Abstract
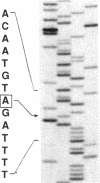
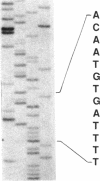
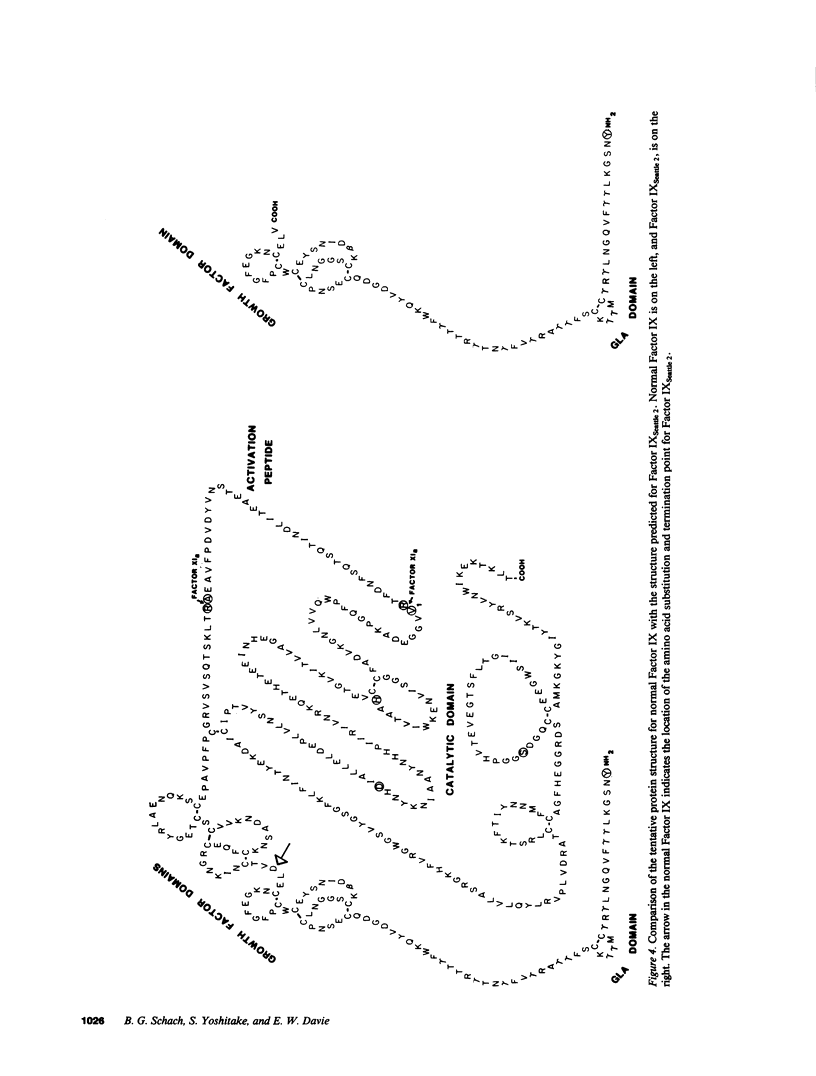
To understand the molecular basis for hemophilia B in patients with little or no circulating Factor IX antigen, a patient who had less than 0.2% circulating Factor IX antigen (Factor IXSeattle 2) was selected for analysis of his Factor IX gene. Genomic DNA fragments from the abnormal gene were cloned into bacteriophage lambda vectors and recombinant phage were identified using radiolabeled genomic probes obtained from the normal Factor IX gene. The exons and flanking regions of the abnormal gene were sequenced by the dideoxy chain-termination method and this sequence was compared with that of the normal gene. Only one significant difference was observed, the deletion of a single adenine nucleotide in exon V. This resulted in a frameshift that converted an aspartic acid at position 85 in the protein to a valine and the formation of a stop signal at position 86. These data indicate that the gene for Factor IXSeattle 2 codes for an 85 residue polypeptide that terminates after the first epidermal growth factor domain. Thus, the putative Factor IXSeattle 2 polypeptide lacks the second epidermal growth factor domain, the activation peptide, and the catalytic domain present in the normal protein. This provides an explanation for the coagulation disorder in this patient and represents the first report of a single nucleotide deletion and frameshift resulting in hemophilia B.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley A. K., Rees D. J., Rizza C., Brownlee G. G. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position -4. Cell. 1986 May 9;45(3):343–348. doi: 10.1016/0092-8674(86)90319-3. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. L., Thompson A. R. Partial factor IX protein in a pedigree with hemophilia B due to a partial gene deletion. J Clin Invest. 1986 Apr;77(4):1194–1200. doi: 10.1172/JCI112421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. L., Weinmann A. F., Thompson A. R. Calcium-specific immunoassays for factor IX: reduced levels of antigen in patients with vitamin K disorders. J Lab Clin Med. 1986 Mar;107(3):269–278. [PubMed] [Google Scholar]
- Brownlee G. G. The molecular genetics of haemophilia A and B. J Cell Sci Suppl. 1986;4:445–458. doi: 10.1242/jcs.1986.supplement_4.24. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Alberti A., Kan Y. W. A beta-thalassemia lesion abolishes the same Mst II site as the sickle mutation. Nucleic Acids Res. 1983 Nov 25;11(22):7789–7794. doi: 10.1093/nar/11.22.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Yoshitake S., Chance P. F., Bray G. L., Thompson A. R., Scott C. R., Kurachi K. An intragenic deletion of the factor IX gene in a family with hemophilia B. J Clin Invest. 1985 Dec;76(6):2161–2164. doi: 10.1172/JCI112222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Gould K. G., Rees D. J., Brownlee G. G. Molecular cloning of the gene for human anti-haemophilic factor IX. Nature. 1982 Sep 9;299(5879):178–180. doi: 10.1038/299178a0. [DOI] [PubMed] [Google Scholar]
- Davis L. M., McGraw R. A., Ware J. L., Roberts H. R., Stafford D. W. Factor IXAlabama: a point mutation in a clotting protein results in hemophilia B. Blood. 1987 Jan;69(1):140–143. [PubMed] [Google Scholar]
- Diuguid D. L., Rabiet M. J., Furie B. C., Liebman H. A., Furie B. Molecular basis of hemophilia B: a defective enzyme due to an unprocessed propeptide is caused by a point mutation in the factor IX precursor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5803–5807. doi: 10.1073/pnas.83.16.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Galliano M., Minchiotti L., Iadarola P., Zapponi M. C., Ferri G., Castellani A. A. Structural characterization of a chain termination mutant of human serum albumin. J Biol Chem. 1986 Mar 25;261(9):4283–4287. [PubMed] [Google Scholar]
- Giannelli F., Choo K. H., Rees D. J., Boyd Y., Rizza C. R., Brownlee G. G. Gene deletions in patients with haemophilia B and anti-factor IX antibodies. Nature. 1983 May 12;303(5913):181–182. doi: 10.1038/303181a0. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Ripley L. S. Structural intermediates of deletion mutagenesis: a role for palindromic DNA. Proc Natl Acad Sci U S A. 1984 Jan;81(2):512–516. doi: 10.1073/pnas.81.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran P., Lavelle D. E., Kaul R., Mohandas T., Warren S. T. Isolation and characterization of human factor IX cDNA: identification of Taq I polymorphism and regional assignment. Somat Cell Mol Genet. 1984 Sep;10(5):465–473. doi: 10.1007/BF01534851. [DOI] [PubMed] [Google Scholar]
- Jaye M., de la Salle H., Schamber F., Balland A., Kohli V., Findeli A., Tolstoshev P., Lecocq J. P. Isolation of a human anti-haemophilic factor IX cDNA clone using a unique 52-base synthetic oligonucleotide probe deduced from the amino acid sequence of bovine factor IX. Nucleic Acids Res. 1983 Apr 25;11(8):2325–2335. doi: 10.1093/nar/11.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Boehm C. D., Sexton J. P., Antonarakis S. E. beta-Thalassemia due to a deletion of the nucleotide which is substituted in the beta S-globin gene. Am J Hum Genet. 1983 Sep;35(5):1028–1033. [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Matsunaga E., Takihara Y., Nakamura T., Takagi Y., Lin S., Lee H. Structural analysis of a beta-thalassemia gene found in Taiwan. J Biol Chem. 1983 Mar 10;258(5):2748–2749. [PubMed] [Google Scholar]
- Kinniburgh A. J., Maquat L. E., Schedl T., Rachmilewitz E., Ross J. mRNA-deficient beta o-thalassemia results from a single nucleotide deletion. Nucleic Acids Res. 1982 Sep 25;10(18):5421–5427. doi: 10.1093/nar/10.18.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Alexander P. S. The base substitution fidelity of eucaryotic DNA polymerases. Mispairing frequencies, site preferences, insertion preferences, and base substitution by dislocation. J Biol Chem. 1986 Jan 5;261(1):160–166. [PubMed] [Google Scholar]
- Kunkel T. A. Frameshift mutagenesis by eucaryotic DNA polymerases in vitro. J Biol Chem. 1986 Oct 15;261(29):13581–13587. [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- McGraw R. A., Davis L. M., Lundblad R. L., Stafford D. W., Roberts H. R. Structure and function of factor IX: defects in haemophilia B. Clin Haematol. 1985 Jun;14(2):359–383. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Anagnou N. P., Ley T. J. Advances in thalassemia research. Blood. 1984 Apr;63(4):738–758. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Noyes C. M., Griffith M. J., Roberts H. R., Lundblad R. L. Identification of the molecular defect in factor IX Chapel Hill: substitution of histidine for arginine at position 145. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4200–4202. doi: 10.1073/pnas.80.14.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake I. R., Furlong B. L., Bloom A. L. Carrier detection by direct gene analysis in a family with haemophilia B (factor IX deficiency). Lancet. 1984 Feb 4;1(8371):242–243. doi: 10.1016/s0140-6736(84)90123-5. [DOI] [PubMed] [Google Scholar]
- Rees D. J., Rizza C. R., Brownlee G. G. Haemophilia B caused by a point mutation in a donor splice junction of the human factor IX gene. Nature. 1985 Aug 15;316(6029):643–645. doi: 10.1038/316643a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Danforth B. N., Glickman B. W. Mechanisms of spontaneous mutagenesis: an analysis of the spectrum of spontaneous mutation in the Escherichia coli lacI gene. J Mol Biol. 1986 May 20;189(2):273–284. doi: 10.1016/0022-2836(86)90509-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Thompson A. R. Monoclonal antibody to an epitope on the heavy chain of factor IX missing in three hemophilia-B patients. Blood. 1983 Nov;62(5):1027–1034. [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]