Dear Editor,
CRISPR interference (CRISPRi) is a recently developed tool used to study single guide RNA (gRNA)-mediated sequence-specific repression of transcription in both prokaryotic and eukaryotic cells1,2,3,4,5. Transcription initiation and elongation of a gene can be interfered by the presence of gRNA:DNA hetero-duplex/dCas9 (a catalytically inactive form of Cas9) complex in its promoter and exons. If Krüppel associated box (KRAB) domain is fused to dCas9, repression of target gene is more efficient. Likewise, fusion of a transcription activator such as VP16 (CRISPR-on) can increase target gene expression through three to four gene-specific gRNAs (up to seven) that recognize the proximal promoter of a target gene in cultured cells and in vivo2. We applied both CRISPRi and CRISPR-on tools in worm and zebrafish, and demonstrated here that our dCas9 fusion systems modify gene expression at or near their endogenous expression location(s) through target-specific gRNAs (ts-gRNAs).
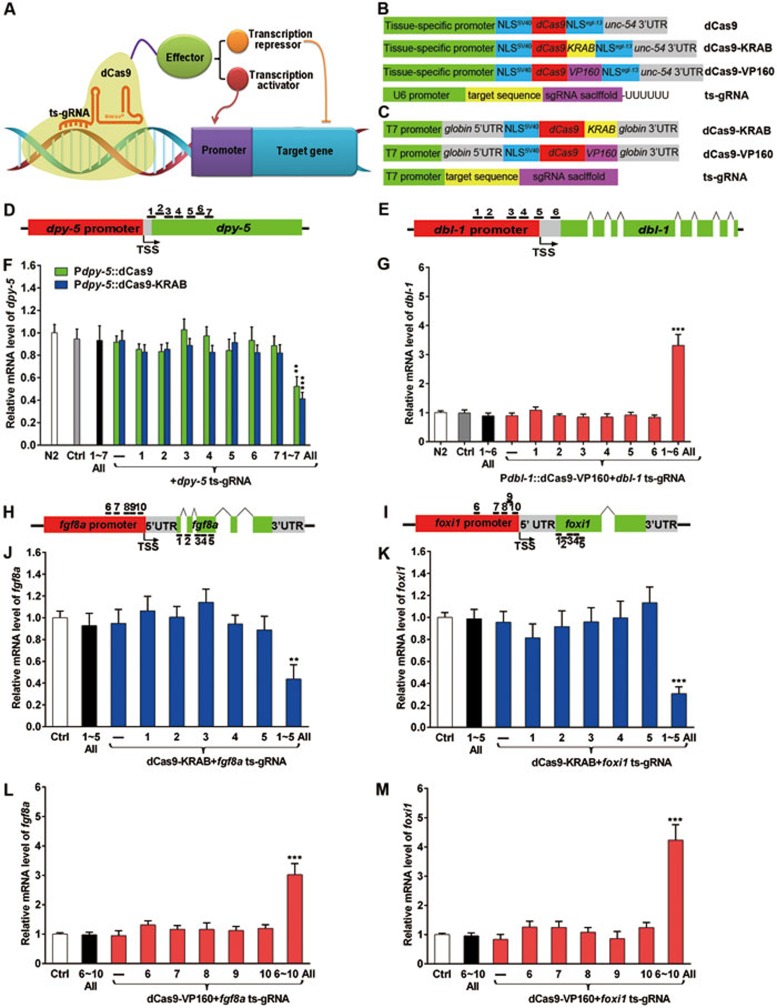
We fused dCas9 to the KRAB domain (repressor) or the VP160 domain containing 10 tandem VP16 units (activator; Figure 1A). In C. elegans, dCas9 constructs were driven by tissue-specific promoters while ts-gRNAs were under control of a U6-type RNA polymerase III promoter (Figure 1B). Different combinations of the DNA constructs were injected into adult hermaphrodite gonads to generate transgenic worms. For zebrafish experiments, dCas9-KRAB and dCas9-VP160 mRNAs and ts-gRNAs were synthesized in vitro and injected into one-cell stage embryos (Figure 1C), and the resulted embryos were analyzed by real-time RT-PCR and in situ hybridization. Flanking sequences, including the nuclear localization sequences (NLSs) and UTRs, were indicated in Figure 1B and 1C or described previously6.
Figure 1.
The dCas9 fusion systems can be used to suppress or activate gene expression in both worm and zebrafish embryos. (A) dCas9 is fused to an effector and guided by ts-gRNAs to suppress or activate target genes. (B) Constructs of dCas9 fusion systems used in C. elegans. ts-gRNA is driven by U6 promoter. All dCas9 constructs contain NLSs flanking dCas9-coding region that is driven by dpy-5 or dbl-1 promoter. (C) Constructs of dCas9 system used in zebrafish. All dCas9 constructs contain NLSs attached to dCas9-coding region. ts-gRNA and dCas9 mRNA were in vitro synthesized by T7 RNA polymerase and the RNAs were injected in zebrafish experiments. (D) dpy-5 locus of C. elegans, where TSS (an arrow), dpy-5 ORF (green bar), dpy-5 promoter (red bar) and seven dpy-5ts-gRNA targeting sites (1-7, short black lines) are indicated. All seven ts-gRNAs target the non-template DNA strand. (E) dbl-1 locus of C. elegans. Among the six dbl-1ts-gRNAs (1-6, short black lines), ts-gRNAs 1, 2, 3, 4 and 6 target the template strand while ts-gRNA5 targets the non-template strand of dbl-1. Spaces in the green bar represent seven dbl-1 introns. (F) qRT-PCR reveals requirement of multiple ts-gRNAs to suppress dpy-5 expression. N2, wild-type worms (white bar); Ctrl, Pcol-10::mCherry worms (grey bar); 1-7All, pRF-4[rol-6(su1006)] worms carrying all seven ts-gRNA plasmids (black bar). The ts-gRNA plasmids expressing none, a single gRNA (one of seven target sequences) or all seven gRNAs (1-7All) were co-expressed with Pdpy-5::dCas9 or Pdpy-5::dCas9-KRAB in N2 worms. (G) qRT-PCR reveals requirement of multiple ts-gRNAs to activate dbl-1 expression. 1-6All, pRF-4[rol-6(su1006)] worms carrying all six ts-gRNA plasmids (black bar). Different combinations of ts-gRNA and Pdbl-1::dCas9-VP160 plasmids as indicated were co-expressed in N2 worms. (H) The zebrafish fgf8a locus. fgf8ats-gRNAs 1-6 target the non-template DNA strand and fgf8ats-gRNA 7-10 targets the template DNA strand. (I) The zebrafish foxi1 locus, foxi1ts-gRNAs 1-5, 7 and 8 target the non-template DNA strand while foxi1ts-gRNAs 6, 9 and 10 target the template DNA strand. (J, K) qRT-PCR reveals requirement of multiple ts-gRNAs in suppressing fgf8a (J) or foxi1 (K) expression. Ctrl, uninjected embryos (white bar); 1-5All, injecting all five ts-gRNAs only (black bar); (−), injecting RNA transcribed from empty gRNA vector (without target sequence) in the presence of dCas9-KRAB mRNA. (L, M) qRT-PCR reveals requirement of multiple ts-gRNAs in activating fgf8a (J) or foxi1 (K) expression. 6-10All, injecting all five ts-gRNAs only (black bar); (−), injecting RNA transcribed from empty gRNA vector (without target sequence in the presence of dCas9-VP160 mRNA. For worm experiments (F, G), total RNA was isolated from synchronized young adult transgenic worms of each group and pooled together for qRT-PCR analysis. For zebrafish experiments (J-M), RNAs are injected into wild-type zebrafish embryos at the 1-cell stage. A total of 30 embryos for each group were collected at 11 hpf and their total RNAs were isolated and pooled together for qRT-PCR analysis. In all qRT-PCR experiments, data shown represent means ± SEM of three independent experiments normalized to wild-type/control animals (N2 in F and G; uninjected embryos in J-M). **P < 0.01; ***P < 0.001 (unpaired Student's t-test)
Decrease in dpy-5 expression levels produces short body and dumpy phenotype, known as Dpy. Dbl-1 is a TGF-β family member expressed primarily in neurons and regulates lon-1 to adjust body length. When dbl-1 is overexpressed, worm body length is elongated (Lon phenotype). We targeted dpy-5 by selecting seven ts-gRNAs that recognize its non-template DNA strand of coding region spanning about 400 bp downstream of the transcription start site of dpy-5 (Figure 1D). Transgenic worms carrying Pdpy-5::dCas9 or Pdpy-5::dCas9-KRAB and one of seven dpy-5ts-gRNAs did not exhibit obvious changes in dpy-5 expression and body length (Figure 1F and Supplementary information, Table S1a). When all seven dpy-5ts-gRNAs(1-7) were included, suppression of dpy-5 expression and Dpy phenotype occurred (Figure 1F and Supplementary information, Figure S1aA–S1aC and S1aE). It appeared that Pdpy-5::dCas9-KRAB and dpy-5ts-gRNAs(1-7) combination showed slightly stronger effect on dpy-5 expression than that of Pdpy-5::dCas9 and dpy-5ts-gRNAs(1-7) combination, although both combinations resulted in the Dpy phenotype (Figure 1F and Supplementary information, Figure S1aA–S1aC, S1aE and Table S1a). We also found that combined with dCas9-KRAB, at least five ts-gRNAs were required to knockdown dpy-5 (Supplementary information, Figure S1bA and S1bC). These results suggest that recruiting multiple dCas9/dpy-5ts-gRNA complexes to exonic region of dpy-5 may account for a synergistic suppression. To directly visualize tissue-specific gene suppression, we generated Pdpy-30::NLSSV40-GFP-NLSegl-13; Pdpy-30::NLSSV40-mCherry-NLSegl-13 transgenic worms expressing GFP and mCherry in all cells (Supplementary information, Figure S1cA–S1cD). When dCas9-KRAB driven by an intestine-specific promoter Pges-1 and five gfpts-gRNAs driven by PU6 promoter were introduced into double-transgenic animals, GFP was largely diminished in intestine nuclei while mCherry signal remained in most animals examined (92.7%, n = 83; Supplementary information, Figure S1cE–S1cH), suggesting that tissue- and gene-specific knockdown is achievable with our dCas9 fusion system. Using Pdpy-7::GFP worms that only express GFP in the hypodermis, we found that only dCas9-KRAB driven by the hypodermis-specific dpy-7 promoter, not that by the intestine-specific Pges-1, reduced GFP expression in hypodermal cells (Supplementary information, Figure S1d), indicating that dCas9 functions in a cell-autonomous manner.
To test whether dbl-1ts-gRNAs and dCas9-VP160 combination could activate dbl-1 expression, we generated transgenic worms carrying dCas9-VP160 and one of six dbl-1ts-gRNAs or all of them (Figure 1E). We observed a significant elevation of dbl-1 expression and the Lon phenotype (Supplementary information, Figure S1aA, S1aD, S1aF and Table S1b) only when dCas9-VP160 and all six dbl-1ts-gRNAs (1-6) were present (Figure 1G and Supplementary information, Figure S1bB and S1bD). In addition, using transgenic worms carrying a copy of integrated pud-2.2-promoter-driven GFP (Ppud-2.2::GFP) that expresses GFP strongly and uniformly in the intestine and weakly in the hypodermis, we were able to directly monitor changes of GFP in these two tissues. Ppud-2.2::GFP worms carrying Pges-1::dCas9 or Pges-1::dCas9-KRAB and all five gfpts-gRNAs (1-5) showed suppressed gfp expression (Supplementary information, Figure S1e). Ppud-2.2::GFP worms carrying Pges-1::dCas9-VP160 and all five pud-2.2ts-gRNAs (6-10) that recognize pud-2.2 promoter showed significantly enhanced GFP expression mainly in the intestine (Supplementary information, Figure S1e and S1fC-S1fH). When hsp-16.2 (a ubiquitously expressed gene) promoter was used to drive dCas9-VP160 in the presence of all five pud-2.2ts-gRNAs (6-10) in Ppud-2.2::GFP worms, an enhanced GFP expression was also mainly observed in the intestine and epidermis upon heat shock (Supplementary information, Figure S1g), suggesting that ts-gRNAs and dCas9-VP160 tend to modify gene expression in place(s) where the gene is transcriptionally active.
In zebrafish, we targeted fgf8a and foxi1, both of which are required for the induction of the otic placode7,8. We designed five fgf8ats-gRNAs and five foxi1ts-gRNAs to suppress fgf8a and foxi1 expression, respectively. These ts-gRNAs recognize the non-template strand of fgf8a or foxi1 coding region (Figure 1H and 1I). When all five fgf8ats-gRNAs were co-injected with dCas9-KRAB mRNA into one-cell stage wild-type fertilized eggs, the resulted embryos showed reduced fgf8a expression at 11 hpf (Figure 1J and Supplementary information, Figure S1hC) and smaller otic vesicle at 32 hpf (Supplementary information, Figure S1iA–S1iB). Similarly, we observed that foxi1 expression and otic vesicle size were reduced when dCas9-KRAB mRNA and all five foxi1ts-gRNAs (1-5) were injected (Figure 1K, Supplementary information, Figure S1hD, S1iA and S1iC). If dCas9-VP160 mRNA and fgf8ats-gRNAs (6-10) or foxi1ts-gRNAs (6-10) were co-injected, an elevated expression of fgf8a or foxi1 was observed (Figure 1L and 1M and Supplementary information, Figure S1hE and S1hF), leading to enlarged otic vesicles (Supplementary information, Figure S1iD–S1iF), and enhanced fgf8a or foxi1 expression appeared to be near their endogenous expression locations (Supplementary information, Figure S1j). We also detected slightly changed expression of pea3 and pax8, downstream genes of Fgf8a and Foxi1, respectively, by in situ hybridization (Supplementary information, Figure S1k). Thus, our dCas9 fusion system works in both worm and zebrafish to adjust endogenous gene expression in a tissue-specific fashion. Presumably, the gene specificity is determined by an array of ts-gRNAs spreading across a short range at the target gene locus.
Zebrafish and C. elegans are well-established model animals with many loss-of-function and gain-of-function tools to regulate gene expression. To compare the efficiency of our dCas9 fusion systems with that of other widely used tools, we used dpy-5 RNAi to knockdown dpy-5 expression, and found that the loss-of-function efficiency was higher in dpy-5 RNAi worms than that of worms expressing Pdpy-5::dCas9/dCas9-KRAB and dpy-5ts-gRNAs (1-7; Supplementary information, Figure S1lA). This result was further confirmed by measuring worm body lengths (Supplementary information, Figure S1lC and Table S1c). We also measured dbl-1 expression levels and body lengths of worms overexpressing dCas9-VP160 and dbl-1ts-gRNAs (1-6), and worms expressing Pdbl-1::Dbl-1. Although dbl-1 expression level was higher in Pdbl-1::Dbl-1 transgenic worms than that of worms expressing dCas9-VP160 and dbl-1ts-gRNAs (1-6), the body lengths were comparable (Supplementary information, Figure S1lB and S1lD). In zebrafish experiments, pea3 and pax8 expression levels in embryos co-expressing fgf8a or foxi1 were comparable to those injected with dCas9-VP160 mRNA and ts-gRNAs (Supplementary information, Figure S1m). Injecting mRNA directly into zebrafish fertilized eggs swamped all embryonic cells with abundant fgf8a or foxi1 mRNA (data not shown). However, in embryos injected with dCas9-VP160 mRNA and ts-gRNAs, fgf8a or foxi1 mRNA was only observed in and near the original expression places (Supplementary information, Figure S1j). It therefore appeared that enhanced pea3 or pax8 expression in latter experiment groups might be more physiologically relevant (due to the on-site overexpression of fgf8a or foxi1) than that of conventional mRNA injection. On the other hand, the reduction of pea3 and pax8 expression level was moderate, compared to that of using fgf8a or foxi1 morpholino antisense oligo (MO; Supplementary information, Figure S1m). However, MO-mediated knockdown has been frequently criticized to cause somewhat too severe phenotypes that could not match the corresponding mutants due to its toxicity, off-target effects, or some unknown reasons9. Therefore, the modulation of endogenous gene expression by our dCas9 fusion systems may be used to reveal in vivo gene functions more appropriately.
In cultured cells, transcriptionally quiescent genes could be activated by dCas9-effector and their ts-gRNAs, contrary to our results. We believe that different genome modification(s) in culture cells, i.e., epigenetic states (open chromatin state, CpG methylation, and so on), may account for the discrepancy10,11. The strand-specific repression by ts-gRNAs of a gene was explained to be the consequence of steric inhibition of RNA polymerase II activity in prokaryotes5. At a transcriptionally active gene locus, the transcriptional machinery constantly unwinds the promoter and proximal exons to recurrently produce transcripts. The active state of a gene thus likely provides more chances for dCas9/ts-gRNAs to gain access to their target DNA sequences. In fact, using multiple ts-gRNAs to adjust the endogenous gene expression level or to conduct large-scale genetic screens in mammalian cells was also thought to better reduce the off-target effects2,4,10,12,13,14. However, different from previous findings that Cas9 and a single ts-gRNA are enough to induce target gene knockdown in many cases5, we found that an individual ts-gRNA and dCas9 or its derivatives could barely change target gene expression, which may potentially compromise the simple/easy application of our fusion systems. Nevertheless, with multiple dCas9-gRNA:DNA complexes formed simultaneously in a close range on target gene locus, the possible instability of a single dCas9/ts-gRNA:DNA complex is no longer a limiting factor.
Unlike worm experiments, in which DNA constructs of dCas9 or its derivatives and multiple ts-gRNAs (Supplementary information, Table S1d) were injected, we used in vitro-synthesized RNAs to modulate the expression of target genes in zebrafish (Supplementary information, Figure S1h and Table S1e). Due to the stability issue of synthetic RNAs in vivo, injected RNAs may at best modulate actively expressed target genes during early embryogenesis. The transient suppression or activation of target genes therefore led to a mild morphological phenotype(s) (Supplementary information, Figure S1i). To achieve long-lasting and spatiotemporally controllable effects, transgenic fish carrying ubiquitous and/or inducible dCas9-KRAB, dCas9-VP160 and multiple gene-specific ts-gRNAs are desirable. To avoid injecting too many ts-gRNA-encoding constructs at a time, a plasmid containing tandem ts-gRNA sequences that could automatically release individual ts-gRNAs when transcribed in vivo would be an ideal option15. Despite the lack of yet-to-be-developed amendment, we have demonstrated that our dCas9 fusion systems are a noteworthy alternative to modulate endogenous gene expression in whole organisms. In theory, ts-gRNA-guided dCas9 fusion proteins can be used to target and perturb noncoding elements such as enhancers, silencers, insulators, introns, and so on, and with libraries of ts-gRNAs that cover whole genomes, high-throughput analyses to interrogate gene functions and genetic networks will be readily conducted in both animals.
Acknowledgments
We thank Dr Hui CC for comments on the manuscript and Ms Ping Huang for the excellent management of Liu D laboratory. This study was supported by the National Natural Science Foundation of China (31371316), the National Basic Research Program of China (973 Program; 2012CB944503 and 2011CBA01102), the Ministry of Science and Technology of China and the Peking-Tsinghua Center for Life Sciences to DL. PL was a recipient of the PKU President Graduate Scholarship.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
The modular dCas9 fusion system works efficiently to suppress and activate endogenous gene expression in C. elegans
Summary of body length measurement in dpy-5 knockdown experiments
Materials and Methods
References
- Bikard D, Jiang W, Samai P, et al. Nucleic Acids Res. 2013. pp. 7429–7437. [DOI] [PMC free article] [PubMed]
- Cheng AW, Wang H, Yang H, et al. Cell Res. 2013. pp. 1163–1171. [DOI] [PMC free article] [PubMed]
- Gilbert LA, Larson MH, Morsut L, et al. Cell. 2013. pp. 442–451. [DOI] [PMC free article] [PubMed]
- Perez-Pinera P, Kocak DD, Vockley CM, et al. Nat Methods. 2013. pp. 973–976. [DOI] [PMC free article] [PubMed]
- Qi LS, Larson MH, Gilbert LA, et al. Cell. 2013. pp. 1173–1183. [DOI] [PMC free article] [PubMed]
- Liu P, Long L, Xiong K, et al. Cell Res. 2014. pp. 886–889. [DOI] [PMC free article] [PubMed]
- Phillips BT, Bolding K, Riley BB. Dev Biol. 2001. pp. 351–365. [DOI] [PubMed]
- Nissen RM, Yan J, Amsterdam A, et al. Development. 2003. pp. 2543–2554. [DOI] [PubMed]
- Schulte-Merker S, Stainier DYR. Development. 2014. pp. 3103–3104. [DOI] [PubMed]
- Kuscu C, Arslan S, Singh R, et al. Nat Biotechnol. 2014. pp. 677–683. [DOI] [PubMed]
- Wu X, Scott DA, Kriz AJ, et al. Nat Biotechnol. 2014. pp. 670–676. [DOI] [PMC free article] [PubMed]
- Koike-Yusa H, Li Y, Tan EP, et al. Nat Biotechnol. 2014. pp. 267–273. [DOI] [PubMed]
- Mali P, Esvelt KM, Church GM. Nat Methods. 2013. pp. 957–963. [DOI] [PMC free article] [PubMed]
- Wang T, Wei JJ, Sabatini DM, et al. Science. 2014. pp. 80–84. [DOI] [PMC free article] [PubMed]
- Nissim L, Perli SD, Fridkin A, et al. Mol Cell. 2014. pp. 698–710. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The modular dCas9 fusion system works efficiently to suppress and activate endogenous gene expression in C. elegans
Summary of body length measurement in dpy-5 knockdown experiments
Materials and Methods