Abstract
OBJECTIVE
The purpose of this study was to assess the diagnostic performance of supplemental screening molecular breast imaging (MBI) in women with mammographically dense breasts after system modifications to permit radiation dose reduction.
SUBJECTS AND METHODS
A total of 1651 asymptomatic women with mammographically dense breasts on prior mammography underwent screening mammography and adjunct MBI performed with 300-MBq 99mTc-sestamibi and a direct-conversion (cadmium zinc telluride) gamma camera, both interpreted independently. The cancer detection rate, sensitivity, specificity, and positive predictive value of biopsies performed (PPV3) were determined.
RESULTS
In 1585 participants with a complete reference standard, 21 were diagnosed with cancer: two detected by mammography only, 14 by MBI only, three by both modalities, and two by neither. Of 14 participants with cancers detected only by MBI, 11 had invasive disease (median size, 0.9 cm; range, 0.5–4.1 cm). Nine of 11 (82%) were node negative, and two had bilateral cancers. With the addition of MBI to mammography, the overall cancer detection rate (per 1000 screened) increased from 3.2 to 12.0 (p < 0.001) (supplemental yield 8.8). The invasive cancer detection rate increased from 1.9 to 8.8 (p < 0.001) (supplemental yield 6.9), a relative increase of 363%, while the change in DCIS detection was not statistically significant (from 1.3 to 3.2, p =0.250). For mammography alone, sensitivity was 24%; specificity, 89%; and PPV3, 25%. For the combination, sensitivity was 91% (p < 0.001); specificity, 83% (p < 0.001); and PPV3, 28% (p = 0.70). The recall rate increased from 11.0% with mammography alone to 17.6% (p < 0.001) for the combination; the biopsy rate increased from 1.3% for mammography alone to 4.2% (p < 0.001).
CONCLUSION
When added to screening mammography, MBI performed using a radiopharmaceutical activity acceptable for screening (effective dose 2.4 mSv) yielded a supplemental cancer detection rate of 8.8 per 1000 women with mammographically dense breasts.
Keywords: 99mTc-sestamibi, breast cancer, mammographic density, molecular breast imaging, supplemental screening
The performance of digital screening mammography is reduced in women with mammographically dense breasts [1], who constitute approximately 50% of the screening-aged population [2]. Breast density inform legislation, which mandates that women undergoing mammography be informed that they may benefit from supplemental screening if they have mammographically dense breasts, has passed in 19 states and is under review in 4 additional states and at the federal level. This legislation has fueled demand for supplemental breast cancer screening despite the absence of consensus about the best imaging technique and the balance of benefits and harms [3, 4].
The addition of handheld whole-breast screening ultrasound to screening mammography significantly increased the breast cancer detection rate (yield per 1000 screened) in women with mammographically dense breasts by 3.5 in single-center studies and by 4.2–4.4 in multicenter trials [5–7]. However, operator dependence and high false-positive rates associated with screening ultrasound are persistent concerns [3, 8, 9].
In two prospective European multicenter trials evaluating the addition of breast tomosynthesis to digital screening mammography, the supplemental cancer detection rate was 1.9–2.8 [10, 11]. Although neither study population was restricted to women with mammographically dense breasts, one study reported that the supplemental cancer detection rates were similar in the low- versus high-density subgroups (2.8 vs 2.5, p = 0.85) [11, 12].
Like mammography, ultrasound and tomosynthesis are anatomic imaging techniques relying on morphologic differences to distinguish normal from malignant findings. These differences can be more difficult to discern in the dense breast, likely accounting for the small incremental gain in cancer detection with adjunct anatomic techniques. Gadolinium-enhanced MRI provides an anatomic and functional image. The addition of MRI to mammography in women with mammographically dense breasts yields a supplemental cancer detection rate of 11 in women at average risk for breast cancer and 18 in women at increased risk [7]. However, the high additional recall rate (9.0–22.7%), reluctance to undergo MRI, and cost limit feasibility of screening MRI beyond the high-risk population [7, 9, 13, 14].
Dedicated gamma camera imaging provides a functional breast image based on preferential uptake of a radiopharmaceutical (such as 99mTc-sestamibi) in tumors relative to normal tissue, independent of breast density [15, 16]. Unlike older-generation scintillating gamma cameras, known as scintimammography or breast-specific gamma imaging, molecular breast imaging (MBI) directly converts gamma-ray energy to electronic signal through solid-state cadmium zinc telluride (CZT) detectors. The dual-head configuration of the MBI system increases detection of subcentimeter tumors relative to single-head systems [17]. In a pilot study to assess whether MBI-associated gains in cancer detection justified work on MBI system modifications to reduce administered radiation doses, we showed that the addition of MBI to screening mammography using a conventional 740 MBq of dispensed activity of 99mTc-sestamibi increased the cancer detection rate in women with mammographically dense breasts by 7.5 per 1000 screened [18]. After implementation of registered high-sensitivity collimation and a widened energy acceptance window, both specific to MBI, count density and diagnostic accuracy were maintained at a dispensed activity of less than 300 MBq [19–21]. In contrast, successful dose reduction with other breast-specific gamma imaging systems has not been shown [22]. Despite the advantages of gamma imaging in the dense breast, to date, there has been no prospective screening trial evaluating performance characteristics at doses within an acceptable range for screening. The purpose of this study was to evaluate prospectively the performance characteristics of screening MBI as an adjunct to screening digital mammography using a dispensed activity of 300 MBq of 99mTc-sestamibi in women with mammographically dense breasts.
Subjects and Methods
Study Population
Study participants (Table 1) included asymptomatic women presenting for routine screening mammography at the Mayo Clinic Rochester who had heterogeneously or extremely dense breasts (using the BI-RADS density categories) on their most recent mammography or were 50 years old or younger without a prior mammography [12]. Women were excluded if they were enrolled at younger than 50 years without a prior mammography and the study mammography was nondense; pregnant or lactating; had recent breast surgery or biopsy; or were undergoing therapy with tamoxifen, raloxifene, or an aromatase inhibitor.
TABLE 1.
Participant Characteristics
| Characteristic | Eligible Women (n = 1608) | Analysis Seta (n = 1585) |
|---|---|---|
|
| ||
| Mean age at enrollment (y) (range) | 58.1 (30.6–86.1) | 58.0 (30.6–86.1) |
| Race or ethnicity | ||
| White | 1577 (98.1) | 1554 (98.0) |
| Hispanic or Latina | 0 | 0 |
| Black or African American | 5 (0.3) | 5 (0.3) |
| Native Hawaiian or other Pacific Islander | 0 | 0 |
| Asian | 14 (0.9) | 14 (0.9) |
| American Indian or Alaskan Native | 3 (0.2) | 3 (0.2) |
| Unknown or data missing | 9 (0.6) | 9 (0.6) |
| Menopausal status | ||
| Premenopausal | 343 (21.3) | 340 (21.5) |
| Perimenopausal | 184 (11.4) | 183 (11.5) |
| Postmenopausal | 1007 (62.6) | 989 (62.4) |
| Surgical menopause | 74 (4.6) | 73 (4.6) |
| Mammographic breast densityb | ||
| Almost entirely fat | 0 | 0 |
| Scattered fibroglandular densities | 146 (9.1) | 143 (9.0) |
| Heterogeneously dense | 1239 (77.1) | 1221 (77.0) |
| Extremely dense | 223 (13.9) | 221 (13.9) |
| Risk factorsc | ||
| Known mutation in BRCA1 or BRCA2 genes | 2 (0.1) | 2 (0.1) |
| Chest, mediastinal, or axillary irradiationd | 5 (0.3) | 4 (0.3) |
| Personal history of breast cancer | 164 (10.2) | 158 (10.0) |
| ADH, ALH, LCIS, or atypical papilloma | 20 (1.2) | 20 (1.3) |
| Lifetime risk ≥ 20% by Gail or Claus model | 76 (4.7) | 75 (4.7) |
| 5-year Gail model risk ≥ 2.5% | 197 (12.3) | 195 (12.3) |
| 5-year Gail model risk ≥ 1.7% | 352 (21.9) | 348 (22.0) |
| One first-degree relative with history of breast cancer | 47 (2.9) | 47 (3.0) |
| Two second-degree relatives with history of breast cancer | 53 (3.3) | 53 (3.3) |
| None of the above risk factors | 692 (43.0) | 683 (43.1) |
| Mammography before study entry | ||
| < 425 days (14 mo) | 1302 (81.0) | 1287 (81.2) |
| 425–729 days (14 to < 24 mo) | 288 (17.9) | 280 (17.7) |
| 730–1094 days (24 to < 36 mo) | 14 (0.9) | 14 (0.9) |
| 1095–1459 days (36 to < 48 mo) | 1 (0.1) | 1 (0.1) |
| Nonee | 3 (0.2) | 3 (0.2) |
| MBI before study entry | ||
| < 425 days (14 mo) | 0 | 0 |
| 425–729 days (14 to < 24 mo) | 7 (0.4) | 7 (0.4) |
| 730–1094 days (24 to < 36 mo) | 31 (1.9) | 30 (1.9) |
| 1095–1459 days (36 to < 48 mo) | 69 (4.3) | 69 (4.4) |
| ≥ 1460 days (≥ 48 mo) | 68 (4.2) | 68 (4.3) |
| None | 1433 (89.1) | 1411 (89.0) |
Note—Except where otherwise indicated, data in parentheses are percentages. ADH = atypical ductal hyperplasia, ALH = atypical lobular hyperplasia, LCIS = lobular carcinoma in situ, MBI = molecular breast imaging.
Analysis set includes participants in whom both initial screening mammography and MBI studies were completed and cancer status was verified.
Eligibility determined by density assessed on previous mammography before study entry. Mammographic density reported in table refers to density assessed from study mammography.
Although participants may have had multiple risk factors, they are assigned to only one risk category according to the following prioritization: mutation in BRCA1/BRCA2; history of chest, mediastinal, or axillary irradiation; personal history of breast cancer; history of prior biopsy showing ADH, ALH, LCIS, or atypical papilloma; lifetime risk ≥ 20% by Gail or Claus model; 5-year risk ≥ 2.5% by Gail model; 5-year risk ≥ 1.7% by Gail model; at least one first-degree relative with history of breast cancer; at least two second-degree relatives with history of breast cancer.
Chest, mediastinal, or axillary irradiation was administered before age 30 years and at least 8 years before study enrollment.
A total of five women under the age of 50 years with no prior mammography were enrolled; two of five had scattered fibroglandular densities (excluded from analysis) and three of five had heterogeneously dense breasts on the study mammography.
Screening Methods
Two-view digital screening mammography (Selenia, Hologic) and two-view MBI (Discovery NM 750b, GE Healthcare, or LumaGem, Gamma Medica) were performed within 21 days of each other and were independently interpreted by different radiologists blinded to the other imaging test. Biopsies were performed after completion of both tests.
Mammography examinations were interpreted according to routine practice, with access to clinical information and prior mammography results, but readers were blinded to study participation and MBI results. A BI-RADS assessment of 0 was considered test positive; assessments of 1 and 2 were considered test negative [12].
MBI systems were composed of dual-head CZT detectors, with elements of either 1.6- or 2.5-mm pixel size and equipped with specialized registered collimation and a widened energy window (110–154 keV) [19, 20]. Imaging commenced immediately after IV injection of 300 MBq of 99mTc-sestamibi. Bilateral craniocaudal and mediolateral oblique projections were acquired under light compression for 10 minutes per view. Using a validated lexicon, MBI was interpreted in isolation by one of four breast radiologists who were blinded to mammographic and clinical information [23]. Assessments of 1–5, paralleling BI-RADS, were assigned. MBI assessments of 3, 4, or 5 were considered test positive; assessments of 1 or 2 were considered negative. In accordance with the study design, test-positive MBI findings were permitted to trigger additional diagnostic evaluation in the setting of a negative or benign mammography, but a negative or benign MBI was not permitted to change recommendations generated by test-positive mammography. The diagnostic algorithm for workup of test-positive MBI is published elsewhere [18].
Determination of Reference Standard
The positive reference standard was histopathologic diagnosis of breast cancer within 365 days of the study mammography. A secondary analysis was performed using a positive reference standard defined as histopathologic diagnosis of breast cancer within 455 days of the study mammographic imaging. A negative reference standard was determined by benign histopathology, negative or benign imaging at the next annual screening performed at least 330 days after the study mammography, or medical record review or patient interview at 1 year confirming no breast cancer diagnosis. The final histopathology reported for each participant was determined from the more severe of surgical excision or core needle biopsy results. All malignancies and atypical lesions were excised.
Statistical Analysis
The primary unit of analysis is the participant. Correct correlation of imaging assessment and pathologic lesions was determined by a radiologist. In keeping with definitions from other screening trials, cancers positive “only” on a given modality are those not detected on any other modality. Sensitivity of a modality “alone” refers to the number of cancers that would have been detected if only that modality had been used and includes some cancers that were also detected on the other modality [7]. All statistical analyses were independently verified.
For patients with verified cancer status, calculations included cancer detection rate per thousand women screened (yield); number needed to screen (inverse of yield); sensitivity; specificity; recall rate; positive predictive values (PPV1, defined as the number of malignancies per abnormal screening examinations, and PPV3, defined as the number of malignancies per total biopsies performed); and negative predictive values (NPV1 and NPV3). Using the Wilson Score method, we computed the 95% CIs for estimated proportions. Comparisons between modalities of proportions with common denominators were performed using McNemar or Fisher Exact tests when cell counts were small. Comparisons of predictive values were performed using the method of Moskowitz and Pepe [24]. All hypothesis testing and 95% CI computations were two-sided with a significance level of 0.05 (confidence level, 95%). R software, version 3.0 (R Project), was used to perform the analyses.
Results
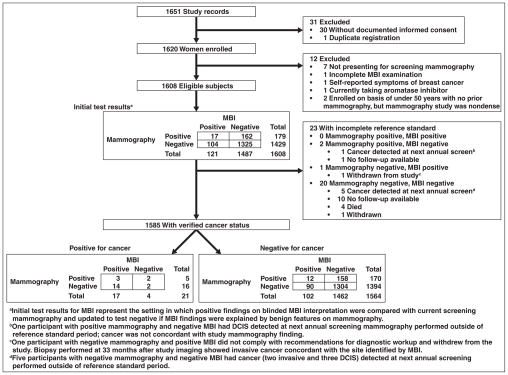
Of 1651 women enrolled between May 2009 and March 2012, 1585 met the eligibility criteria and had the complete reference standard (Fig. 1). Cancer status was verified by pathology findings in 40 (2.5%), negative imaging findings in 1523 (96.1%), clinical examination in two (0.1%), and patient interview in 20 (1.3%). In patients without a pathologic diagnosis, the reference standard was obtained no earlier than 330 days. Participant characteristics are given in Table 1.
Fig. 1.
Flowchart shows study protocol. MBI = molecular breast imaging, DCIS = ductal carcinoma in situ.
Performance characteristics of mammography alone, MBI alone, and the combination are given in Table 2. The addition of MBI increased the cancer detection rate per 1000 screened from 3.2 (95% CI, 1.3–7.4) for mammography alone to 12.0 (95% CI, 7.7–18.6) for the combination (p < 0.001), giving a supplemental yield of 8.8 (95% CI, 4.3–13.3). With adjunct MBI, sensitivity was increased from 23.8% (5/21) for mammography alone to 90.5% (19/21) for the combination (p < 0.001). MBI was more sensitive for invasive cancer (81.3%, 13/16) than mammography (18.8%, 3/16, p = 0.006). Although the specificities of mammography alone and MBI alone were similar (89.1% [1394/1564] vs 93.5% [1462/1564], p < 0.001), the specificity of the combination was 83.4% (1304/1564), less than that for mammography alone (p < 0.001).
TABLE 2.
Performance Characteristics of Mammography and Molecular Breast Imaging (MBI) at Participant Level After 365 Days of Follow-Up
| Characteristic | Mammography Alone | Mammography With Adjunct MBI | Mammography With Adjunct MBI vs Mammography Alone | MBI Alonea | MBI Alone vs Mammography Alone | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No./Total | Estimate (95% CI) | No./Total | Estimate (95% CI) | p | No./Total | Estimate (95% CI) | p | |
|
| ||||||||
| Cancer detection rate (per 1000) | ||||||||
| All densities | 5/1585 | 3.2 (1.3–7.4) | 19/1585 | 12.0 (7.7–18.6) | < 0.001 | 17/1585 | 10.7 (6.7–17.1) | 0.004 |
| Scattered fibroglandular densities | 0/143 | 0 (0–26.2) | 3/143 | 21.0 (7.2–59.9) | 0.500 | 3/143 | 21.0 (7.2–59.9) | 0.500 |
| Heterogeneously dense | 4/1221 | 3.3 (1.3–8.4) | 13/1221 | 10.6 (6.2–18.1) | 0.004 | 11/1221 | 9.0 (5.0–16.0) | 0.065 |
| Extremely dense | 1/221 | 4.5 (0.2–25.2) | 3/221 | 13.6 (4.6–39.1) | 0.500 | 3/221 | 13.6 (4.6–39.1) | 0.500 |
| No. needed to screen | 5/1585 | 317.0 (135.8–741.7) | 19/1585 | 83.4 (53.6–130.1) | < 0.001 | 17/1585 | 93.2 (58.4–149.1) | 0.004 |
| Sensitivity (%) | ||||||||
| All cancers | 5/21 | 23.8 (10.6–45.1) | 19/21 | 90.5 (71.1–97.3) | < 0.001 | 17/21 | 81.0 (60.0–92.3) | 0.004 |
| Scattered fibroglandular densities | 0/3 | 0 (0–56.1) | 3/3 | 100 (43.9–100) | 0.500 | 3/3 | 100 (43.9–100) | 0.500 |
| Heterogeneously dense | 4/14 | 28.6 (11.7–54.6) | 13/14 | 92.9 (68.5–99.6) | 0.004 | 11/14 | 78.6 (52.4–92.4) | 0.065 |
| Extremely dense | 1/4 | 25.0 (1.3–69.9) | 3/4 | 75.0 (30.1–98.7) | 0.500 | 3/4 | 75.0 (30.1–98.7) | 0.500 |
| Invasive cancers | 3/16 | 18.8 (6.6–43.0) | 14/16 | 87.5 (64.0–96.5) | < 0.001 | 13/16 | 81.3 (57.0–93.4) | 0.006 |
| DCIS | 2/5 | 40.0 (11.8–76.9) | 5/5 | 100 (56.6–100) | 0.250 | 4/5 | 80.0 (37.6–99.0) | 0.630 |
| Specificity (%) | 1394/1564 | 89.1 (87.5–90.6) | 1304/1564 | 83.4 (81.4–85.1) | < 0.001 | 1462/1564 | 93.5 (92.1–94.6) | < 0.001 |
| Recall rate (%) | 175/1585 | 11.0 (9.6–12.7) | 279/1585 | 17.6 (15.8–19.6) | < 0.001 | 119/1585 | 7.5 (6.3–8.9) | < 0.001 |
| Biopsy rate (%) | 20/1585 | 1.3 (0.8–1.9) | 67/1585 | 4.2 (3.3–5.3) | < 0.001 | 51/1585 | 3.2 (2.5–4.2) | < 0.001 |
| PPV1 (%) | 5/175 | 2.9 (1.2–6.5) | 19/279 | 6.8 (4.4–10.4) | 0.021 | 17/119 | 14.3 (9.1–21.7) | < 0.001 |
| NPV1 (%) | 1394/1410 | 98.9 (98.2–99.3) | 1304/1306 | 99.8 (99.4–100) | < 0.001 | 1462/1466 | 99.7 (99.3–99.9) | 0.002 |
| PPV3 (%) | 5/20 | 25.0 (11.2–46.9) | 19/67 | 28.4 (19.0–40.1) | 0.700 | 17/51 | 33.3 (22.0–47.0) | 0.460 |
| NPV3 (%) | 1549/1565 | 99.0 (98.3–99.4) | 1516/1518 | 99.9 (99.5–100) | < 0.001 | 1530/1534 | 99.7 (99.3–99.9) | 0.003 |
Note—DCIS = ductal carcinoma in situ, PPV1 = positive predictive value of number of malignancies per abnormal screening examination, PPV3 = PPV of number of malignancies per total biopsies performed, NPV1 = negative predictive value of number of malignancies per abnormal screening examination, NPV3 = NPV of number of malignancies per total biopsies performed.
The study was not designed to directly compare mammography alone to MBI alone. MBI alone findings represent the setting in which test-positive findings on initial blinded MBI interpretation (assessment of 3, 4, or 5) generated a comparison with current screening mammography, and patients were only recalled for diagnostic workup if findings were not explained by benign features on mammography.
Of 1585 participants, 21 were diagnosed with cancer: In two, cancer was detected only with mammography; in 14, cancer was detected only with MBI; in three, cancer was detected with both modalities; and in two, cancer was detected on neither modality (Table 3). Two participants had bilateral cancers seen only on MBI. Of the 21 cancers, 16 (76%) were invasive, of which 13 of 16 (81%) were node negative. The median size of the largest invasive cancer per participant was 0.9 cm (range, 0.3–4.1 cm; mean ± SD, 1.4 ± 1.1 cm). Of 14 MBI-only detected cancers (Figs. 2–4), 11 were invasive with a median size of 0.9 cm (range, 0.5–4.1 cm) and a mean of 1.6 ± 1.2 cm) and nine of these 11 (82%) were node negative. The two mammography-only detected cancers included one invasive cancer (0.3-cm invasive ductal carcinoma) and one ductal carcinoma in situ (0.5 cm) (Fig. 5). Of the two interval cancers detected, both multifocal node-negative grade 1 invasive lobular carcinomas, one was identified on prophylactic mastectomy performed 6 months after study imaging and the other on MRI triggered by an unrelated MBI finding.
TABLE 3.
Summary of 21 Cancers Identified in 21 Study Participants at 365 Days After Study Entry
| Histopathologic Finding | Tumor Size (cm) | Tumor Grade | TNM Category | Age (y) | Menopausal Status | Breast Density |
|---|---|---|---|---|---|---|
|
| ||||||
| Cancers found on MBI only (n = 14) | ||||||
| IDC | 4.1 | 2 | T2N2M0 | 54 | Premenopausal | Scattered fibroglandular densities |
| ILC | 3.6 | 2 | T2N1M0 | 67 | Postmenopausal | Extremely dense |
| IDC multifocal | 2.0 | 1 | T1cN0M0 | 77 | Postmenopausal | Heterogeneously dense |
| IDC | 1.9 | 2 | T1cN0M0 | 65 | Postmenopausal | Heterogeneously dense |
| IDCa | 1.4 | 1 | T1cN0M0 | 63 | Postmenopausal | Scattered fibroglandular densities |
| IDC | 0.9 | 2 | T1aN0M0 | 64 | Postmenopausal | Heterogeneously dense |
| IDCb | 0.9 | 1 | T1bN0M0 | 70 | Postmenopausal | Heterogeneously dense |
| IDC | 0.8 | 1 | T1bN0M0 | 81 | Postmenopausal | Heterogeneously dense |
| IDC multifocal | 0.7 | 3 | T1bN0M0 | 75 | Postmenopausal | Scattered fibroglandular densities |
| ILC | 0.5 | 1 | T1bN0M0 | 73 | Postmenopausal | Heterogeneously dense |
| IDC | 0.5 | 1 | T1aN0M0 | 70 | Postmenopausal | Heterogeneously dense |
| DCIS | 1.6 | Intermediate grade | T1s (DCIS) | 71 | Postmenopausal | Heterogeneously dense |
| DCIS | 0.6 | Intermediate grade | Tis (DCIS) | 64 | Postmenopausal | Heterogeneously dense |
| DCIS multifocal | 0.4 | Low grade | Tis (DCIS) | 49 | Premenopausal | Extremely dense |
| Cancers found on mammography only (n = 2) | ||||||
| IDC | 0.3 | 2 | T1aN0M0 | 49 | Premenopausal | Heterogeneously dense |
| DCIS | 0.5 | Intermediate grade | Tis (DCIS) | 62 | Postmenopausal | Heterogeneously dense |
| Cancers found on both MBI and mammography (n = 3) | ||||||
| IDC | 2.2 | 3 | T2N0M0 | 64 | Postmenopausal | Heterogeneously dense |
| IDC multifocal | 1.6 | 1 | T1cN1M0 | 53 | Perimenopausal | Extremely dense |
| DCIS with microinvasion | 2.0 | High grade | Tis (DCIS) | 74 | Postmenopausal | Heterogeneously dense |
| Cancers found on neither MBI nor mammography | ||||||
| (interval cancers) (n = 2) | ||||||
| ILC multifocal | 0.7 | 1 | T1bN0M0 | 45 | Premenopausal | Extremely dense |
| ILC multifocal | 0.6 | 1 | T1bN0M0 | 69 | Postmenopausal | Heterogeneously dense |
Note—MBI = molecular breast imaging, IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma in situ, DCIS = ductal carcinoma in situ.
In addition to the invasive cancer detected only on MBI, a mammographically occult 5.0-cm high-grade DCIS was detected by MBI in the contralateral breast.
In addition to the invasive cancer detected only on MBI, a mammographically occult 0.8-cm low-grade DCIS was detected by MBI in the contralateral breast.
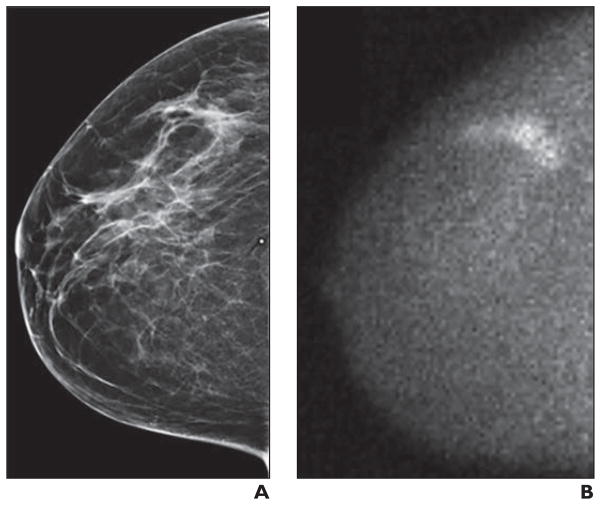
Fig. 2.
Mammographically occult invasive lobular carcinoma in 54-year-old woman detected by molecular breast imaging (MBI).
A, Right craniocaudal image from digital screening mammography was interpreted as negative.
B, Adjunct craniocaudal MBI image was interpreted as multifocal area of marked radiotracer uptake in upper outer right breast. Final pathology was multifocal node-negative grade I invasive lobular carcinoma more than 4 cm in extent on MBI, with largest individual mass being 0.6 cm.
Fig. 4.
Small mammographically occult invasive ductal carcinoma detected by molecular breast imaging (MBI) in 70-year-old woman.
A, Left mediolateral oblique image from digital screening mammography was interpreted as negative.
B, Adjunct left mediolateral oblique image from MBI was interpreted as focal area of mild radiotracer uptake in upper outer left breast (arrow). Final pathology was node-negative 0.5-cm grade I invasive ductal carcinoma.
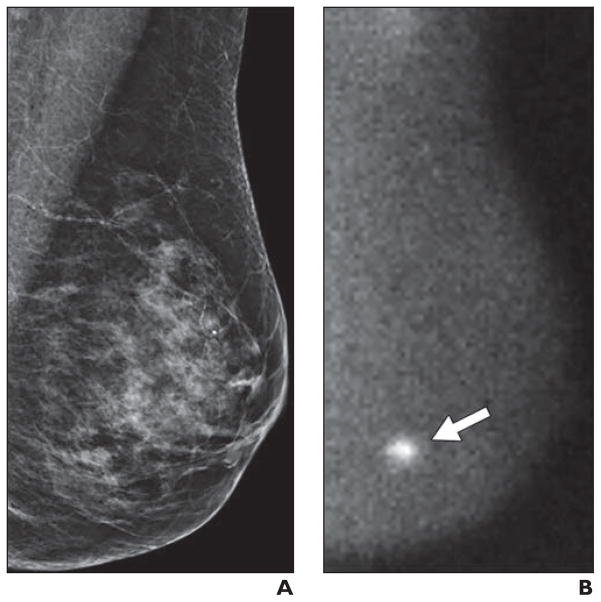
Fig. 5.
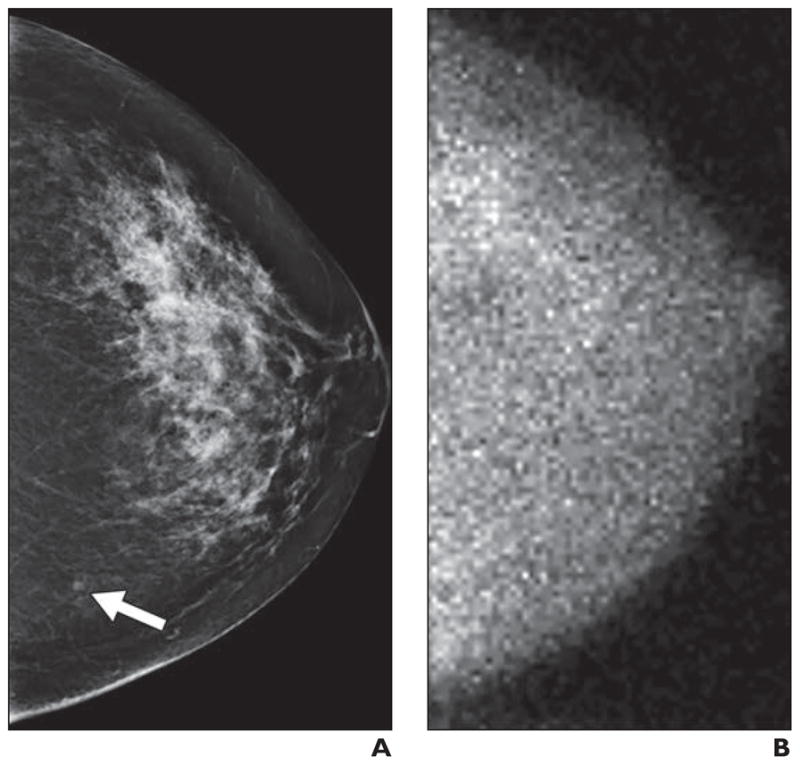
Small molecular breast imaging (MBI)-occult invasive ductal carcinoma detected by mammography in 49-year-old woman.
A, Left craniocaudal image from digital screening mammography was interpreted as showing focal asymmetry in lower inner left breast (arrow) that was less conspicuous on diagnostic evaluation and was recommended for short-interval follow-up in 6 months, at which time biopsy was performed.
B, Adjunct craniocaudal image from MBI obtained at time of screening mammography was interpreted as negative. Final pathology was 0.3-cm grade II invasive ductal carcinoma.
The addition of MBI increased the recall rate from 11.0% (175/1585) with mammography alone to 17.6% (279/1585, p < 0.001) for the combination. The addition of MBI increased the biopsy rate from 1.3% (20/1585) for mammography alone to 4.2% (67/1585, p < 0.001) for the combination. PPV1 was increased from 2.9% (5/175) for mammography alone to 6.8% (19/279) for mammography with adjunct MBI (p = 0.021), whereas the PPV3 for mammography alone (25.0%, 5/20) and mammography with adjunct MBI (28.4%, 19/67) were not statistically different (p = 0.70). Secondary analyses in 1589 patients with the 455-day reference standard are presented in Tables S1 and S2 (which can be seen in the AJR electronic supplement to this article at www.ajronline.org).
Discussion
The supplemental cancer detection rate of MBI was 8.8 cancers per 1000 women, which compares favorably with ultrasound and tomosynthesis. In studies of whole-breast ultrasound, the supplemental cancer detection rate was 3.5–4.4 per 1000 [5–7]. However, operator dependence and high false-positive rates associated with screening ultrasound are persistent concerns [3, 8, 9]. In two prospective multicenter trials evaluating the addition of breast tomosynthesis to digital screening mammography, the supplemental cancer detection rate was 1.9–2.8 [10, 11]. Although neither study population was restricted to women with mammographically dense breasts, one study reported similar cancer detection rates in the low- versus high-density subgroups (2.8 vs 2.5, p = 0.85) [11]. Recent U.S. studies comparing tomosynthesis to digital mammography failed to show a statistically significant increase in the cancer detection rate [25, 26].
The addition of MBI to mammography was more sensitive than mammography alone in detecting cancer (90.5% vs 23.8%, p < 0.001). The absolute increase in sensitivity with supplemental MBI was 67%, compared with 39% (34/36 vs 20/36) for ultrasound; 34% for tomosynthesis (8/8 vs 5/8); and 56% (16/16 vs 7/16) for MRI found in similarly designed studies [7, 11].
Approximately 80% of cancers seen only on MBI were invasive, suggesting that MBI is not selectively detecting clinically unimportant cancers (overdiagnosis). Eighty-two percent (9/11) of the invasive cancers seen only on MBI were node negative, suggesting that MBI contributes to early detection of clinically important cancers. MBI also detected larger and node-positive invasive cancers that were mammographically occult, suggesting a role for MBI in identifying cancers masked on repeated mammographic screenings by dense breast parenchyma. The two cancers detected only on mammography were small (< 5 mm). The interval cancers were likely occult because of the diffuse (nonfocal) pattern of involvement.
One primary concern about supplemental screening is the risk of false-positive findings and unnecessary biopsies [9, 27]. The addition of MBI to mammography raised the recall rate by 6.6%, which compares favorably with the additional recall rates for supplemental handheld ultrasound (15.1%) and MRI (9.0–22.7%) [7]. The addition of MBI to mammography raised the biopsy rate by 2.9%, which also compares favorably with the additional biopsy rates for handheld ultrasound (7.8%) and MRI (8.0%) [7]. PPV1 was significantly higher for MBI and for the combination of MBI and mammography compared with mammography alone. Despite the additional biopsies prompted by MBI, PPV3 was not reduced relative to mammography alone, in contrast to the reduced PPV3 seen with the addition of ultrasound [7]. As has been observed with mammography, ultrasound, and MRI, the specificity of MBI would likely increase with incidence screening when prior MBI studies would be available for comparison [7, 28]. In contrast to MBI, ultrasound, and MRI, tomosynthesis has been found to reduce the rate of recall, although no reduction in biopsy rate has yet been reported [10, 11, 25].
Prior studies of MBI and other nuclear medicine-based breast imaging have used radiation doses deemed too high for serial screening [29]. The reduced dispensed activity of 300 MBq of 99mTc-sestamibi used in this study, after system optimization for low-dose imaging, corresponds to an effective dose of 2.4 mSv. Although this is higher than the average effective dose from digital mammography (~ 0.5 mSv) and the effective dose from digital mammography combined with tomosynthesis (1.2 mSv), which is not considered excessive, this effective dose is below natural background radiation levels (U.S. annual average, 3 mSv) [29] and is well below the threshold of 50–100 mSv at which current national and international recommendations indicate that radiation risk concern is warranted [18, 30, 31]. Furthermore, the actual administered activity in this study was likely less than the target of 300 MBq because of the known adhesion of 99mTc-sestamibi to plastic syringes. On the basis of measurements reported elsewhere, we expect the administered activity was, on average, 20% less than the dispensed activity of 300 MBq, or equivalent to an average administered activity of 240 MBq (corresponding to an effective dose of 2.0 mSv) [32].
Our study has several limitations. First, the imaging study order was not randomized. However, because participants provided consent before imaging and images were interpreted by radiologists blinded to the other study, bias was unlikely. Second, we compared prevalence screen MBI with incident mammography; it is possible that the supplemental yield of MBI would be diluted on subsequent screens. Third, because eligibility was based on density on prior mammography, 9% of study mammography examinations had scattered fibroglandular densities, which may limit generalizability to populations with heterogeneously or extremely dense breasts. Fourth, because Mayo Clinic Rochester provides primary and specialty care, results may not be generalizable to all breast imaging centers. Lastly, we have not shown that detection of small, node-negative mammographically occult cancers translates into mortality reduction, an endpoint requiring years and considerable resources to investigate. We relied on surrogate endpoints of tumor size and lymph node involvement. Numerous studies have shown that tumor size correlates with mortality, with size less than 20 mm conferring a survival advantage [33, 34]. The association between lethality and tumor size is strongest in women who are lymph node–negative [35]. The median size of the 11 invasive tumors detected by MBI only was 9 mm, and nine of these 11 were node negative.
An important next step for MBI technology is a multicenter prospective randomized trial to assess comparative performance measures. MBI can be interpreted rapidly and requires minimal radiologist training to achieve high interobserver agreement and diagnostic accuracy [23]. The compact MBI machine could be readily incorporated into breast imaging practices with access to 99mTc-sestamibi. Ultimately, a multicenter, prospective randomized trial comparing MBI to other supplemental screening modalities is needed to determine which supplemental screening modality offers the best balance of benefit (supplemental detection rate of clinically important cancers) to harms (supplemental recall and biopsy rates for false-positive findings).
In contrast to supplemental screening in dense breasts with ultrasound, for which mammography contributed about half of all cancers detected [36], mammography added little increased cancer detection to MBI, and the two MBI-occult cancers detected by mammography were small and node negative. Nevertheless, mammography remains the only screening modality for which a breast cancer mortality reduction has been shown [37]. We hypothesize that a screening program of biennial MBI alternating with biennial mammography in women with dense breasts would exploit what mammography finds and find what mammography misses, preserving the cancer detection rate found in this study without the additional costs and false-positive findings associated with supplemental screening.
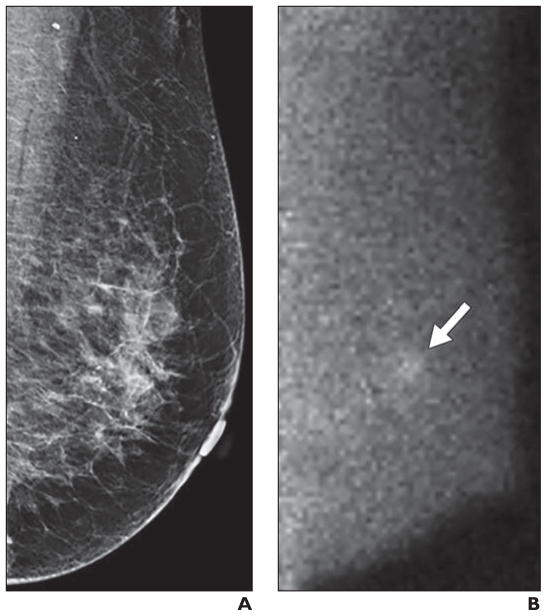
Fig. 3.
Multifocal mammographically occult invasive ductal carcinoma detected by molecular breast imaging (MBI) in 78-year-old woman.
A, Left mediolateral oblique image from digital screening mammography was interpreted as negative.
B, Adjunct left mediolateral oblique image from MBI was interpreted as focal area of marked radiotracer uptake in lower inner left breast (arrow). Final pathology was multifocal node-negative grade I invasive ductal carcinoma, with largest mass (2 cm) visualized by MBI.
Acknowledgments
This work was supported by grant KG090823 from Komen for the Cure and Clinical and Translational Science Awards (CTSA) grant UL1TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
We thank the study coordinators, Lori Johnson, Beth Connelly, Roxanne Pederson, and Tamara Hudson, and the nuclear medicine technologists, Carley Pletta, Karlie Homann, Thuy Tran, and Tiffinee Swanson, who provided essential contributions to this work. We thank Linda Miller for her role as a patient advocate. We thank Meridith Blevins for her statistical support. We thank Stephen Phillips and Dana Whaley who promoted early MBI adoption at our institution.
APPENDIX 1: AJR JOURNAL CLUB
Study Guide
Molecular Breast Imaging at Reduced Radiation Dose for Supplemental Screening in Mammographically Dense Breasts
Alan Mautz1, Joseph J. Budovec2, Margaret Mulligan2
alan.mautz@gmail.com, jbudovec@mcw.edu, mmulliga@mcw.edu*
Introduction
-
1
Why is this study being performed? Do the authors provide an appropriate rationale for performing the study? Is the study timely?
-
2
What does the study set out to prove? How would you state the null hypothesis for this study?
Methods
-
3
How were potential participants screened for inclusion in the study? What were the exclusion criteria?
-
4
What kind of study is described in this article? How did the study design attempt to reduce bias?
-
5
What are the limitations of this study? Are these limitations adequately discussed?
-
6
What statistical methods were used to analyze the data?
Results
-
7
Was the research question answered? Was the null hypothesis proven or refuted?
-
8
What measure is used to quantify the proposed benefit of adding molecular breast imaging (MBI) to screening mammography? How does this compare with other possible screening modalities?
Physics
-
9
Review briefly the mechanism of radiotracer uptake with 99mTc-sestamibi.
-
10
How do direct conversion cameras, such as the cadmium zinc telluride camera used in this study, differ from conventional scintillating gamma cameras?
Discussion
-
11
What impact to clinical practice does this study aim to have? To what extent would this study need to be validated in order for your institution or practice to adopt MBI?
-
12
How might cumulative dose increases by adding MBI to screening women with dense breasts be mitigated long-term?
-
13
Is MBI part of your clinical practice? If not, would you pursue it after reading the results of this study and those included in its references?
-
14
Are there ethical ramifications in the as yet not widespread use of MBI?
Footnotes
The Aroostook Medical Center, Presque Isle, ME
Medical College of Wisconsin, Milwaukee, WI
Please note that the authors of the Study Guide are distinct from those of the companion article.
Presented at the 2013 RSNA annual meeting, Chicago, IL.
Supplemental Data
Available online at www.ajronline.org.
References
- 1.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Breast Cancer Surveillance Consortium website. [Accessed August 6, 2014];Distribution of key variables: BI-RADS breast density. breastscreening.cancer.gov/data/variables/2011/freq_tables_pct.html#density. Published 2009.
- 3.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265:59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
- 4.Yee KM. Breast density laws may spur new screening modalities. [Accessed August 6, 2014];AuntMinnie website. www.auntminnie.com/index.aspx?sec=sup_n&sub=wom&pag=dis&ItemID=99461. Published May 24, 2012.
- 5.Corsetti V, Ferrari A, Ghirardi M, et al. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol Med (Torino) 2006;111:440–448. doi: 10.1007/s11547-006-0040-5. [DOI] [PubMed] [Google Scholar]
- 6.Tohno E, Ueno E, Watanabe H. Ultrasound screening of breast cancer. Breast Cancer. 2009;16:18–22. doi: 10.1007/s12282-008-0082-8. [DOI] [PubMed] [Google Scholar]
- 7.Berg WA, Zhang Z, Lehrer D, et al. ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–1404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Orsi CJ, Sickles EA. To seek perfection or not? That is the question. Radiology. 2012;265:9–11. doi: 10.1148/radiol.12121515. [DOI] [PubMed] [Google Scholar]
- 9.American College of Radiology website. [Accessed June 29, 2014];ACR Statement on Reporting Breast Density in Mammography Reports and Patient Summaries. www.acr.org/About-Us/Media-Center/Position-Statements/Position-Statements-Folder/Statement-on-Reporting-Breast-Density-in-Mammography-Reports-and-Patient-Summaries. Published April 24, 2012.
- 10.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267:47–56. doi: 10.1148/radiol.12121373. [DOI] [PubMed] [Google Scholar]
- 11.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14:583–589. doi: 10.1016/S1470-2045(13)70134-7. [DOI] [PubMed] [Google Scholar]
- 12.D’Orsi CI, Bassett LW, Berg WA. Breast Imaging Reporting and Data System, BI-RADS: mammography. Reston, VA: American College of Radiology; 2003. [DOI] [PubMed] [Google Scholar]
- 13.Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254:79–87. doi: 10.1148/radiol.2541090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 15.Khalkhali I, Baum JK, Villanueva-Meyer J, et al. (99m)Tc sestamibi breast imaging for the examination of patients with dense and fatty breasts: multicenter study. Radiology. 2002;222:149–155. doi: 10.1148/radiol.2221010237. [DOI] [PubMed] [Google Scholar]
- 16.Rechtman LR, Lenihan MJ, Lieberman JH, et al. Breast-specific gamma imaging for the detection of breast cancer in dense versus nondense breasts. AJR. 2014;202:293–298. doi: 10.2214/AJR.13.11585. [DOI] [PubMed] [Google Scholar]
- 17.Hruska CB, Phillips SW, Whaley DH, Rhodes DJ, O’Connor MK. Molecular breast imaging: use of a dual-head dedicated gamma camera to detect small breast tumors. AJR. 2008;191:1805–1815. doi: 10.2214/AJR.07.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes DJ, Hruska CB, Phillips SW, Whaley DH, O’Connor MK. Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts. Radiology. 2011;258:106–118. doi: 10.1148/radiol.10100625. [DOI] [PubMed] [Google Scholar]
- 19.Hruska CB, Weinmann AL, O’Connor MK. Proof of concept for low-dose molecular breast imaging with a dual-head CZT gamma camera. Part I. Evaluation in phantoms. Med Phys. 2012;39:3466–3475. doi: 10.1118/1.4718665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hruska CB, Weinmann AL, Tello Skjerseth CM, et al. Proof of concept for low-dose molecular breast imaging with a dual-head CZT gamma camera. Part II. Evaluation in patients. Med Phys. 2012;39:3476–3483. doi: 10.1118/1.4719959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinmann AL, Hruska CB, O’Connor MK. Design of optimal collimation for dedicated molecular breast imaging systems. Med Phys. 2009;36:845–856. doi: 10.1118/1.3077119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickerscheid D, Lavalaye J, Romijn L, Habraken J. Contrast-noise-ratio (CNR) analysis and optimisation of breast-specific gamma imaging (BSGI) acquisition protocols. EJNMMI Res. 2013;3:21. doi: 10.1186/2191-219X-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conners AL, Hruska CB, Tortorelli CL, et al. Lexicon for standardized interpretation of gamma camera molecular breast imaging: observer agreement and diagnostic accuracy. Eur J Nucl Med Mol Imaging. 2012;39:971–982. doi: 10.1007/s00259-011-2054-z. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz CS, Pepe MS. Comparing the predictive values of diagnostic tests: sample size and analysis for paired study designs. Clin Trials. 2006;3:272–279. doi: 10.1191/1740774506cn147oa. [DOI] [PubMed] [Google Scholar]
- 25.Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R., Jr Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR. 2013;200:1401–1408. doi: 10.2214/AJR.12.9672. [DOI] [PubMed] [Google Scholar]
- 26.Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269:694–700. doi: 10.1148/radiol.13130307. [DOI] [PubMed] [Google Scholar]
- 27.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 29.Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257:246–253. doi: 10.1148/radiol.10100570. [DOI] [PubMed] [Google Scholar]
- 30.United Nations Scientific Committee on the Effects of Atomic Radiation; Nations U, editor. Biological mechanisms of radiation actions at low doses. New York, NY: United Nations; 2012. [Google Scholar]
- 31.Hendee WR, O’Connor MK. Radiation risks of medical imaging: separating fact from fantasy. Radiology. 2012;264:312–321. doi: 10.1148/radiol.12112678. [DOI] [PubMed] [Google Scholar]
- 32.Swanson TN, Troung DT, Paulsen A, Hruska CB, O’Connor MK. Adsorption of 99mTc-sestamibi onto plastic syringes: evaluation of factors affecting the degree of adsorption and their impact on clinical studies. J Nucl Med Technol. 2013;41:247–252. doi: 10.2967/jnmt.113.132159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 34.Fisher ER, Anderson S, Redmond C, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06: 10-year pathologic and clinical prognostic discriminants. Cancer. 1993;71:2507–2514. doi: 10.1002/1097-0142(19930415)71:8<2507::aid-cncr2820710813>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Michaelson JS, Silverstein M, Sgroi D, et al. The effect of tumor size and lymph node status on breast carcinoma lethality. Cancer. 2003;98:2133–2143. doi: 10.1002/cncr.11765. [DOI] [PubMed] [Google Scholar]
- 36.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U S. Preventive Services Task Force. Screening for breast cancer: U.S Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
Background Reading
- 1.Khalkhali I, Baum JK, Villanueva-Meyer J, et al. (99m)Tc sestamibi breast imaging for the examination of patients with dense and fatty breast: multicenter study. Radiology. 2002;222:149–155. doi: 10.1148/radiol.2221010237. [DOI] [PubMed] [Google Scholar]
- 2.Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257:246–253. doi: 10.1148/radiol.10100570. [DOI] [PubMed] [Google Scholar]