Abstract
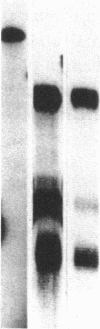
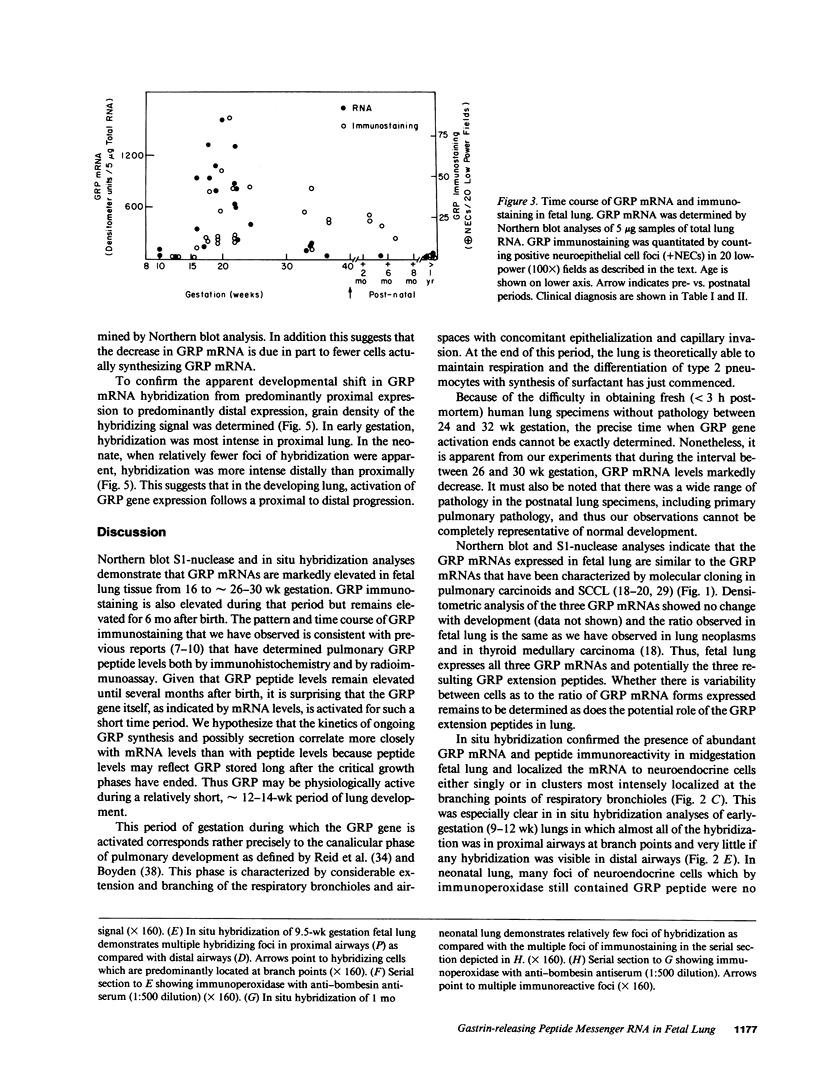
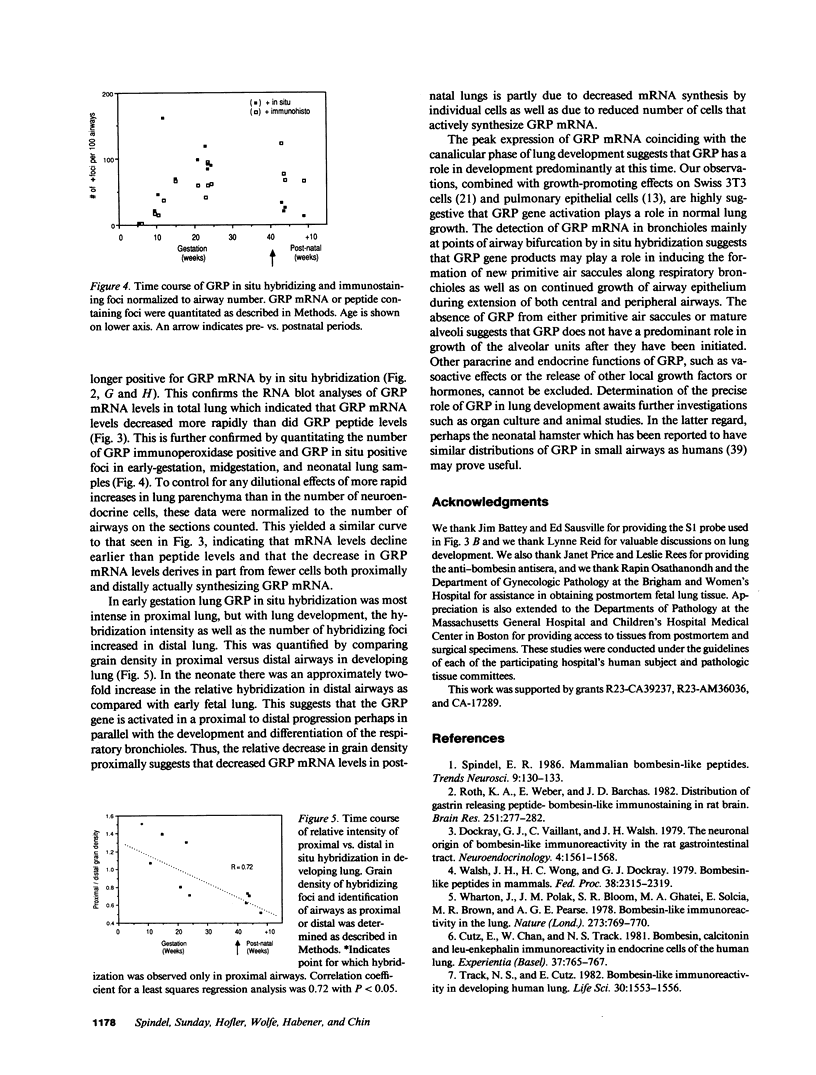
Gastrin-releasing peptide (GRP), the mammalian homologue of the amphibian peptide bombesin, is present in pulmonary neuroendocrine cells and appears to be a growth factor for both normal and neoplastic pulmonary cells. Previously we have reported the cloning of the messenger RNAs (mRNAs) and gene that encode human GRP. We now report that GRP mRNAs are markedly elevated in human fetal lung during the canalicular phase of pulmonary development (from approximately 16 to 30 wk gestation). By RNA blot and in situ hybridization analyses, GRP mRNAs were first detectable in fetal lung at 9-10 wk, plateaued at levels 25-fold higher than in adult lungs from 16 to approximately 30 wk and then declined to near adult levels by 34 wk gestation. By contrast, GRP peptide levels remain elevated until several months after birth. Consistent with this, in situ hybridization and immunohistochemical studies showed that GRP mRNA and peptide consistently colocalized in early gestation lung but that in neonatal lung, many cells that contained GRP peptide no longer contained GRP mRNA. The transient expression of high levels of GRP mRNAs during an approximately 12-wk phase of fetal lung development suggests that the secretion of GRP or its COOH-terminal peptides from pulmonary neuroendocrine cells may play a role in normal lung development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blay J., Irvine R. F., Heslop J. P., Berridge M. J. Reduction of epidermal growth factor receptor affinity by heterologous ligands: evidence for a mechanism involving the breakdown of phosphoinositides and the activation of protein kinase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):377–384. doi: 10.1016/0006-291x(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Cutz E., Chan W., Track N. S. Bombesin, calcitonin and leu-enkephalin immunoreactivity in endocrine cells of human lung. Experientia. 1981 Jul 15;37(7):765–767. doi: 10.1007/BF01967969. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Vaillant C., Walsh J. H. The neuronal origin of bombesin-like immunoreactivity in the rat gastrointestinal tract. Neuroscience. 1979;4(11):1561–1568. doi: 10.1016/0306-4522(79)90019-8. [DOI] [PubMed] [Google Scholar]
- Erisman M. D., Linnoila R. I., Hernandez O., DiAugustine R. P., Lazarus L. H. Human lung small-cell carcinoma contains bombesin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2379–2383. doi: 10.1073/pnas.79.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatei M. A., Sheppard M. N., Henzen-Logman S., Blank M. A., Polak J. M., Bloom S. R. Bombesin and vasoactive intestinal polypeptide in the developing lung: marked changes in acute respiratory distress syndrome. J Clin Endocrinol Metab. 1983 Dec;57(6):1226–1232. doi: 10.1210/jcem-57-6-1226. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Lock J. E., Elde R. P., Thompson T. R. Pulmonary neuroendocrine cells in hyaline membrane disease and bronchopulmonary dysplasia. Pediatr Res. 1982 Jun;16(6):446–454. doi: 10.1203/00006450-198206000-00009. [DOI] [PubMed] [Google Scholar]
- Lehy T., Puccio F., Chariot J., Labeille D. Stimulating effect of bombesin on the growth of gastrointestinal tract and pancreas in suckling rats. Gastroenterology. 1986 Jun;90(6):1942–1949. doi: 10.1016/0016-5085(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Wilson B. S. Specific endocrine tissue marker defined by a monoclonal antibody. Science. 1983 Nov 11;222(4624):628–630. doi: 10.1126/science.6635661. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Ghatei M. A., Bloom S. R., Adrian T. E., Mochizuki T., Yanaihara C., Yanaihara N. Dose-response comparisons of canine plasma gastroenteropancreatic hormone responses to bombesin and the porcine gastrin-releasing peptide (GRP). Regul Pept. 1983 Jan;5(2):125–137. doi: 10.1016/0167-0115(83)90120-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Gazdar A. F., Carney D. N., Minna J. D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981 Dec 11;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Price J., Penman E., Bourne G. L., Rees L. H. Characterisation of bombesin-like immunoreactivity in human fetal lung. Regul Pept. 1983 Dec;7(4):315–322. doi: 10.1016/0167-0115(83)90103-9. [DOI] [PubMed] [Google Scholar]
- Reid L. M. Lung growth in health and disease. Br J Dis Chest. 1984 Apr;78(2):113–134. [PubMed] [Google Scholar]
- Roth K. A., Weber E., Barchas J. D. Distribution of gastrin releasing peptide--bombesin-like immunostaining in rat brain. Brain Res. 1982 Nov 18;251(2):277–282. doi: 10.1016/0006-8993(82)90744-2. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausville E. A., Lebacq-Verheyden A. M., Spindel E. R., Cuttitta F., Gazdar A. F., Battey J. F. Expression of the gastrin-releasing peptide gene in human small cell lung cancer. Evidence for alternative processing resulting in three distinct mRNAs. J Biol Chem. 1986 Feb 15;261(5):2451–2457. [PubMed] [Google Scholar]
- Spindel E. R., Chin W. W., Price J., Rees L. H., Besser G. M., Habener J. F. Cloning and characterization of cDNAs encoding human gastrin-releasing peptide. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5699–5703. doi: 10.1073/pnas.81.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindel E. R., Zilberberg M. D., Chin W. W. Analysis of the gene and multiple messenger ribonucleic acids (mRNAs) encoding human gastrin-releasing peptide: alternate RNA splicing occurs in neural and endocrine tissue. Mol Endocrinol. 1987 Mar;1(3):224–232. doi: 10.1210/mend-1-3-224. [DOI] [PubMed] [Google Scholar]
- Spindel E. R., Zilberberg M. D., Habener J. F., Chin W. W. Two prohormones for gastrin-releasing peptide are encoded by two mRNAs differing by 19 nucleotides. Proc Natl Acad Sci U S A. 1986 Jan;83(1):19–23. doi: 10.1073/pnas.83.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlman M. T., Gray M. E. Ontogeny of neuroendocrine cells in human fetal lung. I. An electron microscopic study. Lab Invest. 1984 Oct;51(4):449–463. [PubMed] [Google Scholar]
- Stahlman M. T., Kasselberg A. G., Orth D. N., Gray M. E. Ontogeny of neuroendocrine cells in human fetal lung. II. An immunohistochemical study. Lab Invest. 1985 Jan;52(1):52–60. [PubMed] [Google Scholar]
- Track N. S., Cutz E. Bombesin-like immunoreactivity in developing human lung. Life Sci. 1982 May 3;30(18):1553–1556. doi: 10.1016/0024-3205(82)90243-0. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Wong H. C., Dockray G. J. Bombesin-like peptides in mammals. Fed Proc. 1979 Aug;38(9):2315–2319. [PubMed] [Google Scholar]
- Weber S., Zuckerman J. E., Bostwick D. G., Bensch K. G., Sikic B. I., Raffin T. A. Gastrin releasing peptide is a selective mitogen for small cell lung carcinoma in vitro. J Clin Invest. 1985 Jan;75(1):306–309. doi: 10.1172/JCI111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Bloom S. R., Ghatei M. A., Solcia E., Brown M. R., Pearse A. G. Bombesin-like immunoreactivity in the lung. Nature. 1978 Jun 29;273(5665):769–770. doi: 10.1038/273769a0. [DOI] [PubMed] [Google Scholar]
- Willey J. C., Lechner J. F., Harris C. C. Bombesin and the C-terminal tetradecapeptide of gastrin-releasing peptide are growth factors for normal human bronchial epithelial cells. Exp Cell Res. 1984 Jul;153(1):245–248. doi: 10.1016/0014-4827(84)90466-x. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]