Abstract
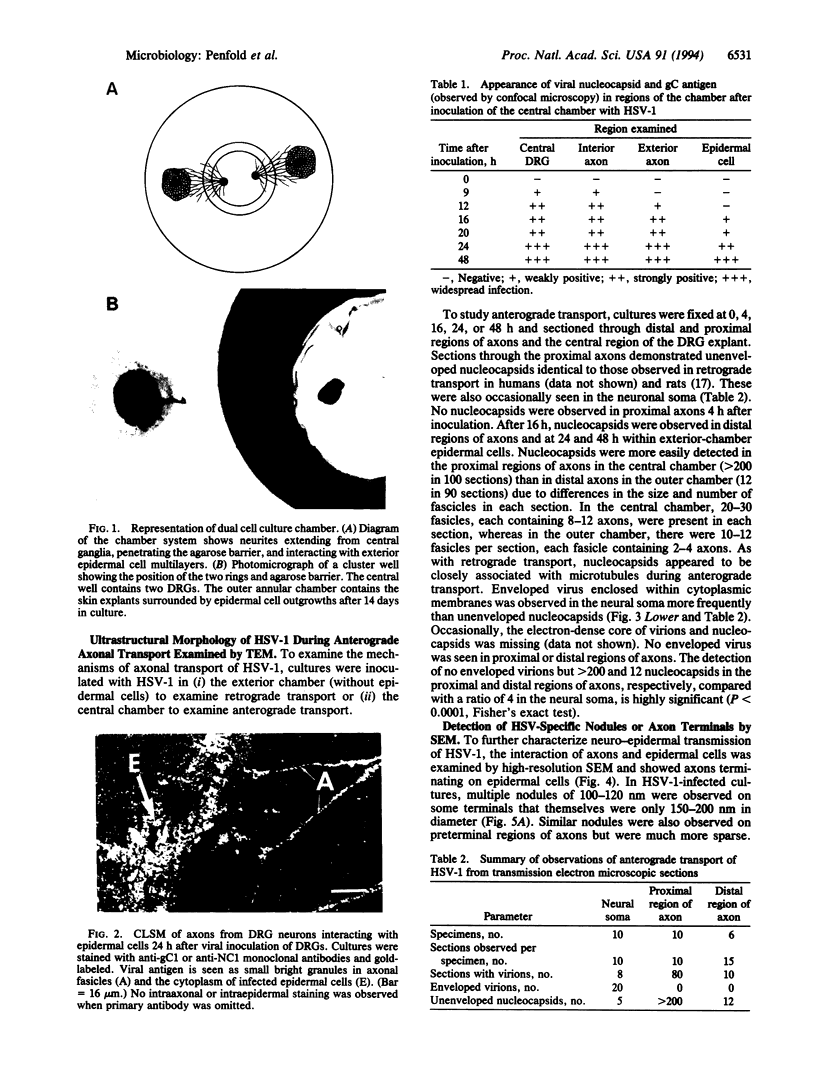
To examine the transmission of herpes simplex virus (HSV) from axon to epidermal cell, an in vitro model was constructed consisting of human fetal dorsal root ganglia cultured in the central chamber of a dual-chamber tissue culture system separated from autologous skin explants in an exterior chamber by concentric steel cylinders adhering to the substratum through silicon grease and agarose. Axons grew through the agarose viral diffusion barrier and terminated on epidermal cells in the exterior chamber. After inoculation of HSV onto dorsal root ganglia, anterograde axonal transport of glycoprotein and nucleocapsid antigen was observed by confocal microscopy to appear in exterior chamber axons within 12 h and in epidermal cells within 16 h, moving at 2-3 mm/h. Although both enveloped and unenveloped nucleocapsids were observed in the neuronal soma by transmission electron microscopy, only nucleocapsids were observed in the axons, closely associated with microtubules. Nodule formation at the surface of HSV-infected axons, becoming more dense at the axon terminus on epidermal cells, and patches of axolemmal HSV glycoprotein D expression were observed by scanning (immuno)electron microscopy, probably representing virus emerging from the axolemma. These findings strongly suggest a specialized mode of viral transport, assembly, and egress in sensory neurons: microtubule-associated intermediate-fast anterograde axonal transport of unenveloped nucleocapsids with separate transport of glycoproteins to the distal regions of the axon and assembly prior to virus emergence at the axon terminus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines J. D., Ward P. L., Campadelli-Fiume G., Roizman B. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J Virol. 1991 Dec;65(12):6414–6424. doi: 10.1128/jvi.65.12.6414-6424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Farabegoli F., Di Gaeta S., Roizman B. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J Virol. 1991 Mar;65(3):1589–1595. doi: 10.1128/jvi.65.3.1589-1595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot R. B. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Diggelmann H., Lawrence W. C., Vernon S. K., Eisenberg R. J. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980 May;34(2):521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco E., Marchisio P. C., Bondanza S., Franzi A. T., Cancedda R., De Luca M. Growth-regulated synthesis and secretion of biologically active nerve growth factor by human keratinocytes. J Biol Chem. 1991 Nov 15;266(32):21718–21722. [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970 Feb;104(2):289–295. [PubMed] [Google Scholar]
- Fraser N. W., Valyi-Nagy T. Viral, neuronal and immune factors which may influence herpes simplex virus (HSV) latency and reactivation. Microb Pathog. 1993 Aug;15(2):83–91. doi: 10.1006/mpat.1993.1059. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Roome A. P. Intra-axonal location of herpes simplex virus particles. J Gen Virol. 1972 Jun;15(3):233–235. doi: 10.1099/0022-1317-15-3-253. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsky L. A., Stevens C. E., Holmes K. K., Ashley R. L., Kiviat N. B., Critchlow C. W., Corey L. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N Engl J Med. 1992 Jun 4;326(23):1533–1539. doi: 10.1056/NEJM199206043262305. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Lycke E., Röyttä M., Svennerholm B., Vahlne A. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow [nocodazole, taxol and erythro-9-3-(2-hydroxynonyl)adenine]. J Gen Virol. 1986 Sep;67(Pt 9):2023–2028. doi: 10.1099/0022-1317-67-9-2023. [DOI] [PubMed] [Google Scholar]
- Lycke E., Hamark B., Johansson M., Krotochwil A., Lycke J., Svennerholm B. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch Virol. 1988;101(1-2):87–104. doi: 10.1007/BF01314654. [DOI] [PubMed] [Google Scholar]
- Lycke E., Kristensson K., Svennerholm B., Vahlne A., Ziegler R. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J Gen Virol. 1984 Jan;65(Pt 1):55–64. doi: 10.1099/0022-1317-65-1-55. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold M. E., Armati P., Cunningham A. L. Fetal skin development in vitro. In Vitro Cell Dev Biol. 1992 Apr;28A(4):223–226. doi: 10.1007/BF02634235. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Rasmussen L. E., Pollard R. B., Arvin A., Merigan T. C. Cellular immunity and herpesvirus infections in cardiac-transplant patients. N Engl J Med. 1977 Jun 16;296(24):1372–1377. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Addison C., McLauchlan J. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J Gen Virol. 1992 Feb;73(Pt 2):277–284. doi: 10.1099/0022-1317-73-2-277. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R. Herpesvirus latency and recurrence. Prog Med Virol. 1986;33:61–77. [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Mitchison T., Steuer E., Reese T. S., Sheetz M. P. Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell. 1985 Dec;43(3 Pt 2):623–632. doi: 10.1016/0092-8674(85)90234-x. [DOI] [PubMed] [Google Scholar]