Abstract
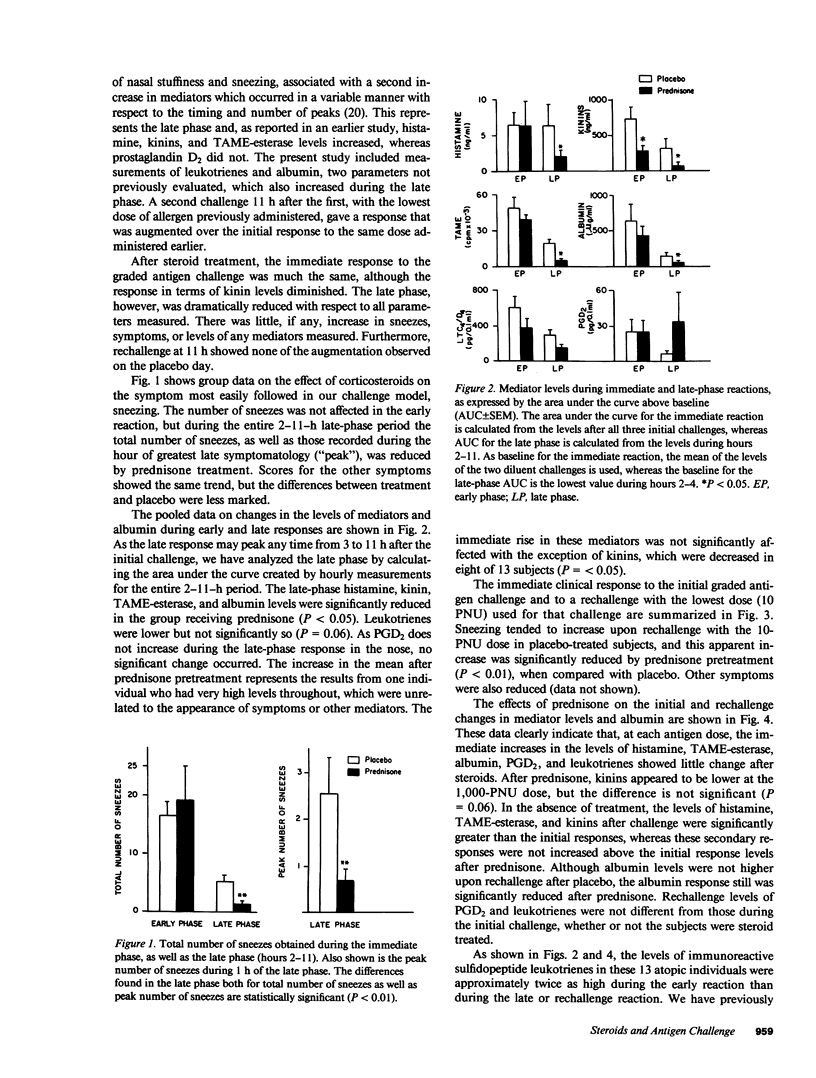
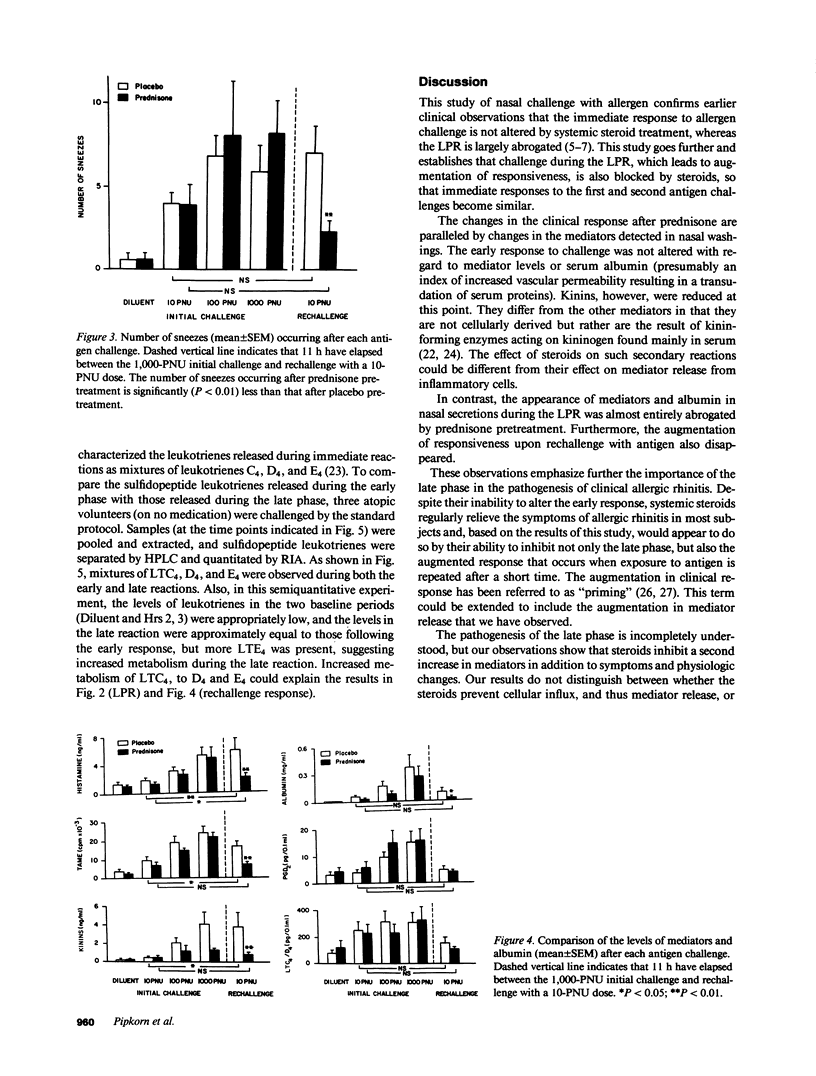
The effect of systemic glucocorticoid treatment on early- and late-phase nasal allergic reactions after allergen challenge was determined in a double-blind, cross-over study in 13 allergic individuals. The subjects were pretreated for 2 d before challenge with 60 mg prednisone per day or a matching placebo. A previously described model using repeated nasal lavages for measuring mediator release in vivo was utilized. Symptom scores obtained repeatedly before, during, and after the challenge and the number and timing of sneezes were recorded. The mediators measured were histamine. N-alpha-p-tosyl-L-arginine methyl ester (TAME)-esterase activity, kinins, PGD2, and LTC4/D4. Albumin was also measured as a marker of plasma transudation. Blood samples were taken for determination of total number of white blood cells, differential count, and total blood histamine content. No effect of steroid therapy was found on the appearance of symptoms or any of the mediators, except a reduction in kinins, in the early phase of the allergic reaction. However, in the late phase, the prednisone reduced the number of sneezes (P less than 0.01), as well as the level of histamine (P less than 0.05), TAME-esterase activity (P less than 0.05), kinins (P less than 0.05), and albumin (P less than 0.05). Only low levels of leukotrienes were found in the late phase, but the quantities of these mediators seemed to be decreased by the glucocorticoid treatment (P = 0.06). PGD2 did not increase during the LPR and thus was not affected by glucocorticosteroids. The immediate response to a second challenge 11 h after the first was also evaluated. Whereas the appearance of mediators was enhanced over the initial response to the same challenge dose in placebo-treated subjects, this enhancement was abrogated after prednisone treatment. As this dose of drug is known to be clinically effective in treating hay fever, the present study confirms the earlier findings of others that short-term systemic glucocorticoid treatment inhibits the late phase but not the immediate phase of antigen challenge. Furthermore, secondary enhancement of immediate responses is inhibited. This study shows that glucocorticoids inhibit the generation or release of inflammatory mediators during the late reaction and the physiologic response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarten C. R., Togias A. G., Naclerio R. M., Lichtenstein L. M., Norman P. S., Proud D. Influx of kininogens into nasal secretions after antigen challenge of allergic individuals. J Clin Invest. 1985 Jul;76(1):191–197. doi: 10.1172/JCI111945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij-Noord H., de Vries K., Sluiter H. J., Orie N. G. Late bronchial obstructive reaction to experimental inhalation of house dust extract. Clin Allergy. 1972 Mar;2(1):43–61. doi: 10.1111/j.1365-2222.1972.tb01267.x. [DOI] [PubMed] [Google Scholar]
- CARRYER H. M., KOELSCHE G. A., PRICKMAN L. E., MAYTUM C. K., LAKE C. F., WILLIAMS H. L. The effect of cortisone of bronchial asthma and hay fever occurring in subjects sensitive to ragweed pollen. J Allergy. 1950 Jul;21(4):282–287. doi: 10.1016/0021-8707(50)90059-1. [DOI] [PubMed] [Google Scholar]
- Connell J. T. Quantitative intranasal pollen challenge. II. Effect of daily pollen challenge, environmental pollen exposure, and placebo challenge on the nasal membrane. J Allergy. 1968 Mar;41(3):123–139. doi: 10.1016/0021-8707(68)90053-1. [DOI] [PubMed] [Google Scholar]
- Connell J. T. Quantitative intranasal pollen challenges. 3. The priming effect in allergic rhinitis. J Allergy. 1969 Jan;43(1):33–44. doi: 10.1016/0021-8707(69)90018-5. [DOI] [PubMed] [Google Scholar]
- Creticos P. S., Peters S. P., Adkinson N. F., Jr, Naclerio R. M., Hayes E. C., Norman P. S., Lichtenstein L. M. Peptide leukotriene release after antigen challenge in patients sensitive to ragweed. N Engl J Med. 1984 Jun 21;310(25):1626–1630. doi: 10.1056/NEJM198406213102502. [DOI] [PubMed] [Google Scholar]
- Gleich G. J. The late phase of the immunoglobulin E-mediated reaction: a link between anaphylaxis and common allergic disease? J Allergy Clin Immunol. 1982 Sep;70(3):160–169. doi: 10.1016/0091-6749(82)90037-9. [DOI] [PubMed] [Google Scholar]
- Naclerio R. M., Meier H. L., Kagey-Sobotka A., Adkinson N. F., Jr, Meyers D. A., Norman P. S., Lichtenstein L. M. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983 Oct;128(4):597–602. doi: 10.1164/arrd.1983.128.4.597. [DOI] [PubMed] [Google Scholar]
- Naclerio R. M., Proud D., Togias A. G., Adkinson N. F., Jr, Meyers D. A., Kagey-Sobotka A., Plaut M., Norman P. S., Lichtenstein L. M. Inflammatory mediators in late antigen-induced rhinitis. N Engl J Med. 1985 Jul 11;313(2):65–70. doi: 10.1056/NEJM198507113130201. [DOI] [PubMed] [Google Scholar]
- Nagy L., Lee T. H., Kay A. B. Neutrophil chemotactic activity in antigen-induced late asthmatic reactions. N Engl J Med. 1982 Mar 4;306(9):497–501. doi: 10.1056/NEJM198203043060901. [DOI] [PubMed] [Google Scholar]
- Pelikan Z. Late and delayed responses of the nasal mucosa to allergen challenge. Ann Allergy. 1978 Jul;41(1):37–47. [PubMed] [Google Scholar]
- Pepys J., Hutchcroft B. J. Bronchial provocation tests in etiologic diagnosis and analysis of asthma. Am Rev Respir Dis. 1975 Dec;112(6):829–859. doi: 10.1164/arrd.1975.112.6.829. [DOI] [PubMed] [Google Scholar]
- Proud D., Togias A., Naclerio R. M., Crush S. A., Norman P. S., Lichtenstein L. M. Kinins are generated in vivo following nasal airway challenge of allergic individuals with allergen. J Clin Invest. 1983 Nov;72(5):1678–1685. doi: 10.1172/JCI111127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. G., Kerigan A. T., Hargreave F. E., Chalmers R., Dolovich J. Late asthmatic responses induced by ragweed pollen allergen. J Allergy Clin Immunol. 1974 Oct;54(4):244–254. doi: 10.1016/0091-6749(74)90067-0. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Lichtenstein L. M., Gillespie E. Inhibition of basophil histamine release by anti-inflammatory steroids. Nature. 1981 Jul 30;292(5822):454–455. doi: 10.1038/292454a0. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Schulman E. S., MacGlashan D. W., Jr, Peters S. P., Hayes E. C., Adams G. K., 3rd, Lichtenstein L. M., Adkinson N. F., Jr Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J Clin Invest. 1983 Jun;71(6):1830–1835. doi: 10.1172/JCI110938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer R. P. The mechanisms of antiinflammatory steroid action in allergic diseases. Annu Rev Pharmacol Toxicol. 1985;25:381–412. doi: 10.1146/annurev.pa.25.040185.002121. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Slott R. I., Zeweiman B. Histologic studies of human skin test responses to ragweed and compound 48/80. II. Effects of corticosteroid therapy. J Allergy Clin Immunol. 1975 Apr;55(4):232–240. doi: 10.1016/0091-6749(75)90142-6. [DOI] [PubMed] [Google Scholar]
- Slott R. I., Zweiman B. A controlled study of the effect of corticosteroids on immediate skin test reactivity. J Allergy Clin Immunol. 1974 Oct;54(4):229–234. doi: 10.1016/0091-6749(74)90065-7. [DOI] [PubMed] [Google Scholar]
- Solley G. O., Gleich G. J., Jordon R. E., Schroeter A. L. The late phase of the immediate wheal and flare skin reaction. Its dependence upon IgE antibodies. J Clin Invest. 1976 Aug;58(2):408–420. doi: 10.1172/JCI108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedmyr N. Effects of glucocorticoids on the airways. Eur J Respir Dis Suppl. 1982;122:48–53. [PubMed] [Google Scholar]
- Taylor G., Shivalkar P. R. 'Arthus-type' reactivity in the nasal airways and skin in pollen sensitive subjects. Clin Allergy. 1971 Dec;1(4):407–414. doi: 10.1111/j.1365-2222.1971.tb00792.x. [DOI] [PubMed] [Google Scholar]


