Abstract
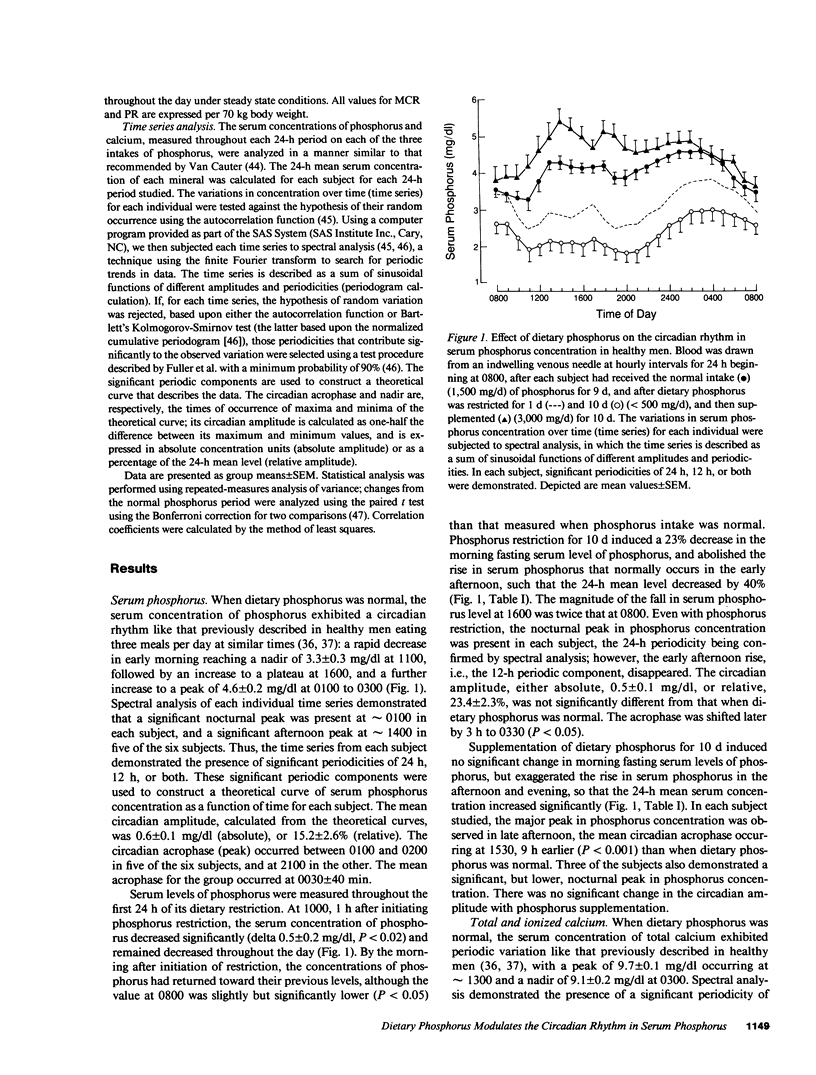
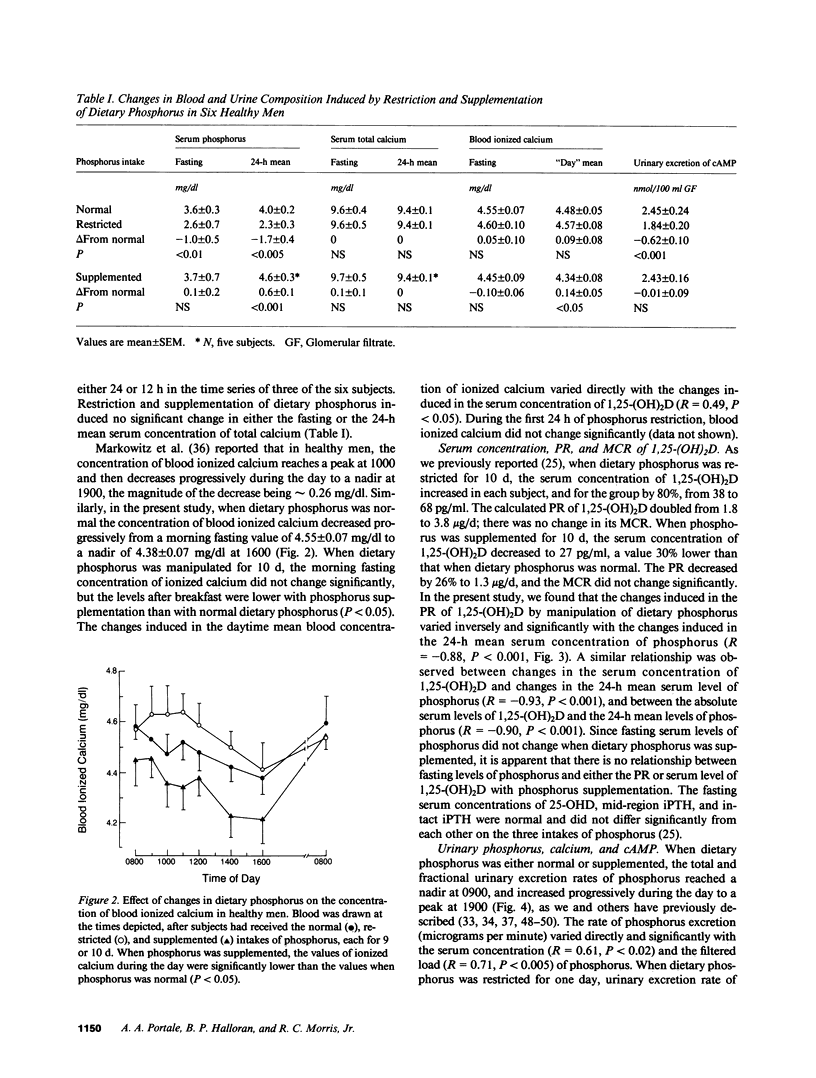
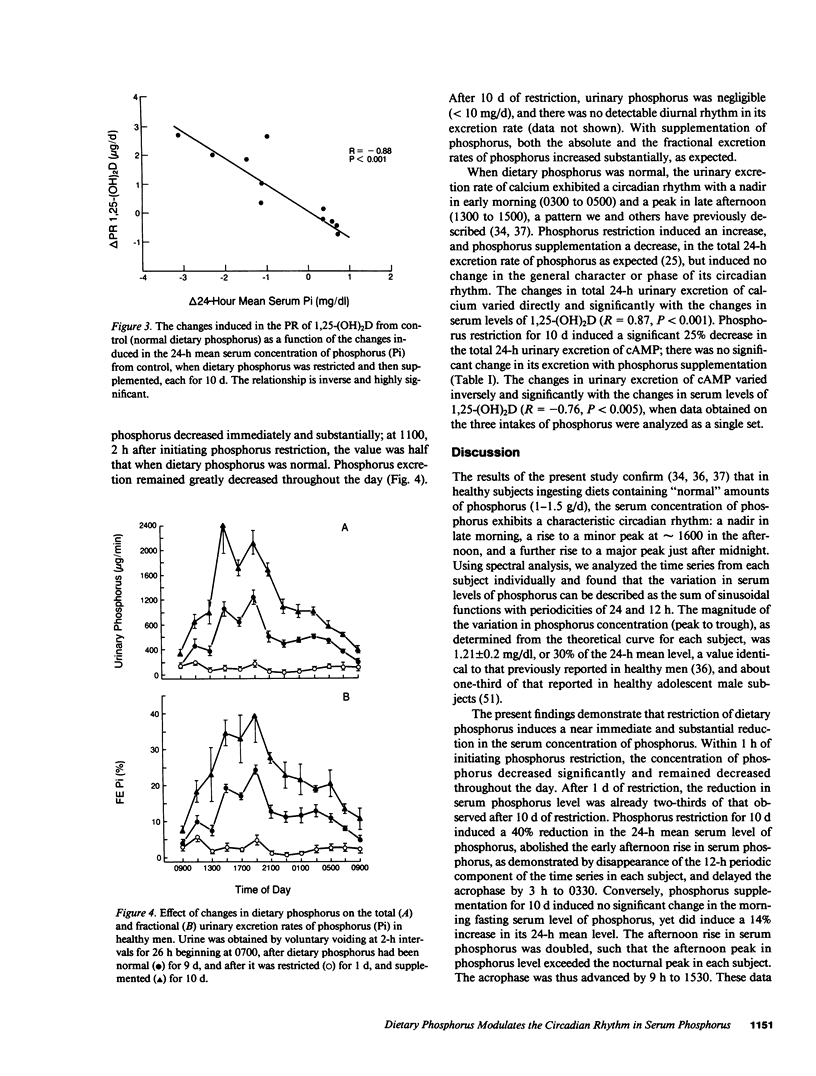
We recently reported that in healthy men, changes in the production rate (PR) of 1,25-dihydroxyvitamin D [1,25-(OH)2D] accounted for the 80% increase and the 30% decrease in its serum concentration that was induced by restriction and supplementation, respectively, of dietary phosphorus. These changes in PR and serum concentration of 1,25-(OH)2D could be mediated by changes in serum concentrations of phosphorus that occur after the morning fasting period. To examine this hypothesis, we measured serum concentrations of phosphorus in blood drawn at hourly intervals for 24 h in six healthy men in whom dietary phosphorus was initially maintained at 1,500 mg/70 kg body weight per day for 9 d, then restricted to 500 mg/d (coupled with orally administered aluminum hydroxide) for 10 d, and then supplemented to 3,000 mg/d for 10 d. When dietary phosphorus was normal, the serum concentration of phosphorus exhibited the normal circadian rhythm: a rapid decrease in early morning to a nadir at 1100, followed by an increase to plateau at 1600 h and a further increase to an acrophase (peak) at 0030 h. The variation in serum levels of phosphorus can be described as the sum of sinusoidal functions with periodicities of 24 and 12 h. Phosphorus restriction for 10 d induced a 40% reduction in the 24-h mean serum level of phosphorus, abolished the early afternoon rise in its serum level (i.e., the 12-h periodic component of the time series), and delayed the acrophase by 3 h to 0330 h. Phosphorus supplementation for 10 d induced a 14% increase in the 24-h mean serum level of phosphorus but no significant change in its morning fasting level, exaggerated the early afternoon rise in serum phosphorus, and advanced the acrophase by 9 h to 1530 h. The changes in the PR of 1,25-(OH)2D induced by restriction and supplementation of dietary phosphorus varied inversely and significantly with those induced in the 24-h mean serum level of phosphorus (R = -0.88, P less than 0.001). These data demonstrate that in healthy men, dietary phosphorus is an important determinant of the serum concentration of phosphorus throughout most of the day. The data suggest that diet-induced changes in serum levels of phosphorus mediate the changes in PR and serum concentration of 1,25(OH)2D.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiba T., Endou H., Koseki C., Sakai F., Horiuchi N., Suda T. Localization of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the mammalian kidney. Biochem Biophys Res Commun. 1980 May 14;94(1):313–318. doi: 10.1016/s0006-291x(80)80222-1. [DOI] [PubMed] [Google Scholar]
- Baxter L. A., DeLuca H. F. Stimulation of 25-hydroxyvitamin D3-1alpha-hydroxylase by phosphate depletion. J Biol Chem. 1976 May 25;251(10):3158–3161. [PubMed] [Google Scholar]
- Bikle D. D., Rasmussen H. The ionic control of 1,25-dihydroxyvitamin D3 production in isolated chick renal tubules. J Clin Invest. 1975 Feb;55(2):292–298. doi: 10.1172/JCI107932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. E., Tsai H. C., Morris R. C., Jr Parathyroidectomy reduces 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the hypocalcemic vitamin D-deficient chick. J Clin Invest. 1977 Dec;60(6):1314–1320. doi: 10.1172/JCI108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. E., Tsai H. C., Morris R. C., Jr Vitamin D status regulates 25-hydroxyvitamin D3-1 alpha-hydroxylase and its responsiveness to parathyroid hormone in the chick. J Clin Invest. 1985 Jan;75(1):155–161. doi: 10.1172/JCI111668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus A. E., Magee J. S., Mallette L. E., Horst R. L., Lang R., Jensen P. S., Gertner J. M., Baron R. A detailed evaluation of oral phosphate therapy in selected patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1983 May;56(5):953–961. doi: 10.1210/jcem-56-5-953. [DOI] [PubMed] [Google Scholar]
- Brunette M. G., Chan M., Ferriere C., Roberts K. D. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978 Nov 16;276(5685):287–289. doi: 10.1038/276287a0. [DOI] [PubMed] [Google Scholar]
- Buchsbaum M., Harris E. K. Diurnal variation in serum and urine electrolytes. J Appl Physiol. 1971 Jan;30(1):27–35. doi: 10.1152/jappl.1971.30.1.27. [DOI] [PubMed] [Google Scholar]
- CARRUTHERS B. M., COPP D. H., MCINTOSH H. W. DIURNAL VARIATION IN URINARY EXCRETION OF CALCIUM AND PHOSPHATE AND ITS RELATION TO BLOOD LEVELS. J Lab Clin Med. 1964 Jun;63:959–968. [PubMed] [Google Scholar]
- Colston K. W., Evans I. M., Galante L., MacIntyre I., Moss D. W. Regulation of vitamin D metabolism: factors influencing the rate of formation of 1,25-dihydroxycholecalciferol by kidney homogenates. Biochem J. 1973 Jul;134(3):817–820. [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gallagher J. C., Riggs B. L., Eisman J., Hamstra A., Arnaud S. B., DeLuca H. F. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest. 1979 Sep;64(3):729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. W. Control of plasma 1,25-(OH)2-vitamin D concentrations by calcium and phosphorus in the rat: effects of hypophysectomy. Calcif Tissue Int. 1981;33(5):485–488. doi: 10.1007/BF02409478. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Garthwaite T. L. Activation of renal 1,25-dihydroxyvitamin D3 synthesis by phosphate deprivation: evidence for a role for growth hormone. Endocrinology. 1985 Jan;116(1):189–193. doi: 10.1210/endo-116-1-189. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Garthwaite T. L., Phillips L. S. Growth hormone and triiodothyronine permit an increase in plasma 1,25(OH)2D concentrations in response to dietary phosphate deprivation in hypophysectomized rats. Calcif Tissue Int. 1983;35(1):100–106. doi: 10.1007/BF02405013. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Napoli J. L. Dietary phosphate deprivation increases 1,25-dihyroxyvitamin D3 synthesis in rat kidney in vitro. J Biol Chem. 1983 Jan 25;258(2):1152–1155. [PubMed] [Google Scholar]
- Gray R. W., Omdahl J. L., Ghazarian J. G., DeLuca H. F. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem. 1972 Dec 10;247(23):7528–7532. [PubMed] [Google Scholar]
- Gray R. W., Wilz D. R., Caldas A. E., Lemann J., Jr The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: studies in healthy subjects in calcium-stone formers and in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1977 Aug;45(2):299–306. doi: 10.1210/jcem-45-2-299. [DOI] [PubMed] [Google Scholar]
- Gray R., Boyle I., DeLuca H. F. Vitamin D metabolism: the role of kidney tissue. Science. 1971 Jun 18;172(3989):1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- Halloran B. P., Portale A. A., Castro M., Morris R. C., Jr, Goldsmith R. S. Serum concentration of 1,25-dihydroxyvitamin D in the human: diurnal variation. J Clin Endocrinol Metab. 1985 Jun;60(6):1104–1110. doi: 10.1210/jcem-60-6-1104. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Midgett R. J., Norman A. W. Regulation of 25-hydroxyvitamin D3-1-hydroxylase in vivo. J Biol Chem. 1974 Dec 10;249(23):7584–7592. [PubMed] [Google Scholar]
- Henry H. L. Regulation of the hydroxylation of 25-hydroxyvitamin D3 in vivo and in primary cultures of chick kidney cells. J Biol Chem. 1979 Apr 25;254(8):2722–2729. [PubMed] [Google Scholar]
- Insogna K. L., Broadus A. E., Gertner J. M. Impaired phosphorus conservation and 1,25 dihydroxyvitamin D generation during phosphorus deprivation in familial hypophosphatemic rickets. J Clin Invest. 1983 Jun;71(6):1562–1569. doi: 10.1172/JCI110912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubiz W., Canterbury J. M., Reiss E., Tyler F. H. Circadian rhythm in serum parathyroid hormone concentration in human subjects: correlation with serum calcium, phosphate, albumin, and growth hormone levels. J Clin Invest. 1972 Aug;51(8):2040–2046. doi: 10.1172/JCI107010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Localization of 25-hydroxyvitamin D3 1 alpha-hydroxylase and 24-hydroxylase along the rat nephron. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1199–1203. doi: 10.1073/pnas.78.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llach F., Massry S. G. On the mechanism of secondary hyperparathyroidism in moderate renal insufficiency. J Clin Endocrinol Metab. 1985 Oct;61(4):601–606. doi: 10.1210/jcem-61-4-601. [DOI] [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Abnormal regulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the X-linked hypophosphatemic mouse. J Clin Invest. 1983 Feb;71(2):400–403. doi: 10.1172/JCI110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin E. G., Kumar R., Heath H., 3rd Hyperphosphatemic tumoral calcinosis: effects of phosphate depletion on vitamin D metabolism, and of acute hypocalcemia on parathyroid hormone secretion and action. J Clin Endocrinol Metab. 1983 Jun;56(6):1319–1322. doi: 10.1210/jcem-56-6-1319. [DOI] [PubMed] [Google Scholar]
- Maierhofer W. J., Gray R. W., Lemann J., Jr Phosphate deprivation increases serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int. 1984 Mar;25(3):571–575. doi: 10.1038/ki.1984.56. [DOI] [PubMed] [Google Scholar]
- Manolagas S. C., Deftos L. J. No diurnal variations in calcitonin and vitamin D2. N Engl J Med. 1985 Jan 10;312(2):122–123. doi: 10.1056/NEJM198501103120218. [DOI] [PubMed] [Google Scholar]
- Markowitz M. E., Rosen J. F., Laxminarayan S., Mizruchi M. Circadian rhythms of blood minerals during adolescence. Pediatr Res. 1984 May;18(5):456–462. doi: 10.1203/00006450-198405000-00013. [DOI] [PubMed] [Google Scholar]
- Markowitz M., Rotkin L., Rosen J. F. Circadian rhythms of blood minerals in humans. Science. 1981 Aug 7;213(4508):672–674. doi: 10.1126/science.7256269. [DOI] [PubMed] [Google Scholar]
- Midgett R. J., Spielvogel A. M., Coburn J. W., Norman A. W. Studies on calciferol metabolism. VII. The renal production of the biologically active form of vitamin D, 1,25-dihydroxycholecalciferol; species, tissue and subcellular distribution. J Clin Endocrinol Metab. 1973 Jun;36(6):1153–1161. doi: 10.1210/jcem-36-6-1153. [DOI] [PubMed] [Google Scholar]
- Ollayos R. W., Winkler A. W. URINARY EXCRETION AND SERUM CONCENTRATION OF INORGANIC PHOSPHATE IN MAN. J Clin Invest. 1943 Mar;22(2):147–154. doi: 10.1172/JCI101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portale A. A., Booth B. E., Halloran B. P., Morris R. C., Jr Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J Clin Invest. 1984 Jun;73(6):1580–1589. doi: 10.1172/JCI111365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portale A. A., Halloran B. P., Murphy M. M., Morris R. C., Jr Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986 Jan;77(1):7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANBURY S. W. Some aspects of disordered renal tubular function. Adv Intern Med. 1958;9:231–282. [PubMed] [Google Scholar]
- Shepard R. M., Horst R. L., Hamstra A. J., DeLuca H. F. Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J. 1979 Jul 15;182(1):55–69. doi: 10.1042/bj1820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIT J. F., LITTLE B., TAIT S. A., FLOOD C. The metabolic clearance rate of aldosterone in pregnant and nonpregnant subjects estimated by both single-injection and constant-infusion methods. J Clin Invest. 1962 Dec;41:2093–2100. doi: 10.1172/JCI104667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIT J. F. REVIEW: THE USE OF ISOTOPIC STEROIDS FOR THE MEASUREMENT OF PRODUCTION RATES IN VIVO. J Clin Endocrinol Metab. 1963 Dec;23:1285–1297. doi: 10.1210/jcem-23-12-1285. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Rat renal 25-hydroxyvitamin D3 1- and 24-hydroxylases: their in vivo regulation. Am J Physiol. 1984 Feb;246(2 Pt 1):E168–E173. doi: 10.1152/ajpendo.1984.246.2.E168. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Trechsel U., Bonjour J. P., Fleisch H. Regulation of the metabolism of 25-hydroxyvitamin D3 in primary cultures of chick kidney cells. J Clin Invest. 1979 Jul;64(1):206–217. doi: 10.1172/JCI109441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E. Method for characterization of 24-h temporal variation of blood components. Am J Physiol. 1979 Sep;237(3):E255–E264. doi: 10.1152/ajpendo.1979.237.3.E255. [DOI] [PubMed] [Google Scholar]
- Van Den Berg C. J., Kumar R., Wilson D. M., Heath H., 3rd, Smith L. H. Orthophosphate therapy decreases urinary calcium excretion and serum 1,25-dihydroxyvitamin D concentrations in idiopathic hypercalciuria. J Clin Endocrinol Metab. 1980 Nov;51(5):998–1001. doi: 10.1210/jcem-51-5-998. [DOI] [PubMed] [Google Scholar]
- WESSON L. G., Jr ELECTROLYTE EXCRETION IN RELATION TO DIURNAL CYCLES OF RENAL FUNCTION. Medicine (Baltimore) 1964 Sep;43:547–592. doi: 10.1097/00005792-196409000-00002. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]


