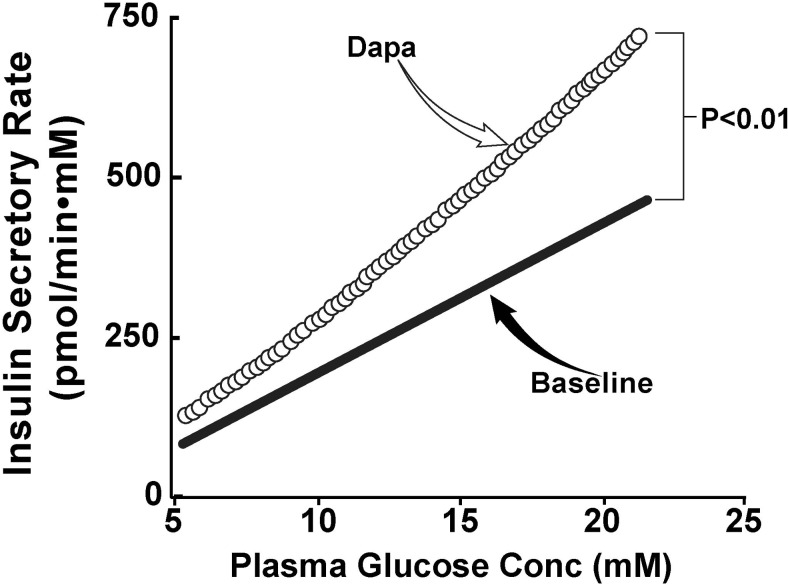
Insulin-stimulated TGD measured with the euglycemic-hyperinsulinemic clamp was comparable at baseline in both groups (4.5 ± 0.5 and 4.3 ± 0.8; P = NS). TGD increased significantly in dapagliflozin-treated (5.2 ± 0.6; P < .05) subjects, whereas no significant change was observed in placebo-treated subjects (4.4 ± 0.6; P < .05 vs dapagliflozin). Thus, the insulin secretion/insulin resistance index (ΔC-Pep0–120/ΔG0–120 ÷ 1/TGD), which represents the “gold standard” measure of β-cell function, increased more than 2-fold in dapagliflozin-treated subjects (0.12 ± 0.04 to 0.26 ± 0.05; P < .01), whereas no significant change was observed in placebo-treated subjects (0.14 ± 0.04 to 0.17 ± 0.03; P < .01 vs dapagliflozin); the increase in ΔC-Pep0–120/ΔG0–120 ÷ IR at 2 weeks relative to baseline in dapagliflozin-treated subjects (0.15 ± 0.03) was significantly greater (P < .05) than that in placebo-treated subjects (0.03 ± 0.03) (Figure 2). Lastly, we measured β-cell glucose sensitivity before and after dapagliflozin treatment. Dapagliflozin caused a significant increase in β-cell glucose sensitivity from 23 ± 5 to 35 ± 5 pmol/min · mm (P < .01) (Figure 3).
Figure 2.
Insulin secretion (A) and β-cell function (B), measured as ΔC-Pep0–120/ΔG0–120 ÷ IR, in dapagliflozin-treated and placebo-treated T2DM patients at baseline and after 2 weeks of treatment. *, P < .05 vs baseline and vs placebo. Dapa, dapagliflozin; Plac, placebo.
Figure 3.
β-Cell glucose sensitivity measured with the Mari model before and after treatment with dapagliflozin (Dapa).
Discussion
The major finding of the present study is that reduction of the plasma glucose concentration by inducing glucosuria in T2DM individuals significantly improved β-cell function (Figure 1). Because the primary action of dapagliflozin is on the kidney and the drug has no known direct action on the β-cell, these findings demonstrate that dapagliflozin treatment improves β-cell function in T2DM by correcting hyperglycemia, ie, reversal of glucotoxicity. Thus, the present study provides the first conclusive evidence in man that chronic hyperglycemia exerts a deleterious effect on β-cell function in T2DM. Consistent with previous results, improvement in hyperglycemia with dapagliflozin for only 2 weeks also improved insulin sensitivity (25). Thus, improvement in the plasma glucose profile corrected two of the core defects present in T2DM individuals.
Dapagliflozin significantly lowered the fasting plasma glucose (by 33 mg/dL), the 2-hour plasma glucose (by 73 mg/dL), and the mean plasma glucose (by 60 mg/dL) during the OGTT. This reduction in plasma glucose levels resulted in a greater than 2-fold increase in β-cell function as measured with ΔC-Pep0–120/ΔG0–120 ÷ IR (P < .001). No change in β-cell function was observed in placebo-treated subjects. These findings are consistent with previous studies from our group in rodents (2) and extend our prior observations by demonstrating that a reduction in plasma glucose concentration, by inhibiting renal sodium glucose reabsorption with a SGLT2 inhibitor, in T2DM patients improves two of the core defects (β-cell dysfunction and insulin resistance). Because SGLT2 is not expressed in the β-cell and since the plasma FFA concentration did not change in dapagliflozin-treated subjects, the improvement in β-cell function after dapagliflozin treatment is the direct result of the amelioration of glucotoxicity (3). Importantly, these results demonstrate that the glucotoxic effect of chronic hyperglycemia on β-cell function in T2DM is reversible. Chronically elevated plasma glucose levels are the major risk factor for diabetic microvascular complications. The results of the present study demonstrate that hyperglycemia also worsens β-cell function, the major factor responsible for the progressive rise in plasma glucose levels in T2DM patients (1, 26). Thus, chronic hyperglycemia is a self-perpetuating cause of the progressive β-cell failure. These results underscore the importance of lowering and maintaining the plasma glucose concentration at or below the treatment goal (ie, HbA1c < 6.5–7.0%) in T2DM individuals.
The glucotoxic effect of chronic hyperglycemia on insulin secretion is well established in experimental systems. In vitro studies using cell culture systems have shown that a small persistent increase in plasma glucose concentration has a deleterious effect on β-cell function (27). In partially pancreatectomized “normal glucose-tolerant” rats, a 16 mg/dL increment in the mean daylong plasma glucose concentration has been shown to markedly impair first-phase insulin secretion (8). Studies that have assessed the effect of elevated plasma glucose levels, created with glucose infusion, on β-cell function have yielded conflicting results. An increase in plasma glucose concentration (+50 mg/dL) for 30 minutes in healthy, normal glucose-tolerant subjects was shown to inhibit first-phase insulin secretion, measured with the hyperglycemic clamp (28). More prolonged elevation of the fasting plasma glucose concentration for 62 hours decreased β-cell function by 36% in nondiabetic subjects (29). Other studies (30) have reported increased β-cell function after 42 hours of glucose infusion in healthy subjects. Methodological differences in the various protocols and differences in the study populations may, in part, explain these conflicting results. For example, in the study by Boden et al (29), the plasma glucose concentration was allowed to return to the preinfusion level for approximately 2 hours before the repeat hyperglycemic challenge. Thus, the glucotoxic effects of hyperglycemia may have been washed out, leaving the β-cell in a hypersensitive state when presented with a repeat glucose stimulus. It is also possible that the glucotoxic effect of chronic hyperglycemia only will manifest itself in genetically predisposed individuals, as we have shown for chronically elevated plasma FFA levels (31). Other evidence in support of a glucotoxic effect of chronic hyperglycemia on β-cell function includes the improvement in insulin secretion in type 2 diabetic subjects after correction of hyperglycemia with insulin (32), and loss of the normal oscillatory β-cell response to glucose in normal glucose-tolerant subjects after short-term exposure to hyperglycemia (33).
In conclusion, our results demonstrate that, for the first time in man in vivo, glucotoxicity plays an important role in the β-cell dysfunction in T2DM and that reduction in plasma glucose levels with an intervention, glucosuria, that does not affect other metabolic parameters results in improved β-cell function.
Acknowledgments
This study was supported by National Institutes of Health Grant 5R01DK240923 (to R.A.D.). Bristol-Myers Squibb provided the dapagliflozin and matching placebo. The studies were carried out in the Bartter Clinical Research Unit of the South Texas Veterans Health Care System-Audie Murphy Division. The salary of R.A.D. is supported in part by the South Texas Veterans Health Care System-Audie L. Murphy Division.
A.Me., A.Ma., C.S., J.X., G.D., A.C., D.T., and S.U.M. generated the data; M.A.-G. analyzed the data and wrote the manuscript; and R.A.D. reviewed and revised the manuscript.
Disclosure Summary: A.Me., A.Ma., C.S., J.X., G.D., A.C., D.T., S.U.M., and M.A.-G. have nothing to declare. R.A.D. serves on the Advisory Board of Astra Zeneca, Novo Nordisk, Janssen, Lexicon, and Boehringer-Ingelheim; receives research support from Bristol Myers Squibb, Boehringer-Ingelheim, Takeda, and Astra Zeneca; and serves on the Speakers Bureau of Novo-Nordisk, and Astra Zeneca.
Footnotes
- EGP
- endogenous glucose production
- FFA
- free fatty acid
- FPG
- fasting plasma glucose
- HbA1c
- glycated hemoglobin
- IR
- insulin resistance
- NS
- not significant
- OGTT
- oral glucose tolerance test
- SGLT2
- sodium-glucose cotransporter 2
- T2DM
- type 2 diabetes mellitus
- TGD
- total glucose disposal.
References
- 1. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest. 1987;80:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. [DOI] [PubMed] [Google Scholar]
- 4. Dohan FC, Lukens FD. Experimental diabetes produced by the administration of glucose. Endocrinology. 1948;42:244–262. [DOI] [PubMed] [Google Scholar]
- 5. Zini E, Osto M, Franchini M, et al. Hyperglycaemia but not hyperlipidaemia causes β cell dysfunction and β cell loss in the domestic cat. Diabetologia. 2009;52:336–346. [DOI] [PubMed] [Google Scholar]
- 6. Imamura T, Koffler M, Helderman JH, et al. Severe diabetes induced in subtotally depancreatized dogs by sustained hyperglycemia. Diabetes. 1988;37:600–609. [DOI] [PubMed] [Google Scholar]
- 7. Green AS, Chen X, Macko AR, et al. Chronic pulsatile hyperglycemia reduces insulin secretion and increases accumulation of reactive oxygen species in fetal sheep islets. J Endocrinol. 2012;212:327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solomon TP, Knudsen SH, Karstoft K, Winding K, Holst JJ, Pedersen BK. Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. J Clin Endocrinol Metab. 2012;97:4682–4691. [DOI] [PubMed] [Google Scholar]
- 10. Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353–363. [DOI] [PubMed] [Google Scholar]
- 11. Mayorov AY, Naumenkova IV, Antsiferov MB, Dedov II. Influence of insulin treatment on insulin sensitivity in insulin requiring type 2 diabetes patients. Diabetes Res Clin Pract. 2005;68(suppl 1):S54–S59. [DOI] [PubMed] [Google Scholar]
- 12. Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E1775–E1781. [DOI] [PubMed] [Google Scholar]
- 14. Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–531. [DOI] [PubMed] [Google Scholar]
- 15. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 16. Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24. [DOI] [PubMed] [Google Scholar]
- 17. Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity. 2008;16:1901–1907. [DOI] [PubMed] [Google Scholar]
- 18. Abdul-Ghani MA, Matsuda M, Jani R, et al. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2008;295:E401–E406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giannini C, Weiss R, Cali A, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012;61:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. β-Cell dysfunction and glucose intolerance: results from the San Antonio Metabolism (SAM) study. Diabetologia 2004;47:31–39. [DOI] [PubMed] [Google Scholar]
- 21. DeFronzo RA, Banerji MA, Bray GA, et al. Determinants of glucose tolerance in impaired glucose tolerance at baseline in the Actos Now for Prevention of Diabetes (ACT NOW) study. Diabetologia. 2010;53:435–445. [DOI] [PubMed] [Google Scholar]
- 22. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve β-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007;292:E871–E883. [DOI] [PubMed] [Google Scholar]
- 23. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500. [DOI] [PubMed] [Google Scholar]
- 24. Mari A, Tura A, Natali A, et al. Impaired β cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia. 2010;53:749–756. [DOI] [PubMed] [Google Scholar]
- 25. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeFronzo RA. Lilly lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–687. [DOI] [PubMed] [Google Scholar]
- 27. Leahy JL, Bonner-Weir S, Weir GC. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest. 1988;81:1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toschi E, Camastra S, Sironi AM, et al. Effect of acute hyperglycemia on insulin secretion in humans. Diabetes. 2002;51(suppl 1):S130–S133. [DOI] [PubMed] [Google Scholar]
- 29. Boden G, Ruiz J, Kim CJ, Chen X. Effects of prolonged glucose infusion on insulin secretion, clearance, and action in normal subjects. Am J Physiol. 1996;270:E251–E258. [DOI] [PubMed] [Google Scholar]
- 30. Byrne MM, Sturis J, Polonsky KS. Insulin secretion and clearance during low-dose graded glucose infusion. Am J Physiol. 1995;268:E21–E27. [DOI] [PubMed] [Google Scholar]
- 31. Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474. [DOI] [PubMed] [Google Scholar]
- 32. Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222–234. [DOI] [PubMed] [Google Scholar]
- 33. Meyer J, Sturis J, Katschinski M, Arnold R, Göke B, Byrne MM. Acute hyperglycemia alters the ability of the normal β-cell to sense and respond to glucose. Am J Physiol Endocrinol Metab. 2002;282:E917–E922. [DOI] [PubMed] [Google Scholar]