Abstract
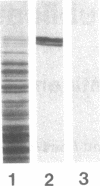
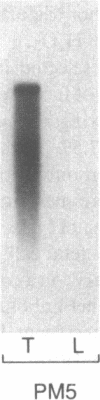
The thyroid microsomal antigen (MSA) in autoimmune thyroid disease is a protein of approximately 107 kD. We screened a human thyroid cDNA library constructed in the expression vector lambda gt11 with anti-107-kD monoclonal antibodies. Of five clones obtained, the recombinant beta-galactosidase fusion protein from one clone (PM-5) was confirmed to react with the monoclonal antiserum. The complementary DNA (cDNA) insert from PM-5 (0.8 kb) was used as a probe on Northern blot analysis to estimate the size of the mRNA coding for the MSA. The 2.9-kb messenger RNA (mRNA) species observed was the same size as that coding for human thyroid peroxidase (TPO). The probe did not bind to human liver mRNA, indicating the thyroid-specific nature of the PM-5-related mRNA. The nucleotide sequence of PM-5 (842 bp) was determined and consisted of a single open reading frame. Comparison of the nucleotide sequence of PM-5 with that presently available for pig TPO indicates 84% homology. In conclusion, a cDNA clone representing part of the microsomal antigen has been isolated. Sequence homology with porcine TPO, as well as identity in the size of the mRNA species for both the microsomal antigen and TPO, indicate that the microsomal antigen is, at least in part, TPO.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banga J. P., Pryce G., Hammond L., Roitt I. M. Structural features of the autoantigens involved in thyroid autoimmune disease: the thyroid microsomal/microvillar antigen. Mol Immunol. 1985 Jun;22(6):629–642. doi: 10.1016/0161-5890(85)90092-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Czarnocka B., Ruf J., Ferrand M., Carayon P., Lissitzky S. Purification of the human thyroid peroxidase and its identification as the microsomal antigen involved in autoimmune thyroid diseases. FEBS Lett. 1985 Oct 7;190(1):147–152. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- Hamada N., Grimm C., Mori H., DeGroot L. J. Identification of a thyroid microsomal antigen by Western blot and immunoprecipitation. J Clin Endocrinol Metab. 1985 Jul;61(1):120–128. doi: 10.1210/jcem-61-1-120. [DOI] [PubMed] [Google Scholar]
- Hirayu H., Seto P., Magnusson R. P., Filetti S., Rapoport B. Molecular cloning and partial characterization of a new autoimmune thyroid disease-related antigen. J Clin Endocrinol Metab. 1987 Mar;64(3):578–584. doi: 10.1210/jcem-64-3-578. [DOI] [PubMed] [Google Scholar]
- Kajita Y., Morgan D., Parkes A. B., Rees Smith B. Labelling and immunoprecipitation of thyroid microsomal antigen. FEBS Lett. 1985 Aug 5;187(2):334–338. doi: 10.1016/0014-5793(85)81271-0. [DOI] [PubMed] [Google Scholar]
- Magnusson R. P., Gestautas J., Seto P., Taurog A., Rapoport B. Isolation and characterization of a cDNA clone for porcine thyroid peroxidase. FEBS Lett. 1986 Nov 24;208(2):391–396. doi: 10.1016/0014-5793(86)81055-9. [DOI] [PubMed] [Google Scholar]
- Portmann L., Hamada N., Heinrich G., DeGroot L. J. Anti-thyroid peroxidase antibody in patients with autoimmune thyroid disease: possible identity with anti-microsomal antibody. J Clin Endocrinol Metab. 1985 Nov;61(5):1001–1003. doi: 10.1210/jcem-61-5-1001. [DOI] [PubMed] [Google Scholar]
- ROITT I. M., DONIACH D., CAMPBELL P. N., HUDSON R. V. Auto-antibodies in Hashimoto's disease (lymphadenoid goitre). Lancet. 1956 Oct 20;271(6947):820–821. doi: 10.1016/s0140-6736(56)92249-8. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., McGregor A. M. Autoimmune thyroid disease: developments in our understanding. Endocr Rev. 1984 Spring;5(2):309–355. doi: 10.1210/edrv-5-2-309. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]