Background: Calcium-mediated feedback to phototransduction is critical for modulating cone responses under different lighting conditions.
Results: The calcium-binding protein recoverin potentiates dim light sensitivity, whereas increasing expression of its target, GRK1, delays response shutoff in cones.
Conclusion: Recoverin and GRK1 levels modulate cone phototransduction.
Significance: Cone pigment inactivation regulates cone responses in dim light but not in bright light.
Keywords: Animal Model, Calcium-binding Protein, G Protein-coupled Receptor (GPCR), Photoreceptor, Phototransduction, Cone Photoreceptors, Light Adaptation, Recoverin, Rhodopsin Kinase
Abstract
Cone photoreceptors function under daylight conditions and are essential for color perception and vision with high temporal and spatial resolution. A remarkable feature of cones is that, unlike rods, they remain responsive in bright light. In rods, light triggers a decline in intracellular calcium, which exerts a well studied negative feedback on phototransduction that includes calcium-dependent inhibition of rhodopsin kinase (GRK1) by recoverin. Rods and cones share the same isoforms of recoverin and GRK1, and photoactivation also triggers a calcium decline in cones. However, the molecular mechanisms by which calcium exerts negative feedback on cone phototransduction through recoverin and GRK1 are not well understood. Here, we examined this question using mice expressing various levels of GRK1 or lacking recoverin. We show that although GRK1 is required for the timely inactivation of mouse cone photoresponse, gradually increasing its expression progressively delays the cone response recovery. This surprising result is in contrast with the known effect of increasing GRK1 expression in rods. Notably, the kinetics of cone responses converge and become independent of GRK1 levels for flashes activating more than ∼1% of cone pigment. Thus, mouse cone response recovery in bright light is independent of pigment phosphorylation and likely reflects the spontaneous decay of photoactivated visual pigment. We also find that recoverin potentiates the sensitivity of cones in dim light conditions but does not contribute to their capacity to function in bright light.
Introduction
Rod and cone photoreceptors utilize a G protein signal transduction cascade, called phototransduction, to signal the presence of light. Rod and cone cells express homologous or identical proteins in their phototransduction cascades. However, they exhibit important functional differences (1), fitting their respective roles as dim and bright light photoreceptors. Rods saturate even in moderately bright light and remain nonfunctional during most of the day (2). In contrast, cones have a remarkable ability to adjust their sensitivity and remain photosensitive even in extremely bright light (3) to allow us to see throughout the day. The molecular mechanisms that underlie this functional difference are still largely unknown.
Adaptation in both rods and cones is mediated by light-induced decline in their outer segment free calcium ([Ca2+]i) (4, 5). In the dark, the influx of calcium through cGMP-gated transduction channels is matched by its extrusion via rod- or cone-specific Na+/(Ca2+, K+) exchangers (6). Activation of the phototransduction cascade by light results in the closure of cGMP channels and a block of the influx of calcium. The continued calcium extrusion causes a decline in [Ca2+]i in the outer segment of the cell. This triggers the calcium-mediated negative feedback on phototransduction, which is required for the timely termination of the photoresponse and for adaptation to background light (reviewed in Ref. 7).
One mechanism by which calcium modulates phototransduction in mammalian rods involves the calcium-binding protein recoverin, a member of the EF-hand superfamily (8). At the high calcium levels present in darkness, recoverin prolongs flash responses in rods, most likely by inhibiting GRK13 (9). The resulting delayed visual pigment phosphorylation by GRK1 delays the rod flash response recovery under dim light when [Ca2+]i is high but has little effect during light adaptation when [Ca2+]i is lowered (10). The role of recoverin in modulating mammalian cone phototransduction in darkness and during light adaptation is not known. Notably, mammalian rods and cones share the same isoforms of recoverin (11, 12) and, in the case of mouse, GRK1 (13). In addition, calcium modulates the sites and extent of pigment phosphorylation in zebrafish cones but not in rods (14). Thus, recoverin and the expression level of GRK1 in cones is likely to control the rate of cone pigment inactivation. Considering that the inactivation of cone visual pigment appears to be the rate-limiting step for the shutoff of the cone photoresponse (15), the modulation of this step by recoverin and GRK1 expression could potentially exert a strong effect on the function of mammalian cones. Indeed, the lack of functional GRK1 severely delays the recovery of cone dim flash responses in both mice (13) and humans (16).
Here, we present cone physiological recordings from recoverin knock-out (Rec−/−), as well as GRK1 knock-out (GRK1−/−) and transgenic mice (GRK1+) that overexpress GRK1. We demonstrate that recoverin and GRK affect the sensitivity and response kinetics of mouse cones in darkness but not in bright background light. We conclude that pigment inactivation modulates cone function in dim light conditions but not in bright light conditions, where its spontaneous decay is likely to dominate phototransduction inactivation.
EXPERIMENTAL PROCEDURES
Animals
All experimental protocols were in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the institutional Animal Studies Committee at Washington University. Recoverin knock-out mice (Rec−/−) and guanylyl cyclase activating protein (GCAP) 1/2 knock-out mice (GCAPs−/−) have been described previously (10, 17). To facilitate single-cell suction recordings and to remove the rod component in the response of transretinal electroretinogram (ERG), all cone recordings were done from mice in rod transducin α knock-out (Gnat1−/−) background (generously provided by Janis Lem, Tufts University) (18). The generation and characterization of the GRK1-overexpressing Bac transgenic mice have been detailed elsewhere (19).
Immunostaining and Estimation of Relative Cone GRK1 Level in GRK1+ Mice
Fixed GRK1+ and WT mouse eyes were juxtaposed on the same mold side by side in series 2–4/mold, and cryosectioned (6 μm thick) and double stained with PNA and 8585 GRK1 antibody according to previously described procedures (19, 20). Confocal 12-bit 1-micron thick layered stacks were obtained and analyzed using a combination of LSM software and ImageJ. Cone outer segments were identified by their association with PNA staining. PNA-positive outer segments attributable to individual cones on cross-sections of GRK1+ and WT retinas were manually selected and examined for the GRK1 epitope pixel density using the ImageJ Measure tool. To validate the overall estimation method for obtaining relative expression levels of GRK, the nearby PNA-negative regions corresponding to rod outer segments were selected and compared between GRK+ and WT eyes. To correct for any bias, the measurements were normalized for the overall pixel density of PNA across the samples.
Electrophysiology
The function of mouse cones was evaluated by single-cell suction recordings from individual cones and by transretinal ERG recordings from isolated retinas. Mice were maintained in a 12-h light/12-h dark cycle and dark-adapted overnight prior to experiments. Following euthanasia, eyes were marked for orientation, removed under dim red light, and hemisected. The retinas were isolated under infrared light. For single-cell recordings, the M cone-rich dorsal retina was isolated, sliced with a razor blade, and stored in Locke solution at 4 °C. Recordings were done from a small piece of retina placed in a recording chamber fit to an inverted microscope. The retina was perfused with ∼37 °C Locke solution containing 112 mm NaCl, 3.6 mm KCl, 2.4 mm MgCl2, 1.2 mm CaCl2, 10 mm HEPES, 20 mm NaHCO3, 3 mm Na2-succinate, 0.5 mm sodium glutamate, 10 mm glucose, and equilibrated with 95% O2, 5% CO2, pH 7.4. Suction recordings were done by drawing the cell body of a single cone into the recording electrode as previously described (21–23). The suction electrode was filled with solution containing 140 mm NaCl, 3.6 mm KCl, 2.4 mm MgCl2, 1.2 mm CaCl2, 3 mm HEPES, 10 mm glucose, pH 7.4. Responses were amplified by a current to voltage converter (Axopatch 200B; Molecular Devices, Sunnyvale, CA), low pass filtered by an eight-pole Bessel filter (KROHN-HITE) with a cutoff frequency of 30 Hz, digitized at 1 kHz, and stored on a computer using pClamp 8.2 acquisition software (Molecular Devices).
Transretinal ERG recordings were performed from a quarter of the isolated dorsal retina, mounted on filter paper photoreceptor-side up and placed on the recording chamber with an electrode connected to the bottom. The second electrode was placed above the retina near the photoreceptor layer. To increase the stability and duration of recordings, the perfusion solution was kept at slightly lower temperature, ∼34 °C, than for single-cell recordings. The perfusion Locke solution also contained 2 mm l-glutamate to block the higher order component of the photoresponse (24). The electrode solution under the retina contained, in addition, 10 mm BaCl2 to suppress the glial component of the transretinal response (25, 26). Photoresponses were amplified by a differential amplifier (DP-311; Warner Instruments). Saturated M-cone transretinal photoresponses were obtained with white light that was long pass filtered (>410 nm, Edmund GG455).
For all recordings and for all figures, test flashes were given at t = 0. Normalized flash sensitivity, SF, was calculated as the ratio of dim flash response amplitude and flash intensity normalized by the saturated dark-adapted response for each retina. Intensity-response data were fit by the Naka-Rushton equation,
 |
where R is the transient peak amplitude of the response, Rmax is the amplitude of the saturated response, I is flash intensity, and Io is the flash intensity to generate half-maximal response.
The fractional amplitude of residual response in background illumination was fitted with Hill equation as follows,
 |
where Rmax is the maximal response amplitude in background illumination, RmaxDA is the maximal response amplitude in darkness, IB (photons μm−2 s−1) is the background light intensity, IR (photons μm−2 s−1) is the background light intensity required to reduce the maximal amplitude 2-fold, and k is the Hill coefficient.
The decline in photoreceptor sensitivity in background light was fit by the Weber-Fechner equation,
 |
where SF is photoreceptor sensitivity in background light, SFD is photoreceptor sensitivity in darkness, IB (photons μm−2 s−1) is the background light intensity, and IS (photons μm−2 s−1) is the background light intensity required to reduce sensitivity 2-fold. We used an estimated collection area of M-cones in our recording configuration of ac(500) = 0.16 μm2 for single-cell recordings, and Ac(500) = 0.12 μm2 for transretinal recordings (23). Time to peak was estimated as the time from the onset of the flash to the peak of the response from the linear range (<30% Rmax). Integration time was estimated as the integral of the dim flash response normalized to its peak amplitude, and recovery time constant was estimated as the single exponential fit to the late phase (past 50%) of the response recovery.
Statistics
For all parameters, statistical significance was determined by Student's t test with p < 0.05.
RESULTS
Recoverin Modulates the Kinetics and Sensitivity of Single-cell Cone Responses
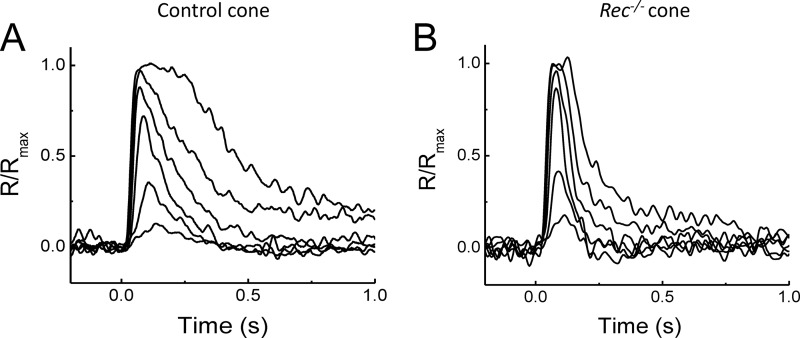
In the mouse retina, rods and cones use the same isoform of recoverin (27). This allowed us to use the recoverin knock-out mice (Rec−/−) generated previously to study the role of recoverin in rods (10, 28), to investigate how recoverin modulates cone phototransduction. To begin characterizing the role of recoverin in mammalian cone phototransduction, we first performed single-cell suction recordings to determine how its deletion affects the responses of mouse cones in dark-adapted conditions. We recorded 500-nm test flash responses from single M-cones selected from the dorsal retina in Gnat1−/− mice (control cones; Fig. 1A) and in Gnat1−/− mice also lacking recoverin (Rec−/− cones; Fig. 1B). The dark current, as measured from the amplitude of saturating flash responses, was not affected by the deletion of recoverin (Table 1). Similarly, the rising phase of the dim flash response in Rec−/− cones was not noticeably different from that in control cones (not shown), suggesting that recoverin does not modulate the activation steps or the amplification of cone phototransduction. However, the lack of recoverin accelerated the onset of response shutoff (Fig. 1, compare A and B) so that the time to peak of the dim flash response was significantly accelerated (p < 0.05) in Rec−/− cones compared with controls (Table 1). The integration time and recovery time constant also appeared to be reduced. Consistent with the acceleration of response shutoff in the absence of recoverin, the flash intensity estimated from the intensity-response curve to produce a half-saturated response (Io) increased by ∼70% (Table 1), indicating a corresponding reduction in sensitivity. However, the low signal to noise ratio of the technically challenging single-cell cone recordings did not allow us to resolve these differences statistically.
FIGURE 1.
Flash response families of single dark-adapted control (A) and Rec−/− (B) M-cones recorded with a suction electrode. Cone responses were evoked by a series of 500-nm test flashes (10 ms in duration). The test flash intensities (in photons μm−2) were 350, 1200, 4800, 1.6 × 103, 4.4 × 103, and 1.5 × 104 for the control cone and 480, 1600, 6600, 2.2 × 103, 5.9 × 103, and 2.9 × 104 for the Rec−/− cone.
TABLE 1.
Parameters of M-cone responses from single-cell suction recordings
The values are shown as means ± S.E. (n).
| Control | Rec−/− | p value | |
|---|---|---|---|
| Io (photons mm−2) | 3200 ± 560 (19) | 5300 ± 1100 (15) | 0.24 |
| Maximal response (pA) | 6.0 ± 0.7 (19) | 5.5 ± 1.2 (15) | 0.98 |
| Time to peak (ms) | 108 ± 4 (19) | 93 ± 4 (13) | 0.02 |
| Integration time (ms) | 125 ± 12 (19) | 99 ± 12 (10) | 0.12 |
| Recovery time constant (ms) | 90 ± 5 (19) | 79 ± 12 (10) | 0.30 |
Recoverin Modulates the Kinetics and Sensitivity on Transretinal Cone Responses
Although suction recording from individual photoreceptors is a very useful tool in investigating the mouse cone phototransduction cascade, the relatively short duration of these recordings limits their usefulness for longer background adaptation experiments. Thus, we switched to transretinal recordings to reinforce our single-cell results and then investigate how the deletion of recoverin affects light adaptation in mouse cones. We compared the cone responses from isolated retinas of Gnat1−/− mice (control retinas) and Gnat1−/− mice also lacking recoverin (Rec−/− retinas). Synaptic transmission was blocked by adding glutamate to the perfusion solution (see “Experimental Procedures” for details). This prevented the signal from traveling to second order neurons in the retina and enabled us to observe the complete response produced by cone photoreceptors. We stimulated with 500-nm light the dorsal part of the retina, populated predominantly with M-cones (29). This allowed us to limit our analysis to M-cones because the contribution of S-cones to the resulting photoresponses would be negligible.
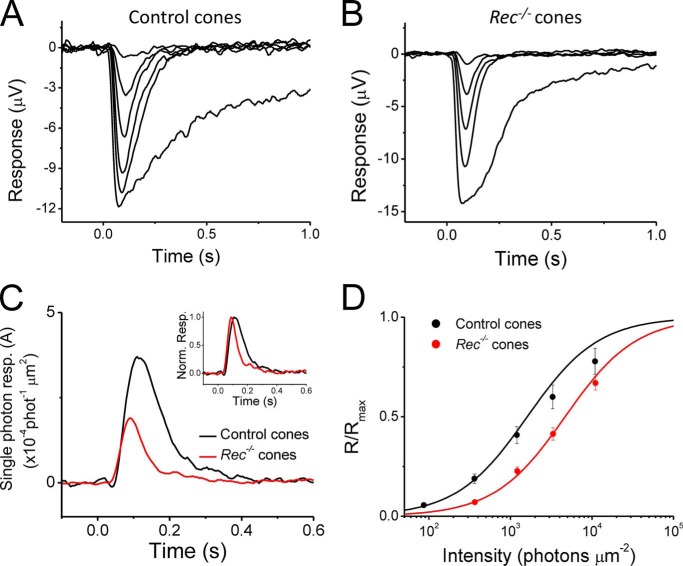
First, to check that the transretinal recordings yield results similar to these from single-cell recordings, we compared the amplitudes and waveforms of flash responses from control and Rec−/− retinas under dark-adapted conditions (Fig. 2). Consistent with the single-cell recordings (Fig. 1), we found that the deletion of recoverin accelerated the transretinal cone flash response (compare Fig. 2, A and B; see also inset of Fig. 2C and Table 2). Specifically, the cone response time to peak was significantly decreased, from 114 ± 4 ms in control retinas to 95 ± 2 ms in Rec−/− retinas (Table 2). The cone integration time was also significantly decreased by 2.2-fold, from 176 ± 21 ms in controls retinas to 81 ± 9 ms in Rec−/− retinas (Table 2). Thus, the acceleration in cone response kinetics upon the deletions of recoverin observed by transretinal ERG recordings were consistent with our single-cell recordings above. Furthermore, the estimated fractional response to a single cone pigment activation also declined from 0.32 ± 0.06% (n = 16) in control retinas to 0.16 ± 0.02% (n = 7) in Rec−/− retinas (Fig. 2C). The sensitivity of cones as measured with transretinal ERG recordings also decreased by nearly 2-fold in the absence of recoverin (Table 2). Thus, the better signal to noise ratio in the transretinal recordings allowed us to resolve the statistically significant difference in response kinetics and sensitivity between control and Rec−/− cones. Notably, the 2-fold decrease in single-photon response amplitude and sensitivity of recoverin-deficient cones is in contrast to the lack of change in these parameters observed in recoverin-deficient rods (10).
FIGURE 2.
A and B, flash response families of dark-adapted control (A) and Rec−/− M-cones (B) from transretinal ERG recordings. Cone responses were evoked by a series of 500-nm test flashes (10 ms in duration) with intensities (in photons μm−2) 87, 360, 1200, 3300, and 1.1 × 104 for control and 360, 1200, 3300, and 1.1 × 104 for the Rec−/− retina. The largest response in each case was triggered by unattenuated white flash. C, fractional single photon responses of control (black) and Rec−/− (red) cones. The cone collecting area was estimated to be 0.12 μm2 (see Equation 6 and “Experimental Procedures” for details). Inset, normalized dim flash responses of control (black) and Rec−/− (red) cones. D, intensity-response data for control (black, n = 16) and Rec/− (red, n = 7) cones, fit with the Naka-Rushton equation (Equation 1). See Table 2 for parameter values. Norm., normalized; Resp., response.
TABLE 2.
Parameters of M-cone responses from transretinal ERG recordings
The values are shown as means ± S.E. (n). SFD and IS are sensitivity in darkness and the background intensity, which gives half the flash sensitivity in Fig. 4D. IR and k are parameters obtained from the fit with Equation 2 in Fig. 4C.
| Control | Rec−/− | |
|---|---|---|
| Io (photons μm−2) | 2,300 ± 331 (16) | 4,000 ± 354 (7)a |
| Maximal response (μV) | 14.4 ± 0.7 (16) | 12.3 ± 1.0 (7) |
| Time to peak (ms) | 114 ± 4 (16) | 95 ± 2 (7)a |
| Integration time (ms) | 176 ± 21 (16) | 81 ± 9 (7)b |
| Recovery time constant (ms) | 122 ± 16 (16) | 61 ± 8 (7)b |
| SFD (photons μm−2 s−) | 3.9E-04 ± 6.7E-05 (16) | 1.9E-04 ± 1.9E-05 (7)a |
| Is (photons μm−2 s−) | 10,000 ± 27,000 (16) | 29,000 ± 5,600 (7)b |
| IR (photons μm−2 s−) | 130,000 ± 60,000 (9) | 370,000 ± 100,000 (7) |
| k (Hill coefficient) | 0.54 ± 0.03 (9) | 0.62 ± 0.04 (7) |
ap < 0.005.
b p < 0.05.
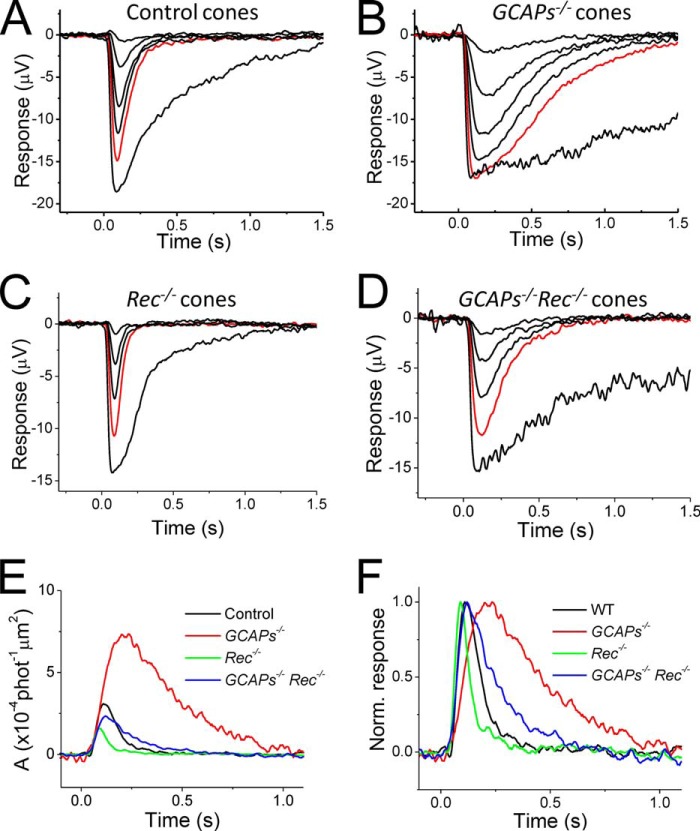
We tested whether the acceleration of response inactivation observed in Rec−/− cones is a direct cause of the lack of modulation of cone phototransduction, rather than simply a result of reduced calcium buffering in the absence of the calcium-binding recoverin, in turn modulating another calcium-dependent phototransduction reaction. In both rods (17) and cones (23), the dominant mechanism for calcium modulation of phototransduction is the synthesis of cGMP by guanylyl cyclase. Because this reaction in both rods and cones is regulated by GCAPs, we compared the effect of recoverin deletion on the single photon response amplitude and kinetics in GCAP-expressing and GCAP-deficient cones (Fig. 3, A–D), an approach used previously by Makino et al. in rods (10). Removal of the major calcium-dependent feedback by deleting GCAPs would be expected to suppress any calcium-buffering effect caused by the removal of recoverin. However, deletion of GCAPs did not block the recoverin-dependent modulation of the single photon response amplitude or kinetics (Fig. 3, E and F). We conclude that recoverin exerts its dominant effect on the function of cones by directly modulating their phototransduction cascade rather than by simple buffering of calcium.
FIGURE 3.
A–D, flash response families of dark-adapted control (A), GCAPs−/− (B), Rec−/− (C), and GCAPs−/−Rec−/− (D) M-cones from transretinal recordings. Red traces in each panel correspond to responses evoked by identical intensity of 500-nm light. E and F, fractional single photon response (E) and normalized responses (F) of control (black), Rec−/− (red), GCAPs−/− (green), and GCAPs−/−Rec−/− (blue) M-cones. For comparison, the responses of GCAP-deficient cones (B and D) are reproduced from Ref. 23, and C is reproduced from Fig. 2B.
Recoverin Modulates Cone Light Adaptation
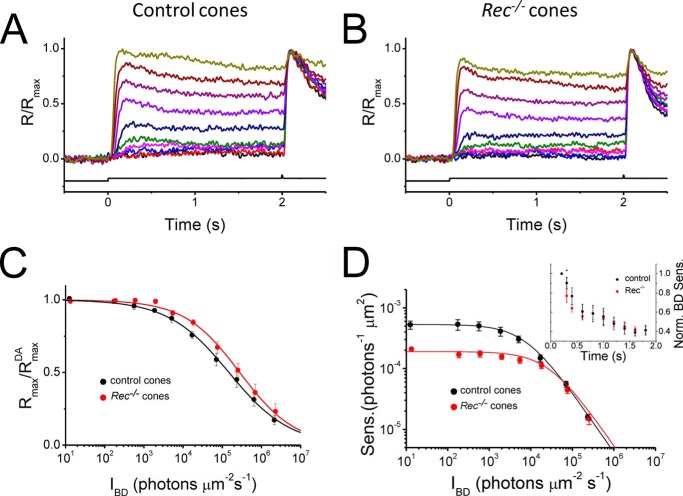
To establish whether recoverin modulates cone function in the presence of background light, we investigated how steady background light affects the residual maximal response and sensitivity of cones from control and Rec−/− retinas. In both, background lights of increasing intensity produced larger and larger step responses that consisted of an initial peak followed by partial relaxation caused by adaptation (Fig. 4, A and B). The residual maximal response was measured by saturating test flashes delivered 2 s after the onset of the background light. Through the opposing actions of response suppression by the background light and phototransduction adaptation, the maximal residual response declined gradually with increasing background light in both control and Rec−/− retinas (Fig. 4C). Using Equation 2, we found that the background intensity that reduced the maximum cone response 2-fold (IR) increased from 130,000 photons μm−2 s−1 in control retinas to 370,000 photons μm−2 s−1 in Rec−/− retinas (Table 2). Such a shift to brighter light is not surprising considering the decreased sensitivity of Rec−/− cones (Fig. 2) and does not likely reflect enhanced background adaptation in the absence of recoverin. Indeed, the rate of decline of the maximum cone response amplitude with increasing background light was comparable between controls and Rec−/− cone responses and could be described by Hill coefficients from Equation 2 of 0.54 and 0.62, respectively (Fig. 4C and Table 2). These results indicate that the operating range of mouse cones was not affected significantly by the deletion of recoverin.
FIGURE 4.
Effect of background light on cone operating range in control and Rec−/− retinas. A and B, maximal M-cone response amplitudes under a series of background lights were measured by a white test flash 2 s after the onset of 500-nm background light in control (A) and Rec−/− (B) retinas. The time course of light stimulation is shown on the bottom of each panel. The step light intensities (500-nm photons μm−2 s−1) were 13, 170, 560, 1.9 × 103, 5.1 × 103, 1.7 × 104, 7.0 × 104, 2.3 × 105, 6.4 × 105, and 2.1 × 106. C, normalized maximal M-cone responses as a function of background intensity, IBD, for control (black circles) and Rec−/− (red circles) retinas. D, averaged flash sensitivity (SF) as a function of background intensity for control (black circles) and Rec−/− (red circles) M-cones. Solid curves are best fitting Weber-Fechner functions with IS of 10,000 (control) and 29,000 (Rec−/−) photons μm−2 s−1. Inset, sensitivity change in background light in control (black, n = 6) and Rec−/− (red, n = 6) cones. Step light sensitivity, 1/IR (from Equation 2), was normalized to its peak value, as a function of time after onset of the background light. * indicates p < 0.05. Error bars show S.E. Sens., sensitivity; Norm., normalized.
We next investigated how background light affects the sensitivity of mouse cones in the absence of recoverin. In both control and Rec−/− retinas, cone sensitivity declined upon exposure to steady background light. Notably, its initial decline was detectably faster in Rec−/− cones compared with controls (Fig, 4D, inset), consistent with the removal of recoverin-mediated inhibition of cone pigment shutoff. At steady state, cone sensitivity decline for both control and recoverin-deficient cones (Fig. 4D) could be described by the Weber-Fechner relation (Equation 3). The intensity of the background light required to reduce cone sensitivity 2-fold (IS) increased from 10,000 photons μm−2 s−1 in control retinas to 29,000 photons μm−2 s−1 in Rec−/− retinas (Table 2). The effect of recoverin deletion was most pronounced at lower background lights, where it was dominated by the lower dark-adapted sensitivity of Rec−/− cones. With increasing background lights, the difference between control and recoverin-deficient retinas gradually declined, and their sensitivities became identical for intensities over 20,000 photons μm−2 s−1. This result is consistent with the expected relief of GRK1 inhibition by recoverin with declining [Ca2+]i and indicates that the modulation of cone phototransduction by recoverin is limited to dark-adapted and dim light conditions, where calcium levels are relatively high.
The Expression Level of GRK1 Modulates the Kinetics of Cone Responses
Our results above clearly demonstrate that recoverin directly modulates the mammalian cone phototransduction cascade, most likely by inhibiting the inactivation of the visual pigment by GRK1. In dark-adapted cones, the extent of modulation of this inactivation should depend on the molar ratio of recoverin and GRK1. Thus, we next sought to examine whether the expression level of GRK1 also modulates cone phototransduction. The shared expression of GRK1 in mouse rods and cones (30, 31) makes it extremely difficult to measure precisely its expression levels specifically in cones. However, previous studies have rigorously determined the overall expression of the GRK1 transgene driven by its own promoter and flanking sequences in the BAC construct in retinas of GRK1+ mice to be 3-fold higher than in wild type controls (19, 20). To confirm higher expression levels of GRK1 in GRK1+ cones as compared with wild type controls, GRK1 immunofluorescence signals in cones were compared between control and GRK1+ retinal sections. As seen in Fig. 5A, the GRK1+ retina appeared to have higher expression levels of GRK1 in both their PNA-negative and PNA-positive regions as compared with WT retina. To provide further validation of this method of comparison, we placed GRK1+ and WT cross-sections side by side on the same mold and slide so that all of the treatment and washing conditions and exposure would be identical. Analysis of confocal micrographs of the stained sections revealed higher pixel density of GRK1 staining across both PNA-negative regions, representing rod outer segments, and PNA-positive regions, representing cone outer segments of GRK1+ retina (Fig. 5B). Taken together, these results indicate that GRK1 is indeed overexpressed in the cones of GRK1+ mice. Quantitation of pixel density suggests overexpression to roughly the same extent as in their rods.
FIGURE 5.

Relative expression of GRK1 in rods and cones of WT and GRK1+ retinas. A, cryosections of photoreceptor outer segments from WT and GRK1+ retinas, juxtaposed, then fixed, and stained with cone marker PNA (green) and GRK1 antibody (red) and imaged with confocal microscope. The positions of the cones are marked by white arrows. B, relative estimate of expression levels of GRK1 epitopes in rods and cones in WT and GRK1+ mouse outer segments based on quantitative immunostaining. After double staining WT and GRK1+ sections for GRK1 and PNA, the average pixel densities (APDU) of GRK1 immunofluorescence were quantified for PNA-negative rod (R) and PNA-positive cone (C) outer segment sections using the ImageJ Select and Measure tools in confocal stacks. The GRK1+/WT ratios and statistical significance were unaffected by normalization against the PNA pixel volume and densities: rods, 2.6/1 (p < 10−12); and cones, 2.8/1 (p < 10−7). Error bars show S.E.
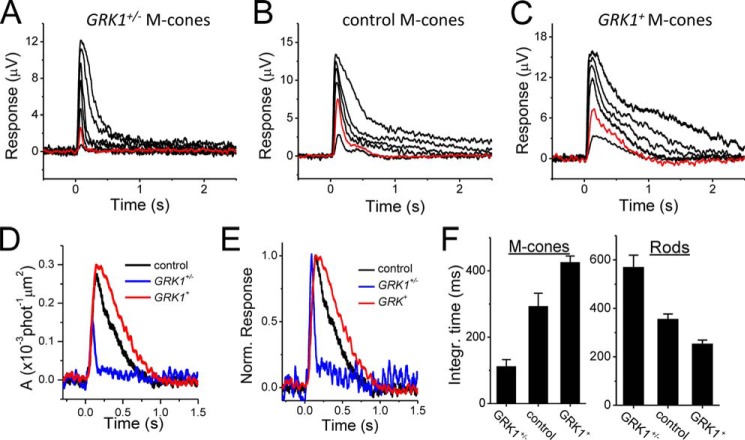
To determine the effect of GRK1 expression in cones, we compared the M-cone flash response kinetics in GRK1 heterozygous mice (GRK1+/−) that express less than half the normal amount of GRK1 (32), wild type controls, and transgenic mice overexpressing GRK1 (GRK1+). In rods, the underexpression of GRK1 in GRK1+/− mice slows down the dim flash response slightly (32, 33), whereas overexpression speeds it up (20, 34). Surprisingly, we observed an opposite trend in cones: GRK1+/− cones displayed an acceleration of the response shutoff (Fig. 6, compare A and B), whereas overexpression of GRK1 resulted in a delayed flash response in GRK1+ cones (Fig. 6C). The rising phase of the cone flash responses was identical in all three strains (Fig. 6D), indicating that the activation of the phototransduction cascade was not affected by the level of GRK1. In contrast, the deceleration of the cone response shutoff with increasing expression of GRK1 could clearly be observed by comparing the normalized dim flash responses from GRK1+/−, control, and GRK+ mice (Fig. 6E). Comparison of the integration times of cone responses from these strains revealed the same trend (Fig. 6F, left panel). In contrast, as we have shown previously, integration time decreased with increasing GRK1 expression level in mouse rods in the same retinas (Fig. 6F, right panel).
FIGURE 6.
A–C, flash response families of GRK1+/− (A), control (B), and GRK1+ (C) M-cones from transretinal recordings. Flash intensities (500-nm photons μm−2) in each panel are 630, 2300, 7400, 2.1 × 103, 5.3 × 103, and 1.9 × 105. Red traces in each panel correspond to responses evoked by identical intensity of 500-nm light. D and E, estimated single-photon responses (D) and normalized dim flash response (E) of GRK1+/− (blue), control (black), and GRK1+ (red) M-cones. F, averaged dim flash response integration time for M-cones (left) and rods (right) of GRK1+/− (n = 8), control (n = 9), and GRK1+ (n = 6) mice. For comparison, rod data in Fig. 2F are reproduced from Ref. 20. Error bars show S.E. Integr., integration; Norm., normalized; phot, photons.
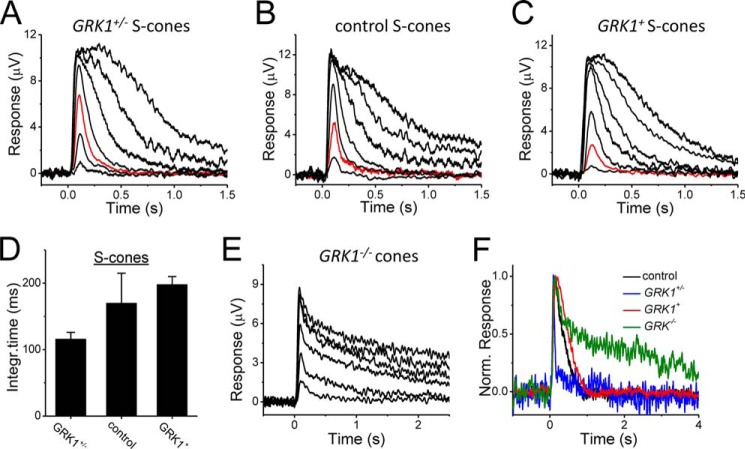
We next investigated whether this contrast with rods on the effects of GRK1 expression on the flash response kinetics extends to S-cones. To do that, we performed transretinal recordings from ventral S-cone rich mouse retina using ultraviolet (360 nm) light. Although this stimulus wavelength would not restrict the response only to S-cones, it would favor excitation of S-cones over M-cones (21). We found that, similar to the case in M-cones, increasing the expression of GRK1 slowed down the response inactivation in S-cones as well (Fig. 7, A–D). Together, these results reveal key differences between rods and cones with respect to pigment deactivation by GRK1 and arrestin binding.
FIGURE 7.
A–C, flash response families obtained from ventral retina of GRK1+/− (A), control (B), and GRK1+ (C) S-cones from transretinal recordings. Red traces in each panel indicate responses evoked by identical intensity of UV light. D, averaged dim flash response integration time for S-cones of GRK1+/− (n = 11), control (n = 7), and GRK1+ (n = 5) mice. E, cone flash response family from dorsal GRK−/− retina stimulated with 500-nm light. F, normalized dim flash responses of control (black), GRK1+/− (blue), GRK1+ (red) (all reproduced from Fig. 6), and GRK1−/− (green) M-cones. Error bars show S.E. Integr., integration; Norm., normalized.
In light of the novel finding that reducing GRK1 concentration accelerated response shutoff in M- and S-cones, we also investigated the kinetics of mouse M-cone responses in GRK1−/− mouse retina. Consistent with the results of Nikonov et al. (21), we also found that GRK1-deficient M-cones on Gnat1−/− background have pronounced slower response inactivation (Fig. 7, E and F). Thus, our results indicate that although cone dim flash response kinetics accelerate with decreasing expression of GRK1, its complete absence greatly delays cone response inactivation.
The Modulation of Cone Response Kinetics by GRK1 Expression Is Light-dependent
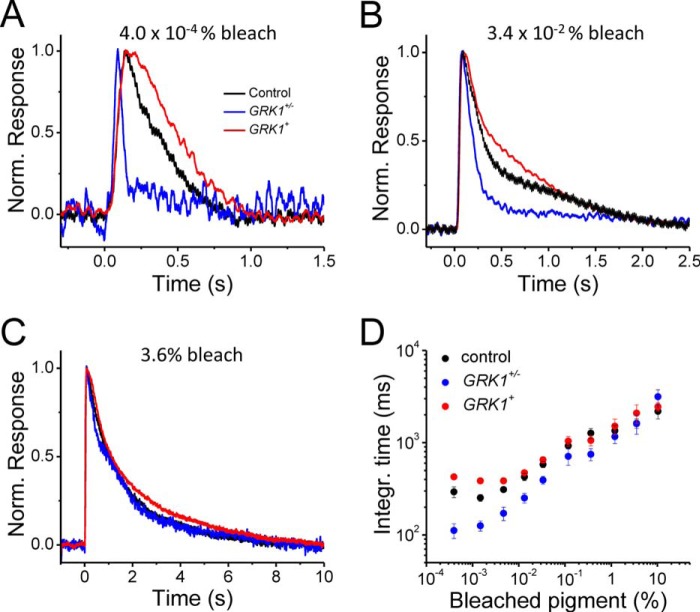
Visual pigment phosphorylation by GRK1 plays a key role in the inactivation of photoactivated pigment (35). However, the much higher expression of visual pigment compared with GRK1 in photoreceptors (100:1) (36) implies that the capacity of GRK1 to inactivate the pigment could be saturated with bright enough light. Beyond that point, the response inactivation would be dominated not by the active termination of the phototransduction cascade, but by the relatively slow decay of photoactivated visual pigment to free opsin. Thus, we investigated whether the dependence of cone flash response kinetics on the expression level of GRK1 is affected by the level of pigment activation. As described above, increasing the expression of GRK1 led to slowing down of the response inactivation for dim flashes that activate an estimated 80 pigments (0.0004% bleach) per cone (Fig. 8A). The same trend could be observed for ∼100-fold brighter flashes, activating 0.034% of the cone pigment (Fig. 8B). However, increasing the flash strength another 100-fold to 3.6% bleach resulted in responses with similar inactivation kinetics regardless of GRK1 expression level (Fig. 8C). A systematic analysis of the dependence of response integration time of flash intensity revealed that, as expected, at low light levels the expression level of GRK1 regulated the kinetics of cone response inactivation (Fig. 8D). However, response kinetics converged and became independent of GRK1 levels for 1% and brighter bleaches. Thus, the expression level of GRK1 modulated cone response inactivation only at relatively dim light intensities.
FIGURE 8.
A–C, normalized transretinal M-cone responses to flashes bleaching 0.0004% (A), 0.034% (B), and 3.6% (C) of visual pigments per cone. Traces colored in black, red, and blue in each panel represent control, GRK1+, and GRK1+/− retinas, respectively. D, integration time as a function of the fraction of bleached visual pigments per cone (%). Integration time is defined as the area under the normalized photoresponse. Error bars show S.E.
DISCUSSION
It is now well established that the mammalian rod response is regulated by both GRK1 and its calcium regulator recoverin (Refs. 10 and 33; but see also Ref. 34). However, the corresponding regulation in mammalian cones is poorly understood. Here we demonstrate that recoverin modulates the cone phototransduction cascade in darkness and in dim background light. We also show that the properties of mouse cone responses are affected by altering the expression level of GRK1 but in a direction opposite to that previously found in rods.
Recoverin Potentiates Mammalian Cone Sensitivity in Dim Light
The possible calcium-dependent modulation of mammalian cone phototransduction by recoverin has not been investigated. Studies from lower vertebrate cones and mouse rods provide some interesting insights. Fish and amphibian photoreceptors use two separate calcium modulators of rhodopsin kinase: recoverin (or S-modulin) in rods (8, 27) and visinin in cones (38). Notably, the expression of the corresponding calcium modulator of GRK is estimated to be 20 times higher in cones compared with rods (39). Furthermore, the same study found that recoverin is 2-fold more abundant than GRK1 in the outer segments of carp rods, whereas visinin is 5-fold more abundant than the corresponding level of cone opsin kinase (GRK7) in carp cones. This higher ratio of calcium regulator to GRK7 in cones has been suggested to produce a more effective inhibition of GRK in cones compared with rods. However, to date this issue has not been investigated in mammalian photoreceptors. Here, we sought to determine the role of recoverin in cone photoreceptors by investigating the function of recoverin-deficient mouse cones.
Our single-cell and transretinal recording demonstrate that in dark-adapted mouse cones, the deletion of recoverin results in acceleration of the recovery of the flash response, demonstrating a role of recoverin in modulating the mammalian cone phototransduction cascade. Thus, recoverin acts to increase the sensitivity of the cone response under dark-adapted conditions, when calcium levels are high. This result is consistent with in vitro evidence that calcium-bound recoverin inhibits GRK1 (40). By slowing cone pigment inactivation, more transducin molecules are activated, and the sensitivity is increased. This effect disappears as the calcium level drops under bright background light (Fig. 4D). This finding is qualitatively comparable with the observed effect of recoverin deletion in mouse rods (10) and is to be expected considering the shared use of the same recoverin (27) and GRK1 (30) genes in mouse rods and cones. The deletion of recoverin in mouse rods was found to accelerate the integration time of the dim flash response to 63% of its wild type value (10), whereas our transretinal recordings revealed a corresponding acceleration to 46% in mouse cones (from 176 to 81 ms; Table 2). However, one notable difference was the acceleration of the time to peak of the dim flash response in cones (Tables 1 and 2) that was not observed in rods (10). This result is consistent with a recent study from carp retina that found stronger inhibition of GRK7 in cones compared with GRK1 in rods (39) and suggests that under dim light conditions, recoverin might play a more dominant role in phototransduction inactivation in cones compared with rods.
Another notable difference between rods and cones was the effects of recoverin deletion on response amplitude and on sensitivity in dark-adapted conditions. In rods, the lack of recoverin does not affect the single photon response amplitude or flash sensitivity (10). In contrast, we find that the deletion of recoverin in cones decreases the estimated cone single photon response (Fig. 2) and cone sensitivity (Tables 1 and 2) by ∼50%. This result is consistent with amphibian studies that have shown that response shutoff is dominated by the recoverin-mediated inactivation of the visual pigment in cones but not in rods (15, 41). Removal of the stronger inhibition of GRK1 in cones would therefore accelerate the shutoff to the point of reducing the overall amplitude of the response. Our results show that by slowing pigment inactivation, recoverin boosts the sensitivity of mammalian cones in dim background light, possibly enhancing their ability to detect light near threshold.
The ability of mammalian cones to function over a wide range of light intensities and, unlike rods, remain able to respond to light is key for our daytime vision. However, the mechanisms that mediate light adaptation in mammalian cones are poorly understood. A recent study showed that the regulation of synthesis of cGMP by guanylyl cyclase in cones is regulated by GCAPs, similar to the case in rods (23). However, the calcium-mediated feedback on cGMP synthesis by GCAPs is not stronger in cones and therefore cannot account for the wider functional range of cones. Here, we examined the role of recoverin, another calcium-dependent mechanism of modulating mammalian phototransduction, in the background adaptation of cones. We found that the deletion of recoverin affected largely the sensitivity in darkness and in dim background light (Fig. 4), indicating that the inhibition of GRK1 by recoverin enhances the ability of cones to detect light near their threshold. However, the difference between control and Rec−/− cones gradually disappeared with increasing background light (Fig. 4) so that the functional range of cones in bright background light was unchanged. This result is reminiscent of the finding in rods (10) and indicates that a disparity in the strength of recoverin modulation cannot explain the differences in operating range between mammalian rods and cones.
The Expression Level of GRK1 Modulates Mammalian Cone Response Kinetics in Dim Light
Rhodopsin is phosphorylated at multiple sites by GRK1 and the timely phosphorylation of photoactivated rhodopsin and arrestin binding fine-tunes its lifetime and controls the reproducibility of the rod dim flash responses (42). The role of pigment phosphorylation in shaping the cone light response is much less understood. Studies with zebrafish (14) and carp (43) cones have shown that cone pigments can be phosphorylated in a light-dependent manner. Fish rods and cones use different isoforms of rhodopsin kinase, GRK1 and GRK7, respectively, to phosphorylate their visual pigments. The higher expression and specific activity of GRK7 compared with GRK1 (43) create the potential for much higher rate of pigment phosphorylation in cones compared with rods. In the mouse retina, GRK1 is the only kinase expressed in rods and cones (30). A study with the cone-like photoreceptors in the Nrl−/− mouse has demonstrated that mammalian cone opsin is phosphorylated upon light activation (44). The essential role of GRK1 in the deactivation of rod and cone visual pigments is demonstrated by abnormally prolonged light responses in both rods and cones in GRK1−/− animals (13, 33).
In mouse rods, arrestin-1 competes with GRK1 and hence modifies the GRK1 binding rate (32). Reducing the level of GRK1 in GRK1+/− rods delays termination of the dim flash response, suggesting that GRK1 binding is rate-limiting for rhodopsin deactivation. Consistent with this model, increasing the expression of rhodopsin kinase over a 10-fold range, from 0.3× to 3× that in wild type rods, results in gradual acceleration of the dim flash response inactivation (20). A similar result was obtained with mouse rods overexpressing by 12-fold mutant GRK1 with enhanced activity (34, 45). Contrary to these results found in rods, we show an opposite trend in cones: response termination was faster in GRK1+/− cones and increasing the expression level of GRK1 over a 10-fold range slowed down progressively cone response inactivation for both M-cones (Fig. 6) and S-cones (Fig. 7). It is interesting to note that GRK-driven deceleration in cone response recovery has also been observed in zebrafish cones overexpressing GRK7 (46). As mentioned above, rod and cone pigment inactivation involves GRK1 binding, phosphate attachment and arrestin binding. Based on the competitive nature between GRK1 and arrestin1 binding and the higher concentration of GRK in cones, the results in cones suggest that arrestin binding could be rate-limiting in cone pigment deactivation. In this model, a further increase in GRK1 concentration would impede arrestin binding, thus delaying pigment deactivation. Notably, cones express high levels of arrestin1, as well as a much lower level of arrestin4 (47), and the gradual delay in cone response inactivation with increasing expression of GRK1 is reminiscent of the cumulative effects of the deletion of arrestin1 and arrestin4 in mouse cones (48). How each isoform may participate in this process awaits future investigation.
Another possibility is that GRK1 interferes with the inactivation of another phototransduction reaction unrelated to the visual pigment. GRK1 contains RGS-like domains (49, 50) that could, for instance, interfere with the inactivation of cone transducin, thus slowing down the response termination. The higher level of GRK1 could potentially also compete with transducin and interfere with the activation of the cone phototransduction cascade. However, we did not see evidence for GRK1-dependent reduction in cone phototransduction amplification. Such competition would also not explain the GRK1-dependent delay on the response shutoff.
Finally, it is intriguing that increasing GRK1 levels in cones reduce their recoverin/GRK ratio toward its rod value (39) while at the same time tend to slow down their flash responses, also toward their rod values. The dichotomy of the effects of GRK1 expression in rods and cones reveals a level of complexity of their respective phototransduction cascades that has not been previously appreciated.
Mammalian Cone Responses to Bright Light Are Independent of the Expression of GRK1
The effect of prolongation of the cone responses by GRK1 overexpression gradually declines with increasing bleaches and the difference in response kinetics disappears for flashes activating more than ∼1% of the cone visual pigment (Fig. 8). This observation indicates that the reaction(s) modulated by the expression level of GRK1 are no longer rate-limiting for bright enough bleaches. Such light intensities would exhaust/saturate the capacity of cones to inactivate their phototransduction cascade by GRK1 and arrestin, and termination of the cone light response would likely be dominated by the spontaneous decay of cone visual pigment. Consistent with this notion, the time constant of cone response decay at 3.6% is 2.8 s, comparable with the rate of decay of photoactivated cone pigment (51). Thus, our results are consistent with the notion that the capacity of mammalian cones to inactivate the phototransduction cascade saturates at a relatively dim light, bleaching only ∼1% of their pigment. A similar idea was proposed recently based on studies of the decay of photoactivated pigment in amphibian L-cones (52). The relatively fast decay of photoactivated cone pigment (37) would result in timely response inactivation even after saturating the cone capacity to phosphorylate photoactivated pigment, thus allowing cones to function even in bright background conditions. Finally, such a mechanism could also help maintain normal cone function in bright light even in the absence of properly functioning pigment inactivation machinery. Indeed, patients with Oguchi disease, characterized with abolished GRK1 activity, have abnormally slow dim flash cone response shutoff, indicating defective cone pigment inactivation, but normal cone function in bright light (16).
Acknowledgments
We thank Janis Lem for the Gnat1−/− animals and the members of the Kefalov lab for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants EY19543 and EY19312 (to V. J. K.), EY12703 and EY12155 (to J. C.), and EY02687 (to Washington University Department Ophthalmology). This work was also supported by Research to Prevent Blindness and a grant from the Uehara Memorial Foundation, Japan (to K. S.).
- GRK
- G protein receptor kinase
- GCAP
- guanylyl cyclase activating protein
- ERG
- electroretinogram
- PNA
- peanut agglutinin.
REFERENCES
- 1. Yau K.-W. (1994) Phototransduction mechanism in retinal rods and cones: the Friedenwald Lecture. Invest. Ophthalmol. Vis. Sci. 35, 9–32 [PubMed] [Google Scholar]
- 2. Green D. G. (1971) Light adaptation in the rat retina: evidence for two receptor mechanisms. Science 174, 598–600 [DOI] [PubMed] [Google Scholar]
- 3. Boynton R. M., Whitten D. N. (1970) Visual adaptation in monkey cones: recordings of late receptor potentials. Science 170, 1423–1426 [DOI] [PubMed] [Google Scholar]
- 4. Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. (1988) Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature 334, 67–69 [DOI] [PubMed] [Google Scholar]
- 5. Nakatani K., Yau K. W. (1988) Calcium and light adaptation in retinal rods and cones. Nature 334, 69–71 [DOI] [PubMed] [Google Scholar]
- 6. Altimimi H. F., Schnetkamp P. P. (2007) Na+/Ca2+-K+ exchangers (NCKX): functional properties and physiological roles. Channels (Austin) 1, 62–69 [DOI] [PubMed] [Google Scholar]
- 7. Fain G. L., Matthews H. R., Cornwall M. C., Koutalos Y. (2001) Adaptation in vertebrate photoreceptors. Physiol. Rev. 81, 117–151 [DOI] [PubMed] [Google Scholar]
- 8. Kawamura S. (1993) Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature 362, 855–857 [DOI] [PubMed] [Google Scholar]
- 9. Palczewski K., Polans A. S., Baehr W., Ames J. B. (2000) Ca2+-binding proteins in the retina: structure, function, and the etiology of human visual diseases. Bioessays 22, 337–350 [DOI] [PubMed] [Google Scholar]
- 10. Makino C. L., Dodd R. L., Chen J., Burns M. E., Roca A., Simon M. I., Baylor D. A. (2004) Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J. Gen. Physiol. 123, 729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dizhoor A. M., Chen C. K., Olshevskaya E., Sinelnikova V. V., Phillipov P., Hurley J. B. (1993) Role of the acylated amino terminus of recoverin in Ca2+-dependent membrane interaction. Science 259, 829–832 [DOI] [PubMed] [Google Scholar]
- 12. Milam A. H., Dacey D. M., Dizhoor A. M. (1993) Recoverin immunoreactivity in mammalian cone bipolar cells. Vis. Neurosci. 10, 1–12 [DOI] [PubMed] [Google Scholar]
- 13. Lyubarsky A. L., Chen C., Simon M. I., Pugh E. N., Jr. (2000) Mice lacking G-protein receptor kinase 1 have profoundly slowed recovery of cone-driven retinal responses. J. Neurosci. 20, 2209–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kennedy M. J., Dunn F. A., Hurley J. B. (2004) Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron 41, 915–928 [DOI] [PubMed] [Google Scholar]
- 15. Matthews H. R., Sampath A. P. (2010) Photopigment quenching is Ca2+ dependent and controls response duration in salamander L-cone photoreceptors. J. Gen. Physiol. 135, 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cideciyan A. V., Zhao X., Nielsen L., Khani S. C., Jacobson S. G., Palczewski K. (1998) Null mutation in the rhodopsin kinase gene slows recovery kinetics of rod and cone phototransduction in man. Proc. Natl. Acad. Sci. U.S.A. 95, 328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mendez A., Burns M. E., Sokal I., Dizhoor A. M., Baehr W., Palczewski K., Baylor D. A., Chen J. (2001) Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 98, 9948–9953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calvert P. D., Krasnoperova N. V., Lyubarsky A. L., Isayama T., Nicoló M., Kosaras B., Wong G., Gannon K. S., Margolskee R. F., Sidman R. L., Pugh E. N., Jr., Makino C. L., Lem J. (2000) Phototransduction in transgenic mice after targeted deletion of the rod transducin α-subunit. Proc. Natl. Acad. Sci. U.S.A. 97, 13913–13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whitcomb T., Sakurai K., Brown B. M., Young J. E., Sheflin L., Dlugos C., Craft C. M., Kefalov V. J., Khani S. C. (2010) Effect of g protein-coupled receptor kinase 1 (Grk1) overexpression on rod photoreceptor cell viability. Invest. Ophthalmol. Vis. Sci. 51, 1728–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakurai K., Young J. E., Kefalov V. J., Khani S. C. (2011) Variation in rhodopsin kinase expression alters the dim flash response shut off and the light adaptation in rod photoreceptors. Invest. Ophthalmol. Vis. Sci. 52, 6793–6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nikonov S. S., Kholodenko R., Lem J., Pugh E. N., Jr. (2006) Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J. Gen. Physiol. 127, 359–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi G., Yau K. W., Chen J., Kefalov V. J. (2007) Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J. Neurosci. 27, 10084–10093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakurai K., Chen J., Kefalov V. J. (2011) Role of guanylyl cyclase modulation in mouse cone phototransduction. J. Neurosci. 31, 7991–8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sillman A. J., Ito H., Tomita T. (1969) Studies on the mass receptor potential of the isolated frog retina: I. general properties of the response. Vision Res. 9, 1435–1442 [DOI] [PubMed] [Google Scholar]
- 25. Bolnick D. A., Walter A. E., Sillman A. J. (1979) Barium suppresses slow PIII in perfused bullfrog retina. Vision Res. 19, 1117–1119 [DOI] [PubMed] [Google Scholar]
- 26. Nymark S., Heikkinen H., Haldin C., Donner K., Koskelainen A. (2005) Light responses and light adaptation in rat retinal rods at different temperatures. J. Physiol. 567, 923–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dizhoor A. M., Ray S., Kumar S., Niemi G., Spencer M., Brolley D., Walsh K. A., Philipov P. P., Hurley J. B., Stryer L. (1991) Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science 251, 915–918 [DOI] [PubMed] [Google Scholar]
- 28. Hurley J. B., Chen J. (2001) Evaluation of the contributions of recoverin and GCAPs to rod photoreceptor light adaptation and recovery to the dark state. Prog. Brain Res. 131, 395–405 [DOI] [PubMed] [Google Scholar]
- 29. Röhlich P., van Veen T., Szél A. (1994) Two different visual pigments in one retinal cone cell. Neuron 13, 1159–1166 [DOI] [PubMed] [Google Scholar]
- 30. Weiss E. R., Ducceschi M. H., Horner T. J., Li A., Craft C. M., Osawa S. (2001) Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J. Neurosci. 21, 9175–9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao X., Huang J., Khani S. C., Palczewski K. (1998) Molecular forms of human rhodopsin kinase (GRK1). J. Biol. Chem. 273, 5124–5131 [DOI] [PubMed] [Google Scholar]
- 32. Doan T., Azevedo A. W., Hurley J. B., Rieke F. (2009) Arrestin competition influences the kinetics and variability of the single-photon responses of mammalian rod photoreceptors. J. Neurosci. 29, 11867–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen C. K., Burns M. E., Spencer M., Niemi G. A., Chen J., Hurley J. B., Baylor D. A., Simon M. I. (1999) Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc. Natl. Acad. Sci. U.S.A. 96, 3718–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen C.-K., Woodruff M. L., Chen F. S., Chen Y., Cilluffo M. C., Tranchina D., Fain G. L. (2012) Modulation of mouse rod response decay by rhodopsin kinase and recoverin. J. Neurosci. 32, 15998–16006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maeda T., Imanishi Y., Palczewski K. (2003) Rhodopsin phosphorylation: 30 years later. Prog. Retin. Eye Res. 22, 417–434 [DOI] [PubMed] [Google Scholar]
- 36. Palczewski K., Buczylko J., Van Hooser P., Carr S. A., Huddleston M. J., Crabb J. W. (1992) Identification of the autophosphorylation sites in rhodopsin kinase. J. Biol. Chem. 267, 18991–18998 [PubMed] [Google Scholar]
- 37. Imai H., Imamoto Y., Yoshizawa T., Shichida Y. (1995) Difference in molecular properties between chicken green and rhodopsin as related to the functional difference between cone and rod photoreceptor cells. Biochemistry 34, 10525–10531 [DOI] [PubMed] [Google Scholar]
- 38. Kawamura S., Kuwata O., Yamada M., Matsuda S., Hisatomi O., Tokunaga F. (1996) Photoreceptor protein s26, a cone homologue of S-modulin in frog retina. J. Biol. Chem. 271, 21359–21364 [DOI] [PubMed] [Google Scholar]
- 39. Arinobu D., Tachibanaki S., Kawamura S. (2010) Larger inhibition of visual pigment kinase in cones than in rods. J. Neurochem. 115, 259–268 [DOI] [PubMed] [Google Scholar]
- 40. Kawamura S. (1999) Calcium-dependent regulation of rhodopsin phosphorylation. Novartis Found. Symp. 224, 208–218; discussion 218–224 [DOI] [PubMed] [Google Scholar]
- 41. Zang J., Matthews H. R. (2012) Origin and control of the dominant time constant of salamander cone photoreceptors. J. Gen. Physiol. 140, 219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doan T., Mendez A., Detwiler P. B., Chen J., Rieke F. (2006) Multiple phosphorylation sites confer reproducibility of the rod's single-photon responses. Science 313, 530–533 [DOI] [PubMed] [Google Scholar]
- 43. Tachibanaki S., Tsushima S., Kawamura S. (2001) Low amplification and fast visual pigment phosphorylation as mechanisms characterizing cone photoresponses. Proc. Natl. Acad. Sci. U.S.A. 98, 14044–14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu X., Brown B., Li A., Mears A. J., Swaroop A., Craft C. M. (2003) GRK1-dependent phosphorylation of S and M opsins and their binding to cone arrestin during cone phototransduction in the mouse retina. J. Neurosci. 23, 6152–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gross O. P., Pugh E. N., Jr., Burns M. E. (2012) Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron 76, 370–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vogalis F., Shiraki T., Kojima D., Wada Y., Nishiwaki Y., Jarvinen J. L., Sugiyama J., Kawakami K., Masai I., Kawamura S., Fukada Y., Lamb T. D. (2011) Ectopic expression of cone-specific G-protein-coupled receptor kinase GRK7 in zebrafish rods leads to lower photosensitivity and altered responses. J. Physiol. 589, 2321–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chan S., Rubin W. W., Mendez A., Liu X., Song X., Hanson S. M., Craft C. M., Gurevich V. V., Burns M. E., Chen J. (2007) Functional comparisons of visual arrestins in rod photoreceptors of transgenic mice. Invest. Ophthalmol. Vis. Sci. 48, 1968–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nikonov S. S., Brown B. M., Davis J. A., Zuniga F. I., Bragin A., Pugh E. N., Jr., Craft C. M. (2008) Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron 59, 462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siderovski D. P., Hessel A., Chung S., Mak T. W., Tyers M. (1996) A new family of regulators of G-protein-coupled receptors? Curr. Biol. 6, 211–212 [DOI] [PubMed] [Google Scholar]
- 50. Singh P., Wang B., Maeda T., Palczewski K., Tesmer J. J. (2008) Structures of rhodopsin kinase in different ligand states reveal key elements involved in G protein-coupled receptor kinase activation. J. Biol. Chem. 283, 14053–14062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Okada T., Matsuda T., Kandori H., Fukada Y., Yoshizawa T., Shichida Y. (1994) Circular dichroism of metaiodopsin II and its binding to transducin: a comparative study between meta II intermediates of iodopsin and rhodopsin. Biochemistry 33, 4940–4946 [DOI] [PubMed] [Google Scholar]
- 52. Estevez M. E., Kolesnikov A. V., Ala-Laurila P., Crouch R. K., Govardovskii V. I., Cornwall M. C. (2009) The 9-methyl group of retinal is essential for rapid Meta II decay and phototransduction quenching in red cones. J. Gen. Physiol. 134, 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]