Abstract
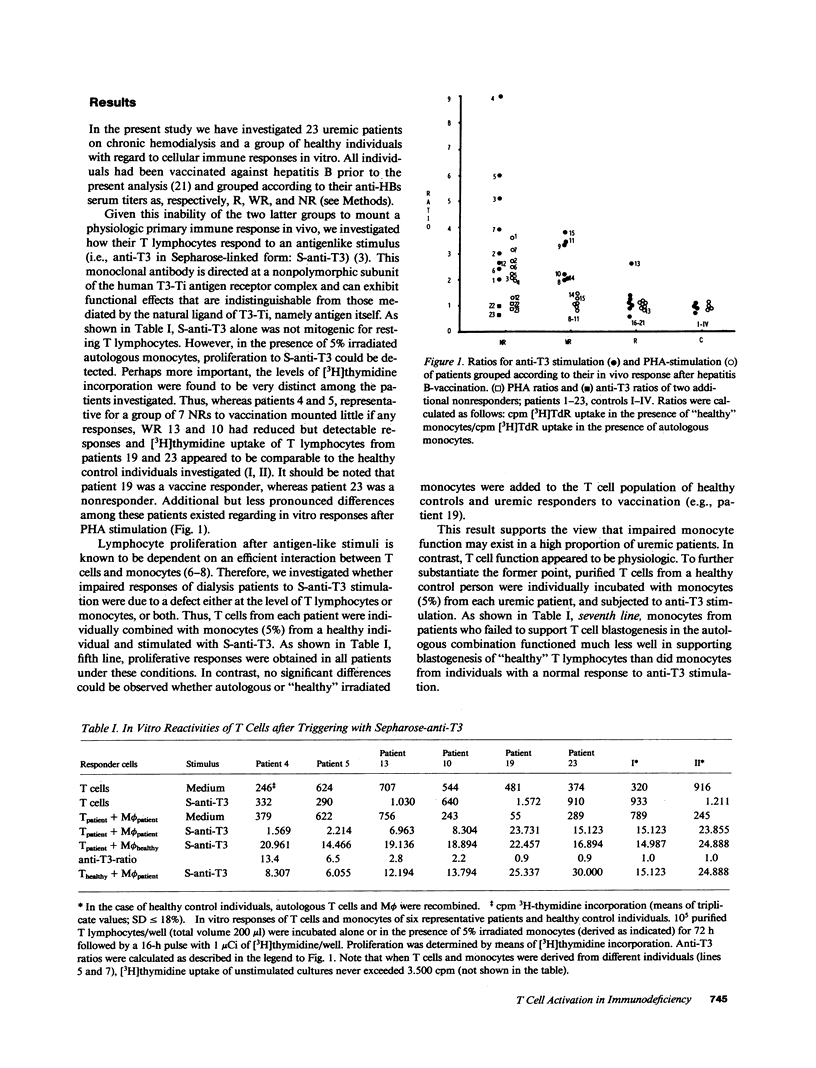
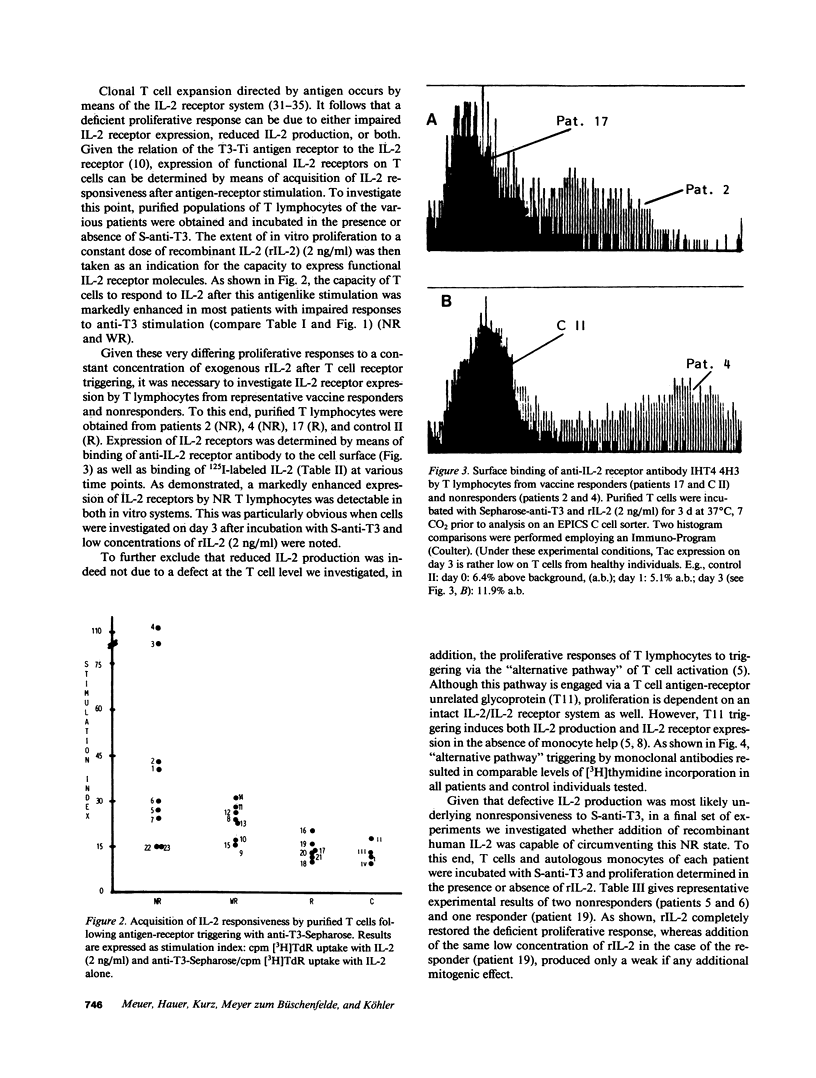
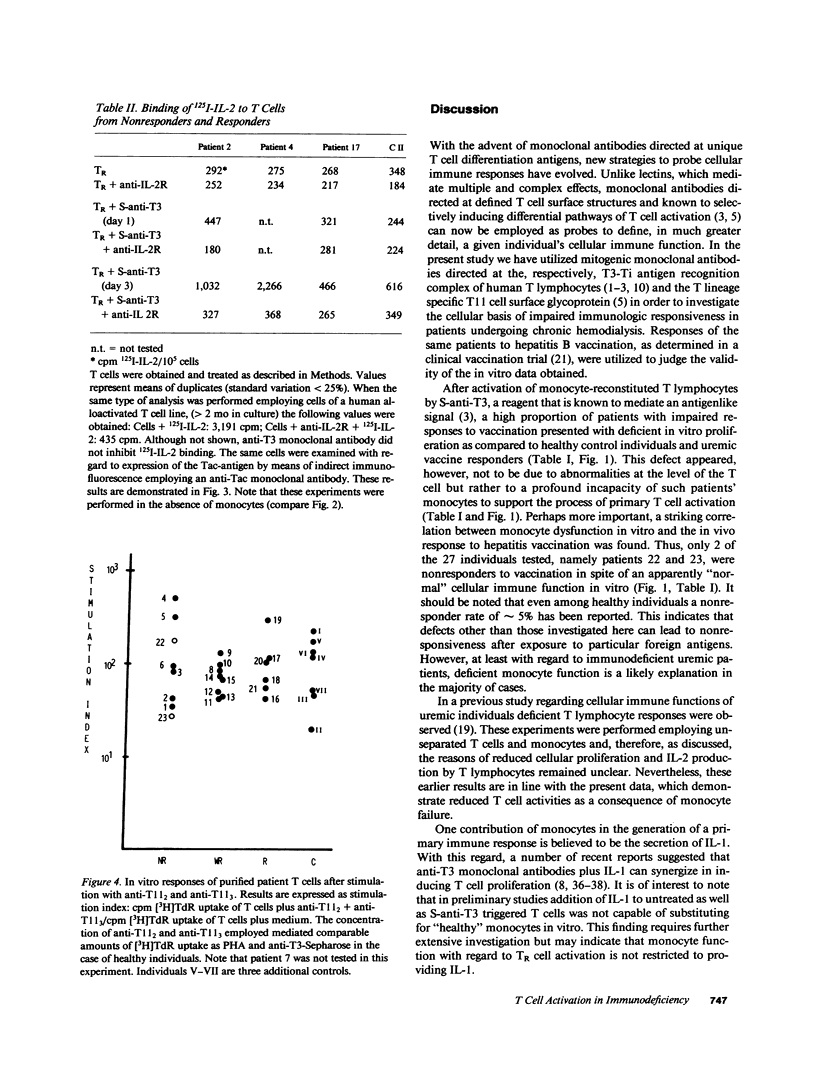
We investigated impaired cellular immune responses of individuals on chronic hemodialysis by using monoclonal antibodies that trigger differential pathways of T cell activation. Reduced cellular reactivity, which exists in a high proportion of such patients, can be attributed to a failure of the monocyte population to support the process of primary T cell activation in vitro. This defect results in a lack of interleukin 2 production, which is critically dependent on a monocyte-derived signal. In contrast, T lymphocyte function was found to be physiologic. Perhaps more important, the degree of monocyte dysfunction in vitro correlated with the same patients' in vivo responses to hepatitis B vaccination. Addition of recombinant human interleukin 2 fully reconstituted their deficient immune response in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramwell S. P., Tsakiris D. J., Briggs J. D., Follett E. A., Stewart J., McWhinnie D. L., Watson M. A., Hamilton D. N., Junor B. J. Dinitrochlorobenzene skin testing predicts response to hepatitis B vaccine in dialysis patients. Lancet. 1985 Jun 22;1(8443):1412–1415. doi: 10.1016/s0140-6736(85)91844-6. [DOI] [PubMed] [Google Scholar]
- Cappel R., Van Beers D., Liesnard C., Dratwa M. Impaired humoral and cell-mediated immune responses in dialyzed patients after influenza vaccination. Nephron. 1983;33(1):21–25. doi: 10.1159/000182898. [DOI] [PubMed] [Google Scholar]
- Cosio F. G., Giebink G. S., Le C. T., Schiffman G. Pneumococcal vaccination in patients with chronic renal disease and renal allograft recipients. Kidney Int. 1981 Aug;20(2):254–258. doi: 10.1038/ki.1981.128. [DOI] [PubMed] [Google Scholar]
- Crosnier J., Jungers P., Couroucé A. M., Laplanche A., Benhamou E., Degos F., Lacour B., Prunet P., Cerisier Y., Guesry P. Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in French haemodialysis units: I, Medical staff. Lancet. 1981 Feb 28;1(8218):455–459. doi: 10.1016/s0140-6736(81)91847-x. [DOI] [PubMed] [Google Scholar]
- Crosnier J., Jungers P., Couroucé A. M., Laplanche A., Benhamou E., Degos F., Lacour B., Prunet P., Cerisier Y., Guesry P. Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in french haemodialysis units: II, Haemodialysis patients. Lancet. 1981 Apr 11;1(8224):797–800. doi: 10.1016/s0140-6736(81)92679-9. [DOI] [PubMed] [Google Scholar]
- De Gast G. C., Houwen B., van der Hem G. K., The T. H. T-lymphocyte number and function and the course of hepatitis B in hemodialysis patients. Infect Immun. 1976 Nov;14(5):1138–1143. doi: 10.1128/iai.14.5.1138-1143.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Hadler S. C., Thompson S. E., Maynard J. E., Ostrow D. G., Altman N., Braff E. H., O'Malley P., Hawkins D., Judson F. N. The prevention of hepatitis B with vaccine. Report of the centers for disease control multi-center efficacy trial among homosexual men. Ann Intern Med. 1982 Sep;97(3):362–366. doi: 10.7326/0003-4819-97-3-362. [DOI] [PubMed] [Google Scholar]
- Gootenberg J. E., Ruscetti F. W., Mier J. W., Gazdar A., Gallo R. C. Human cutaneous T cell lymphoma and leukemia cell lines produce and respond to T cell growth factor. J Exp Med. 1981 Nov 1;154(5):1403–1418. doi: 10.1084/jem.154.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Jilg W., Schmidt M., Deinhardt F., Zachoval R. Hepatitis B vaccination: how long does protection last? Lancet. 1984 Aug 25;2(8400):458–458. doi: 10.1016/s0140-6736(84)92926-x. [DOI] [PubMed] [Google Scholar]
- Kurz P., Köhler H., Meuer S., Hütteroth T., Meyer zum Büschenfelde K. H. Impaired cellular immune responses in chronic renal failure: evidence for a T cell defect. Kidney Int. 1986 Jun;29(6):1209–1214. doi: 10.1038/ki.1986.129. [DOI] [PubMed] [Google Scholar]
- Köhler H., Arnold W., Renschin G., Dormeyer H. H., Meyer zum Büschenfelde K. H. Active hepatitis B vaccination of dialysis patients and medical staff. Kidney Int. 1984 Jan;25(1):124–128. doi: 10.1038/ki.1984.18. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Manger B., Weiss A., Weyand C., Goronzy J., Stobo J. D. T cell activation: differences in the signals required for IL 2 production by nonactivated and activated T cells. J Immunol. 1985 Dec;135(6):3669–3673. [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Cooper D. A., Hodgdon J. C., Hussey R. E., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Identification of the receptor for antigen and major histocompatibility complex on human inducer T lymphocytes. Science. 1983 Dec 16;222(4629):1239–1242. doi: 10.1126/science.6606228. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Meyer zum Büschenfelde K. H. T cell receptor triggering induces responsiveness to interleukin 1 and interleukin 2 but does not lead to T cell proliferation. J Immunol. 1986 Jun 1;136(11):4106–4112. [PubMed] [Google Scholar]
- Miller T. E., Stewart E. Host immune status in uraemia. I. Cell-mediated immune mechanisms. Clin Exp Immunol. 1980 Jul;41(1):115–122. [PMC free article] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Nakhla L. S., Goggin M. J. Lymphocyte transformation in chronic renal failure. Immunology. 1973 Feb;24(2):229–235. [PMC free article] [PubMed] [Google Scholar]
- Palacios R. Mechanisms by which accessory cells contribute in growth of resting T lymphocytes initiated by OKT3 antibody. Eur J Immunol. 1985 Jul;15(7):645–651. doi: 10.1002/eji.1830150702. [DOI] [PubMed] [Google Scholar]
- Quadracci L. J., Ringdén O., Krzymanski M. The effect of uremia and transplantation on lymphocyte subpopulations. Kidney Int. 1976 Aug;10(2):179–184. doi: 10.1038/ki.1976.93. [DOI] [PubMed] [Google Scholar]
- Schwab R., Crow M. K., Russo C., Weksler M. E. Requirements for T cell activation by OKT3 monoclonal antibody: role of modulation of T3 molecules and interleukin 1. J Immunol. 1985 Sep;135(3):1714–1718. [PubMed] [Google Scholar]
- Smith K. A., Baker P. E., Gillis S., Ruscetti F. W. Functional and molecular characteristics of T-cell growth factor. Mol Immunol. 1980 May;17(5):579–589. doi: 10.1016/0161-5890(80)90156-x. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. E., Alter H. J., Taylor P. E., Zang E. A., Harley E. J., Szmuness W. Hepatitis B vaccine in patients receiving hemodialysis. Immunogenicity and efficacy. N Engl J Med. 1984 Aug 23;311(8):496–501. doi: 10.1056/NEJM198408233110803. [DOI] [PubMed] [Google Scholar]
- Stewart E., Miller T. E. Host immune status in uraemia. II. Serum factors and lymphocyte transformation. Clin Exp Immunol. 1980 Jul;41(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Harley E. J., Zang E. A., Oleszko W. R., William D. C., Sadovsky R., Morrison J. M., Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980 Oct 9;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Wakasugi H., Harel A., Dokhelar M. C., Fradelizi D., Tursz T. Accessory function and interleukin 1 production by human leukemic cell lines. J Immunol. 1984 Jun;132(6):2939–2947. [PubMed] [Google Scholar]
- Williams J. M., Deloria D., Hansen J. A., Dinarello C. A., Loertscher R., Shapiro H. M., Strom T. B. The events of primary T cell activation can be staged by use of Sepharose-bound anti-T3 (64.1) monoclonal antibody and purified interleukin 1. J Immunol. 1985 Oct;135(4):2249–2255. [PubMed] [Google Scholar]


