Abstract
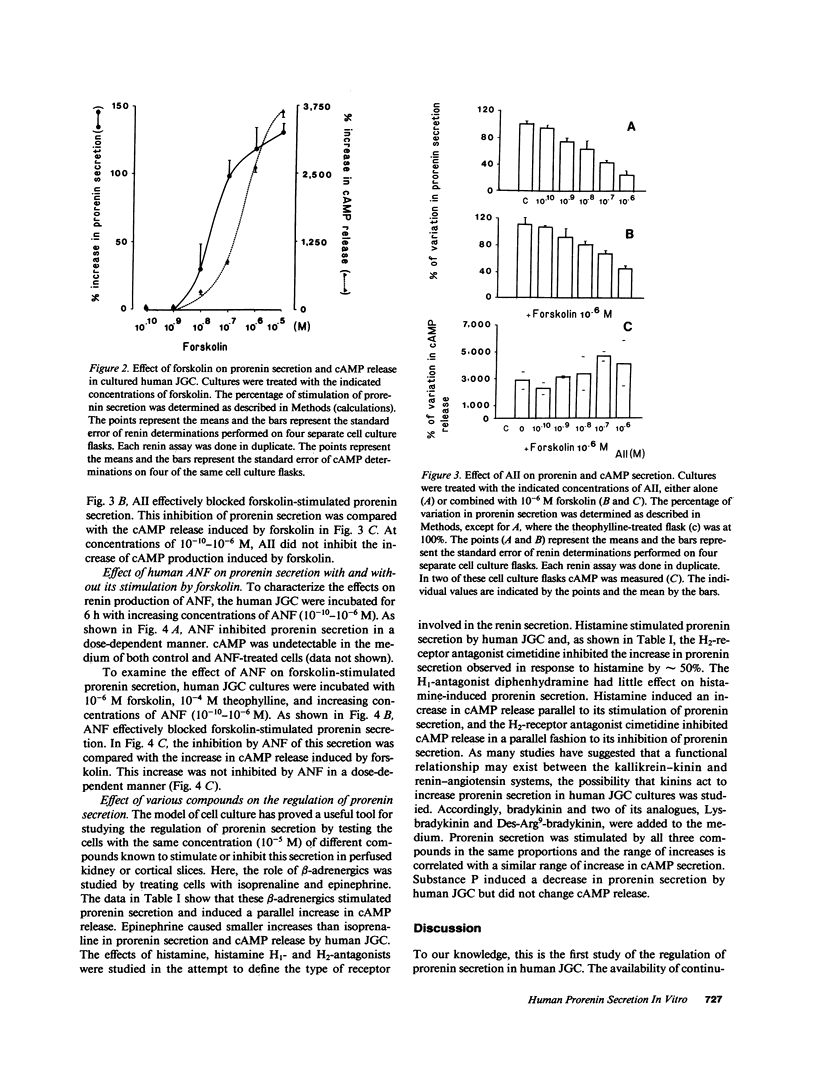
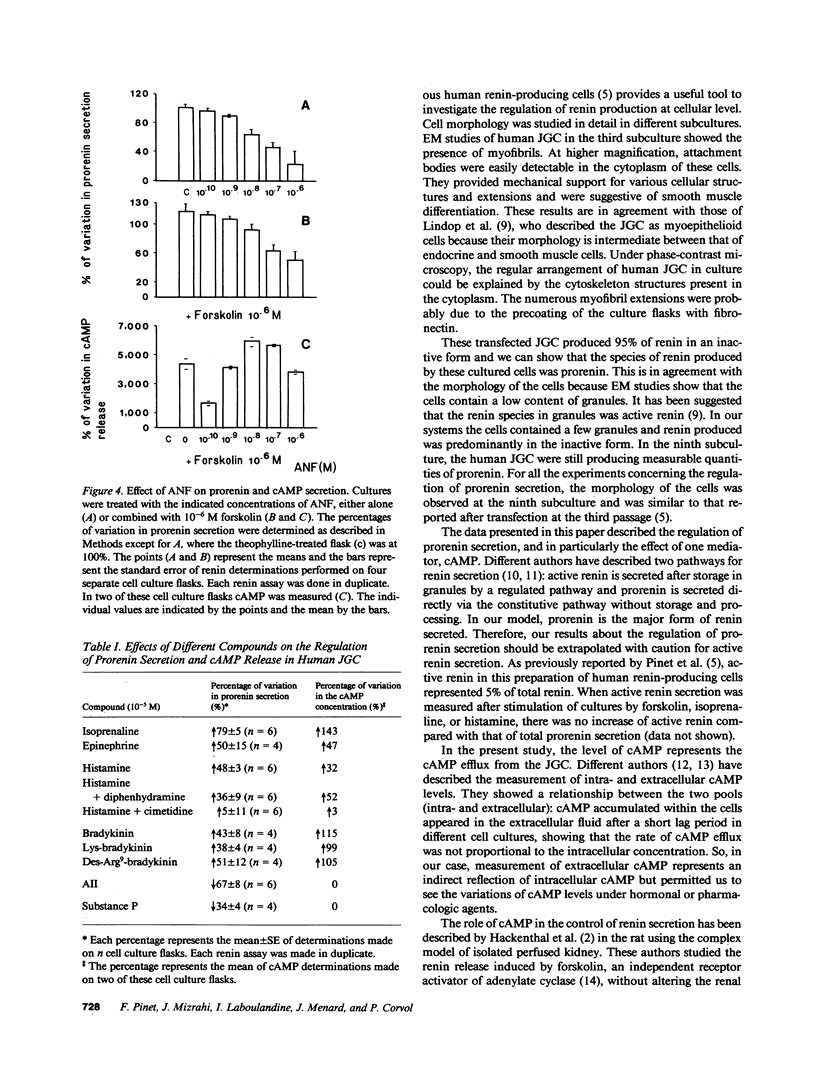
The regulation of renin secretion was studied in continuous culture of human juxtaglomerular cells (JGC), which provided a permanent source of human renin production (Pinet, F., M. T. Corvol, F. Dench, J. Bourguignon, J. Feunteun, J. Ménard, and P. Corvol, 1985, Proc. Natl. Acad. Sci. USA, 82:8503-8507). 95% of the renin species secreted was prorenin, and therefore this study concerned primarily prorenin secretion. Renin production was stable, since the cells had been maintained in culture for more than two years. In culture, these human cells formed colonies of smooth musclelike cells, and electron microscopy showed the presence of cytoskeleton structures including myofibrils and attachment bodies. This human model was used to investigate the control of prorenin secretion in vitro at cellular level. Various pharmacological agents known to stimulate or inhibit renin secretion were tested in the cell cultures. The variations in prorenin secretion were measured in the supernatant. Forskolin, an independent receptor activator of adenylate cyclase, stimulated prorenin secretion in a dose-dependent manner and this stimulation was mediated by 3',5' cyclic-AMP (cAMP). Angiotensin II (AII) was found to inhibit prorenin secretion directly in a dose-dependent manner and atrial natriuretic factor (ANF), whose effects on human JGC were characterized for the first time, was also shown to exert such inhibition. When the effects of this inhibition by AII and ANF were tested on forskolin-mediated stimulation of prorenin secretion, the latter was inhibited and no change occurred in cAMP release. When JGC were treated with histamine, bradykinin, or one or two bradykinin analogues, the responses suggested that in these cells, H2-histamine receptors and kinin receptors are dependent on adenylate cyclase. One peptide, substance P, had an inhibitory effect on prorenin secretion but it was less important than AII and ANF. The present results demonstrate that the adenylate cyclase system of human JGC remains intact during culture and supports the hypothesis that cAMP is the second messenger and Cai2+, the final messenger involved in renin secretion. The cell system used here permits the evaluation of cellular responses and intracellular events in granulated cells in a human model.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Beierwaltes W. H., Prada J., Carretero O. A. Kinin stimulation of renin release in isolated rat glomeruli. Am J Physiol. 1985 Jun;248(6 Pt 2):F757–F761. doi: 10.1152/ajprenal.1985.248.6.F757. [DOI] [PubMed] [Google Scholar]
- Burnett J. C., Jr, Granger J. P., Opgenorth T. J. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984 Nov;247(5 Pt 2):F863–F866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- Cailla H. L., Racine-Weisbuch M. S., Delaage M. A. Adenosine 3',5' cyclic monophosphate assay at 10-15 mole level. Anal Biochem. 1973 Dec;56(2):394–407. doi: 10.1016/0003-2697(73)90205-4. [DOI] [PubMed] [Google Scholar]
- Catanzaro D. F., Mullins J. J., Morris B. J. The biosynthetic pathway of renin in mouse submandibular gland. J Biol Chem. 1983 Jun 25;258(12):7364–7368. [PubMed] [Google Scholar]
- Churchill P. C., Churchill M. C. Isoproterenol-stimulated renin secretion in the rat: second messenger roles of Ca and cyclic AMP. Life Sci. 1982 Apr 12;30(15):1313–1319. doi: 10.1016/0024-3205(82)90694-4. [DOI] [PubMed] [Google Scholar]
- Churchill P. C. Second messengers in renin secretion. Am J Physiol. 1985 Aug;249(2 Pt 2):F175–F184. doi: 10.1152/ajprenal.1985.249.2.F175. [DOI] [PubMed] [Google Scholar]
- Fray J. C., Lush D. J., Valentine A. N. Cellular mechanisms of renin secretion. Fed Proc. 1983 Dec;42(15):3150–3154. [PubMed] [Google Scholar]
- Fray J. C., Park C. S. Forskolin and calcium: interactions in the control of renin secretion and perfusate flow in the isolated rat kidney. J Physiol. 1986 Jun;375:361–375. doi: 10.1113/jphysiol.1986.sp016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullner H. G., Campbell W. B., Pettinger W. A. Effects of substance P on renin release and renal function in anesthetized dogs. Life Sci. 1979 Jan 15;24(3):237–245. doi: 10.1016/0024-3205(79)90225-x. [DOI] [PubMed] [Google Scholar]
- Hackenthal E., Lang R. E., Bührle C. P. Atrial natriuretic factor stimulates renin release from the isolated rat kidney. J Hypertens Suppl. 1985 Dec;3(3):S323–S325. [PubMed] [Google Scholar]
- Hiruma M., Ikemoto F., Yamamoto K. Rat atrial natriuretic factor stimulates renin release from renal cortical slices. Eur J Pharmacol. 1986 Jun 5;125(1):151–153. doi: 10.1016/0014-2999(86)90095-6. [DOI] [PubMed] [Google Scholar]
- Kangawa K., Matsuo H. Purification and complete amino acid sequence of alpha-human atrial natriuretic polypeptide (alpha-hANP). Biochem Biophys Res Commun. 1984 Jan 13;118(1):131–139. doi: 10.1016/0006-291x(84)91077-5. [DOI] [PubMed] [Google Scholar]
- Keeton T. K., Campbell W. B. The pharmacologic alteration of renin release. Pharmacol Rev. 1980 Jun;32(2):81–227. [PubMed] [Google Scholar]
- Kurtz A., Della Bruna R., Pfeilschifter J., Taugner R., Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4769–4773. doi: 10.1073/pnas.83.13.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Pfeilschifter J., Bauer C. Is renin secretion governed by the calcium permeability of the juxtaglomerular cell membrane? Biochem Biophys Res Commun. 1984 Oct 30;124(2):359–366. doi: 10.1016/0006-291x(84)91561-4. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Pfeilschifter J., Hutter A., Bührle C., Nobiling R., Taugner R., Hackenthal E., Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol. 1986 Apr;250(4 Pt 1):C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- Lindop G. B., Gardiner D. S. La cellule humaine sécrétrice de rénine. Etude morphologie. Ann Endocrinol (Paris) 1986;47(3):133–144. [PubMed] [Google Scholar]
- Obana K., Naruse M., Naruse K., Sakurai H., Demura H., Inagami T., Shizume K. Synthetic rat atrial natriuretic factor inhibits in vitro and in vivo renin secretion in rats. Endocrinology. 1985 Sep;117(3):1282–1284. doi: 10.1210/endo-117-3-1282. [DOI] [PubMed] [Google Scholar]
- Opgenorth T. J., Burnett J. C., Jr, Granger J. P., Scriven T. A. Effects of atrial natriuretic peptide on renin secretion in nonfiltering kidney. Am J Physiol. 1986 May;250(5 Pt 2):F798–F801. doi: 10.1152/ajprenal.1986.250.5.F798. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Pinet F., Corvol M. T., Dench F., Bourguignon J., Feunteun J., Menard J., Corvol P. Isolation of renin-producing human cells by transfection with three simian virus 40 mutants. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8503–8507. doi: 10.1073/pnas.82.24.8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt R. E., Ouellette A. J., Dzau V. J. Biosynthesis of renin: multiplicity of active and intermediate forms. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6809–6813. doi: 10.1073/pnas.80.22.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler M. J., Bashor M. M., Spitzer N., Saier M. H., Jr Regulation of adenosine 3':5'-monophosphate efflux from animal cells. J Biol Chem. 1978 Aug 10;253(15):5431–5436. [PubMed] [Google Scholar]
- Schulster D., Orly J., Seidel G., Schramm M. Intracellular cyclic AMP production enhanced by a hormone receptor transferred from a different cell. beta-adrenergic responses in cultured cells conferred by fusion with turkey erythrocytes. J Biol Chem. 1978 Feb 25;253(4):1201–1206. [PubMed] [Google Scholar]
- Schwertschlag U., Hackenthal E. Forskolin stimulates renin release from the isolated perfused rat kidney. Eur J Pharmacol. 1982 Oct 15;84(1-2):111–113. doi: 10.1016/0014-2999(82)90165-0. [DOI] [PubMed] [Google Scholar]
- Schwertschlag U., Hackenthal E. Histamine stimulates renin release from the isolated perfused rat kidney. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):239–242. doi: 10.1007/BF00495872. [DOI] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Sedor J. R., Abboud H. E. Actions and metabolism of histamine in glomeruli and tubules of the human kidney. Kidney Int. 1984 Aug;26(2):144–152. doi: 10.1038/ki.1984.148. [DOI] [PubMed] [Google Scholar]
- Sedor J. R., Abboud H. E. Histamine modulates contraction and cyclic nucleotides in cultured rat mesangial cells. Differential effects mediated by histamine H1 and H2 receptors. J Clin Invest. 1985 May;75(5):1679–1689. doi: 10.1172/JCI111876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G., Ratzenhofer E., Simbruner G., Coradello H., Pollak A., Lubec G. Evidence of H2 receptor activity on glomerular cells. Ren Physiol. 1980;3(1-6):152–155. [PubMed] [Google Scholar]
- Wells J. N., Kramer G. L. Phosphodiesterase inhibitors as tools in cyclic nucleotide research: a precautionary comment. Mol Cell Endocrinol. 1981 Jul;23(1):1–9. doi: 10.1016/0303-7207(81)90112-x. [DOI] [PubMed] [Google Scholar]




