Abstract
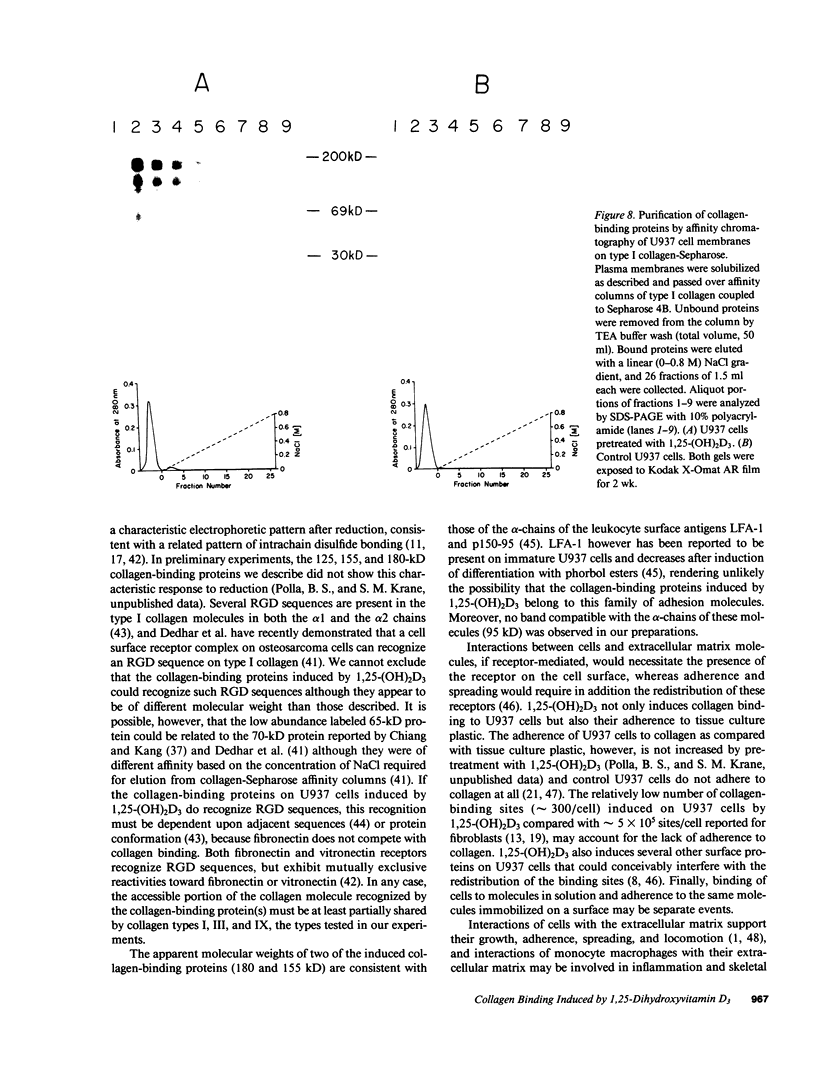
Interactions of cells with components of the extracellular matrix can modulate cellular functions. We measured binding of a major matrix protein to U937 cells, a human promonocytic line. Radioiodinated type I or type III human collagen was bound only to U937 cells differentiated to a more mature phenotype with 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3). Binding was observed at 4 degrees C and was saturable; Scatchard analysis of the binding to 1,25-(OH)2D3-pretreated U937 cells indicated a single class of high-affinity binding sites. Preincubation of U937 cells with interferon gamma did not induce collagen binding. Collagen binding did not appear to be dependent on fibronectin binding. Surface proteins of U937 cells were 125I labeled and cell membrane proteins resolved by affinity chromatography on collagen-Sepharose. Major specifically labeled bands of 180, 155, and 125 kD were identified in membrane fractions from 1,25-(OH)2D3-pretreated U937 cells only. 1,25-(OH)2D3 appears to specifically regulate collagen binding to monocyte precursors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Bhalla A. K., Kurnick J. T., Kradin R. L., Clemens T. L., Holick S. A., Holick M. F., Krane S. M. 1 alpha,25-dihydroxyvitamin D3 induces maturation of the human monocyte cell line U937, and, in association with a factor from human T lymphocytes, augments production of the monokine, mononuclear cell factor. J Clin Invest. 1984 Mar;73(3):731–739. doi: 10.1172/JCI111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Teitelbaum S. L., Reitsma P., Hall A., Pegg L. E., Trial J., Kahn A. J. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5907–5911. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell N. H. Vitamin D-endocrine system. J Clin Invest. 1985 Jul;76(1):1–6. doi: 10.1172/JCI111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A. K., Amento E. P., Clemens T. L., Holick M. F., Krane S. M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983 Dec;57(6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- Bianco C. Fibrin, fibronectin, and macrophages. Ann N Y Acad Sci. 1983 Jun 27;408:602–609. doi: 10.1111/j.1749-6632.1983.tb23277.x. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Kang A. H. Isolation and purification of collagen alpha 1(I) receptor from human platelet membrane. J Biol Chem. 1982 Jul 10;257(13):7581–7586. [PubMed] [Google Scholar]
- Coller B. S. Activation affects access to the platelet receptor for adhesive glycoproteins. J Cell Biol. 1986 Aug;103(2):451–456. doi: 10.1083/jcb.103.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Ricard-Blum S., Kaufmann M. T., Herbage D. Type IX collagen is a potent inducer of PGE2 and interleukin 1 production by human monocyte macrophages. FEBS Lett. 1986 Mar 31;198(2):208–212. doi: 10.1016/0014-5793(86)80406-9. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Trentham D. E., Krane S. M. Collagens act as ligands to stimulate human monocytes to produce mononuclear cell factor (MCF) and prostaglandins (PGE2). Coll Relat Res. 1982 Nov;2(6):523–540. doi: 10.1016/s0174-173x(82)80007-1. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Comoglio P. M., Tarone G. Fibronectin-plasma membrane interaction in the adhesion of hemopoietic cells. J Cell Biol. 1986 Aug;103(2):429–437. doi: 10.1083/jcb.103.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B. Binding of soluble type I collagen molecules to the fibroblast plasma membrane. Cell. 1979 Feb;16(2):265–275. doi: 10.1016/0092-8674(79)90004-7. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. J. Extracellular matrices and the control of cell proliferation and differentiation in vitro. Prog Clin Biol Res. 1984;145:103–128. [PubMed] [Google Scholar]
- Hosein B., Bianco C. Monocyte receptors for fibronectin characterized by a monoclonal antibody that interferes with receptor activity. J Exp Med. 1985 Jul 1;162(1):157–170. doi: 10.1084/jem.162.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Murray J. C., McGoodwin E. B., Martin G. R. Connective tissue structure: cell binding to collagen. J Invest Dermatol. 1978 Jul;71(1):9–11. doi: 10.1111/1523-1747.ep12543641. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Taylor A., Garrels J. I., Hogan B. L. Cell surface-associated proteins which bind native type IV collagen or gelatin. J Biol Chem. 1984 May 10;259(9):5915–5922. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J., von der Mark K. Isolation and characterization of a collagen-binding glycoprotein from chondrocyte membranes. EMBO J. 1983;2(1):45–50. doi: 10.1002/j.1460-2075.1983.tb01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Humphries M. J., Olden K., Yamada K. M. Collagen can modulate cell interactions with fibronectin. J Cell Biol. 1985 Aug;101(2):386–394. doi: 10.1083/jcb.101.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Yamada K. M. Phosphorylation and transformation sensitivity of a major collagen-binding protein of fibroblasts. J Biol Chem. 1986 Jun 5;261(16):7531–7536. [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. The fibronectin receptor on mammalian erythroid precursor cells: characterization and developmental regulation. J Cell Biol. 1986 Feb;102(2):449–456. doi: 10.1083/jcb.102.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Fanning V., Thiagarajan P., Hoxie J., Trinchieri G. Immune interferon and leukocyte-conditioned medium induce normal and leukemic myeloid cells to differentiate along the monocytic pathway. J Exp Med. 1983 Dec 1;158(6):2058–2080. doi: 10.1084/jem.158.6.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla B. S., Healy A. M., Amento E. P., Krane S. M. 1,25-Dihydroxyvitamin D3 maintains adherence of human monocytes and protects them from thermal injury. J Clin Invest. 1986 Apr;77(4):1332–1339. doi: 10.1172/JCI112438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., O'Shea J., Chused T., Takahashi T., Ochoa M., Nutman T. B., Bianco C., Brown E. J. Differentiation stimuli induce receptors for plasma fibronectin on the human myelomonocytic cell line HL-60. Blood. 1984 Oct;64(4):858–866. [PubMed] [Google Scholar]
- Provvedini D. M., Tsoukas C. D., Deftos L. J., Manolagas S. C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983 Sep 16;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rubin K., Gullberg D., Borg T. K., Obrink B. Hepatocyte adhesion to collagen. Isolation of membrane glycoproteins involved in adhesion to collagen. Exp Cell Res. 1986 May;164(1):127–138. doi: 10.1016/0014-4827(86)90460-x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Tsoukas C. D., Provvedini D. M., Manolagas S. C. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984 Jun 29;224(4656):1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- Vartio T., Hedman K., Jansson S. E., Hovi T. The Mr 95,000 gelatin-binding protein in differentiated human macrophages and granulocytes. Blood. 1985 May;65(5):1175–1180. [PubMed] [Google Scholar]
- Vartio T., Hovi T., Vaheri A. Human macrophages synthesize and secrete a major 95,000-dalton gelatin-binding protein distinct from fibronectin. J Biol Chem. 1982 Aug 10;257(15):8862–8866. [PubMed] [Google Scholar]
- Yamada K. M., Akiyama S. K., Hasegawa T., Hasegawa E., Humphries M. J., Kennedy D. W., Nagata K., Urushihara H., Olden K., Chen W. T. Recent advances in research on fibronectin and other cell attachment proteins. J Cell Biochem. 1985;28(2):79–97. doi: 10.1002/jcb.240280202. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Amino acid sequence specificities of an adhesive recognition signal. J Cell Biochem. 1985;28(2):99–104. doi: 10.1002/jcb.240280203. [DOI] [PubMed] [Google Scholar]