Abstract
Pot experiments were conducted to evaluate the damaging effects of salinity on Sesbania sesban plants in the presence and absence of arbuscular mycorrhizal fungi (AMF). The selected morphological, physiological and biochemical parameters of S. sesban were measured. Salinity reduced growth and chlorophyll content drastically while as AMF inoculated plants improved growth. A decrease in the number of nodules, nodule weight and nitrogenase activity was also evident due to salinity stress causing reduction in nitrogen fixation and assimilation potential. AMF inoculation increased these parameters and also ameliorated the salinity stress to some extent. Antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) as well as non enzymatic antioxidants (ascorbic acid and glutathione) also exhibited great variation with salinity treatment. Salinity caused great alterations in the endogenous levels of growth hormones with abscisic acid showing increment. AMF inoculated plants maintained higher levels of growth hormones and also allayed the negative impact of salinity.
Keywords: Sesbania sesban, AMF, Salinity, Nodulation, Systemic acquired resistance
1. Introduction
As sessile organisms plants are frequently encountered by many environmental stresses including abiotic as well as biotic resulting in altered plant growth and metabolism. Salinity is one of the important abiotic environmental factors having great effects on plant growth and development (Barnawal et al., 2014). Increased industrialization and use of saline water for irrigation purposes causes conversion of fertile soils into salt affected soils making the situation much graver. It has been estimated that around 5% to 7% of global land is salt affected (Ruiz-Lozano et al., 2012). This increase in soil salinity induces osmotic stress resulting in altered growth and physiology. Several physio-biochemical processes including photosynthesis, respiration, nitrogen metabolism and ion homeostasis are affected adversely by salinity (Tejera et al., 2004; Porcel et al., 2012).
Exposure of plants to stresses enhances the production as well as accumulation of toxic reactive oxygen species (ROS) including O2−, H2O2 and OH− (Mittler, 2002). Increased production of ROS leads to oxidative damage and causes membrane leakage through lipid peroxidation (Shah et al., 2001). Moreover ROS induced effects are also obvious on the several other macromolecules including proteins, nucleic acids and photosynthetic pigments (Ahmad et al., 2010). In order to avoid salt stress induced oxidative damage plants have developed several protective mechanisms. Synthesis and accumulation of organic osmolytes, enhanced activities of antioxidant enzymes and efficient compartmentalization of toxic ions into other cellular compartments like vacuoles help plants to avert stress induced damage (Parvaiz and Satyawati, 2008; Tong et al., 2004; Liu et al., 2014). Both enzymatic as well as non-enzymatic antioxidants are involved in scavenging of toxic ROS. Enzymatic system comprises of superoxide dismutase (SOD), peroxidases (POD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) while as non enzymatic system includes ascorbic acid, glutathione, phenols, tocopherols etc are among the key antioxidants involved in scavenging of toxic ROS (Mittler, 2002; Ahmad et al., 2010).
Most plants form symbiotic associations with the arbuscular mycorrhizal fungi (AMF) and it has been well studied that AMF has the potential to enhance the rhizospheric soil characteristics considerably thereby affecting plant growth (Navarro et al., 2013; Ahanger et al., 2014). By acting as bio-ameliorators, AMF improves soil structure so as to promote plant growth under normal as well as stressed conditions (Rabie and Almadini, 2005; Cho et al., 2006). AMF enhances growth and mitigates stress by affecting both morpho-physiological and nutritional aspects. A direct beneficial effect of AMF on the plant growth and vigor is well documented (Asghari et al., 2005; Ahanger et al., 2014). In addition of affecting the plant physiological status AMF also alters root morphology so as to increase the absorption of water and nutrients (Aroca et al., 2013; Ahanger et al., 2014). AMF colonized plants show increased absorption as well as efficient utilization of essential mineral nutrients (Neumann and George, 2005; Hart and Forsythe, 2012).
Sesbania sesban Linn, a plant within family Fabaceae is an important medicinal plant and is commonly known as Egyptian sesban and is well widely distributed in several tropical countries (Gomase et al., 2012). According to World Health Organization about 80% of people living in developing countries rely exclusively on traditional medicines for their primary health care needs. Different parts of the Sesbania sesban including leaves, pods and seeds are known for their medicinal value (Mittal et al., 2012). Traditionally leaves of S. sesban have been used as purgative, demulcent, maturant, anthelmintic, inflammation and all pains (Gomase et al., 2012; Mythili and Ravindhran, 2012). Present study was carried out with the aim to evaluate the role of AMF in ameliorating the salinity induced changes in growth and biochemical attributes in S. sesban.
2. Material and methods
2.1. Pot experiment and treatment
Seeds of sesbania (S. sesban [L.] Merr; Syn Sesban aegyptiaca Poiret) were obtained from farms of Faculty of Agriculture, Cairo University, Giza, Egypt. Present study was conducted at the Plant Production Department, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia. The soil used for the experiment has the following properties (%): sand (83.5); clay (8.7); silt (7.8); organic carbon, 0.15; total nitrogen, 0.0062; EC, 7.14 dS/m; and pH 7.5. Equal quantity (450 g) of autoclaved soil was divided among plastic pots. The seeds were surface sterilized with sodium hypochlorite (0.5%, v/v) for 3 min, washed thoroughly with distilled water before germination on blotter. Healthy germinating seeds were transferred to pots (1 plant/pot) with normal soil in one set of experiments and soil of second set of experiment was amended with Arbuscular Mycorrhizal Fungi (AMF). The biofertilizer (Rhizobium leguminosarum bv. viciae Frank) was added to the germinated seeds as thin film of Peat Inoculant (2 × 108 CFU/g) at rate of 4 g inoculant/kg seed. Hoagland’s solution (Hoagland and Arnon, 1950) was used for irrigation with different concentrations of NaCl to get concentration of 0, 75 and 150 mM. The irrigation was carried out every alternate day. The seedlings were grown for eight weeks at 27 ± 1 °C with 12 h light (750 μmol m−2 S−1) and 12 h dark photo-cycle and relative humidity of 70–75% after transplantation. The experiment was laid out in a completely randomized block design with five replications. At the end of pot experiment (8 weeks), the plants were harvested carefully, washed in distilled water, separated into shoots and roots. The samples were dried at 70 °C for 48 h and dry weight was recorded. Leaf samples were used for estimation of photosynthetic pigments, antioxidative enzymes, non enzymatic antioxidants and growth regulators. Fresh root samples were used for observing rhizobial nodules and related mycorrhizal studies.
2.2. The mycorrhizal inoculums
The selected mycorrhizal fungi [Funneliformis mosseae (syn. Glomus mosseae); Rhizophagus intraradices (syn. Glomus intraradices) and Claroideoglomus etunicatum (syn. Glomus etunicatum)] used in the present experiment were isolated as described by Hashem et al. (2014). Fungal inoculums potential was determined by the most probable numbers method (Alexander, 1982) and each trap culture contained 10.2 × 103 propagules per pot (1 Kg capacity). Fungal inoculums consisted of AM fungal spores, hyphae and colonized root fragments. 10 g of trap soil culture (approx. 100 spores/g trap soil, M = 80%)/ pot (1 Kg) was added to the experimental soil as mycorrhizal inoculum.
2.3. Nodulation and nodules activity
Nodules were speared from roots and counted instantly. Fresh weight of nodule was taken instantly after harvesting, whereas dry weight was determined as described above. For estimation of leghemoglobin, nodules (2.0 g) were ground in liquid N2 and 50 mM KPO4 (pH 7.4) buffer that contained 1 mM EDTA was added. The mixture was stirred until it thawed to a homogenate at temperature 2 °C and then transferred to centrifuge tubes. Following centrifugation at 10,000×g for 10 min at 4 °C. The supernatant was collected and mixed with 50 mM KPO4 (pH 7.4) buffer containing 1 mM EDTA and the color intensity was recorded spectrophotometrically at 710 nm (Keilin and Wang, 1945). Nitrogenase activity was measured using the method described by (Bergersen and Turner, 1967). The concentration of NH4+ was measured calorimetrically according to the methods of Bergersen and Turner (1967) and Bergersen (1980) and modified by Minchin et al. (1983).
2.4. Determination of photosynthetic pigments
The photosynthetic pigments were extracted from leaves in dimethyl sulfoxide (DMSO) as described by Hiscox and Israelstam (1979). Absorbance was read at 480, 510, 645, 663 nm using DMSO as blank.
2.5. Extraction and estimation of antioxidative enzymes
Fresh leaves (5 g) were crushed in 100 mM Tris–HCl (pH 7.5) containing 5 mM Dithiothreitol (DTT), 10 mM MgCl2, 1 mM EDTA (Ethylene diaminetetra acetic acid), 5 mM magnesium acetate, 1.5% polyvinylpyrrolidone, 1 mM phenylmethanesulfonyl fluoride (PMSF) and 1 μg ml−1 aprotinin. The homogenate was filtered through cheese cloth and subjected to centrifugation for 15 min at 10,000 rpm. The supernatant collected after centrifugation was used as source of enzyme. Extraction medium of ascorbate peroxidase (APX) contained 2 mM ascorbic acid in addition of the above constituents. All experiments were performed at 4 °C. Protein in the enzyme extract was estimated according to Lowry et al. (1951).
The activity of superoxide dismutase (EC 1.15.1.1) was estimated following Van Rossum et al. (1997) measuring the photoreduction of nitrobluetetrazolium (NBT) at 560 nm and activity was expressed as enzyme unit (EU) mg−1 protein. One unit of SOD was defined as the amount of protein causing 50% decrease of the SOD-inhibitable NBT reduction. The method of Nakano and Asada (1981) was followed for the determination of APX (EC 1.11.1.11) activity. The decrease in absorbance was read at 290 nm and was expressed as enzyme units (EU) mg−l protein. Glutathione reductase [GR] (EC 1.6.4.2) activity was determined according to Carlberg and Mannervik (1985). The decrease in absorbance was monitored at 340 nm for 2 min. The activity of GR was calculated using the extinction coefficient of 6.2 mM−1 cm−1 and expressed as enzyme units (EU) mg−l protein. Catalase [CAT] (EC 1.11.1.6) activity was determined according to the method described by Luck (1974) by measuring change in absorbance at 240 nm. Extinction co-efficient of 36 × 103 mM−l cm−l was used for calculation and expressed as enzyme units (EU) mg−1 protein.
2.6. Extraction and estimation of non-enzymatic antioxidants
Non-enzymatic antioxidant substances including ascorbic acid (AsA), glutathione (GSH), oxidized glutathione (GSSG) were extracted using 1% (w/v) ice-cold trichloroacetic acid (TCA). The homogenate was then centrifuged at 12,000×g for 20 min at 4 °C. For estimation of AsA the method of Law et al. (1983) was adopted, however GSH and GSSG contents were determined following the method of Anderson (1985).
2.7. Extraction and quantification of plant growth regulators
Indole acetic acid (IAA), indole butyric acid (IBA) and abscisic acid (ABA) were extracted in aqueous acetone (80%) supplemented with 10 mg/l butylated hydroxyl toluene and purified using EtOAc and NaHCO3 as described by Kusaba et al. (1998). The purified extract residue was subjected to HPLC on a column of PEGASIL ODS (6 mm i.d. × 150 mm, Senshu Kagaku, Tokyo, Japan). Standard curves of IAA, IBA and ABA ranging from 10 to 200 ng/ml were used as references for quantification. The method described by Lee et al. (1998) was followed for extraction and estimation of gibberellic acid (GA3) by gas chromatograph–mass spectrometer (GC–MS). Standard GA3 was used as references.
2.8. Statistical analysis
Two-way analysis (ANOVA) was used for statistical analysis followed by Duncan’s Multiple Range Test (DMRT). Values presented are the mean ± SE of five replicates. P value at 0.05 was considered as significant.
3. Results
3.1. Growth parameters
Salinity stress reduced the morphological and growth parameters studied while as inoculation of AMF mitigated the salinity stress induced changes (Table 1). Percent reduction in shoot and root length was 21.17% and 40.85% in 75 mM and 150 mM. However AMF inoculated plants showed 23.8% and 47.5% increase in shoot and root length as compared to control. Reduction in fresh weight of shoot and root due to salinity was 25.64% and 48.9% respectively for 75 mM and 57% and 80.1% in for 150 mM NaCl treated plants. AMF induced enhancement in fresh weight of shoot and root was reported to be 37.6% and 29% respectively. In addition salinity induced percent reduction in number of leaves and leaflets was 22% and 59.6% in 150 mM salinity levels (Table 1). Relative to control AMF inoculated plants showed 29.5% and 4.8% increase in number of leaves and leaflets respectively.
Table 1.
Effect of salinity in presence and absence of AM fungi on shoot length (cm), root depth (cm), fresh weight (g), of shoot and fresh weight (g) and dry weight (g) and root of Sesbania sesban (L.) Merr.
| Treatment | Shoot height | Root depth | Shoot fresh wt. | Root fresh wt. | Number of leaf | Number of leaflets |
|---|---|---|---|---|---|---|
| Control | 24.23 | 18.20 | 1.17 | 0.709 | 9.00 | 20.66 |
| AMF | 30.03 | 22.10 | 1.61 | 0.915 | 11.66 | 21.66 |
| 75 mM NaCl | 19.10 | 12.76 | 0.87 | 0.362 | 9.33 | 15.66 |
| 75 + AMF | 22.66 | 15.73 | 1.18 | 0.594 | 8.33 | 18.33 |
| 150 mM NaCl | 14.33 | 7.86 | 0.50 | 0.141 | 7.00 | 8.33 |
| 150 + AMF | 17.40 | 11.30 | 0.82 | 0.397 | 6.66 | 12.66 |
| LSD at: 0.05 | 1.26 | 0.97 | 0.07 | 0.09 | 0.26 | 2.74 |
3.2. Nodulation and nodules activity
Salinity caused a drastic decline in nodulation attributes like number of nodules per plant, fresh and dry weight of nodules, leghemoglobin content and nitrogenase activity (Table 2). However AMF inoculation increased these parameters considerably and also reduced salinity induced decline. Decline in number of nodules per plant, fresh and dry weight of nodules and leghemoglobin content in salinity stressed plants (75 mM NaCl) was 61.3%, 50%, 49.4% and 33.5% respectively. Higher concentration of NaCl (150 mM) caused total inhibition of nodule growth and AMF inoculation mitigated these deleterious effects of salinity (Table 2). Observed percent increase in number of nodules per plant, fresh and dry weight of nodules, leghemoglobin content in AMF inoculated plants was 48.40%, 84.91%, 45.55% and 32.43% respectively. Moreover, nitrogenase activity declined by 54.88% and 100% in low (75 mM NaCl) and high (100 mM NaCl) salinity stresses. However, AMF inoculated salt stressed plants showed only 17.15% (75 mM NaCl + AMF) and 39.45% (150 mM NaCl + AMF) reduction in nitrogenase activity. Relative to control, AMF inoculated plants showed 33.10% increase in nitrogenase activity (Table 2).
Table 2.
Effect of salinity in presence and absence of AM fungi on number of nodules per plant, fresh and dry weight of nodules (mg), leghemoglobin and nitrogenase activity of Sesbania sesban (L.) Merr.
| Treatment | No. of nodules/plant | Fresh wt. (mg) | Dry weight (mg) | Leghemoglobin | Nitrogenase |
|---|---|---|---|---|---|
| Control | 10.33 | 19.66 | 2.59 | 10.73 | 0.583 |
| AMF | 15.33 | 36.33 | 3.77 | 14.21 | 0.776 |
| 75 mM NaCl | 4.00 | 9.66 | 1.31 | 7.13 | 0.263 |
| 75 + AMF | 9.00 | 17.00 | 2.22 | 6.35 | 0.483 |
| 150 mM NaCl | 0.00 | 0.00 | 0.00 | 1.70 | 0.00 |
| 150 + AMF | 4.33 | 10.33 | 1.17 | 5.05 | 0.353 |
| LSD at: 0.05 | 0.25 | 0.48 | 0.16 | 0.75 | 0.11 |
3.3. Chlorophyll
Salinity caused considerable decline in chlorophyll pigments with the effect being more obvious under higher concentrations (100 mM NaCl). At higher salt concentration, percent reduction in chlorophyll a, chlorophyll b and total chlorophyll contents was 72.2%, 62.6% and 70.5% respectively while as AMF inoculated salinity stressed (150 mM NaCl + AMF) plants showed only 41.7%, 37.6% and 15.5% decline respectively (Table 3). Relative to control, AMF increased chlorophyll a, chlorophyll b and total chlorophyll contents by 41%, 25% and 33% respectively.
Table 3.
Effect of salinity in presence and absence of AM fungi on chlorophyll a, chlorophyll b, chlorophyll a/b ratio, total chlorophyll and Carotenoids in Sesbania sesban (L.) Merr.
| Treatment | Pigments system (mg g fresh weight−1) | ||||
|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Chlorophyll a/b ratio | Carotenoids | Total chlorophyll | |
| Control | 0.880 | 0.276 | 3.19 | 0.135 | 1.29 |
| AMF | 1.241 | 0.345 | 3.62 | 0.133 | 1.72 |
| 75 mM NaCl | 0.597 | 0.280 | 2.13 | 0.120 | 0.99 |
| 75 + AMF | 0.851 | 0.304 | 2. 79 | 0.164 | 1.32 |
| 150 mM NaCl | 0.244 | 0.103 | 2.36 | 0.039 | 0.38 |
| 150 + AMF | 0.513 | 0.172 | 2.98 | 0.810 | 1.49 |
| LSD at: 0.05 | 0.052 | 0.034 | 0.011 | 0.002 | 0.048 |
3.4. Antioxidant enzymes
Results regarding activities of antioxidant enzymes are depicted in Fig. 1. Salinity stress caused a significant increase in activities of antioxidant enzymes studied and increase was consistent with the increase in concentration of salt. Exposure to low NaCl (75 mM) concentrations increased SOD, CAT, GR and APX activities by 34.8%, 44.4%, 37.6% and 37% respectively while at higher NaCl concentrations (150 mM) activities increased by 42.36%, 66.88%, 94.8% and 69.9% respectively. AMF alone increased the activities of SOD, CAT, GR and APX by 14.7%, 19.8%, 26.8% and 37% respectively. In combination with salt treatment AMF inoculation further enhanced the activities of antioxidant enzymes studied. At higher concentrations (150 mM) AMF enhanced the activity of SOD, CAT, GR and APX by 53.5%, 78.2%, 113.1% and 80.3% respectively.
Figure 1.
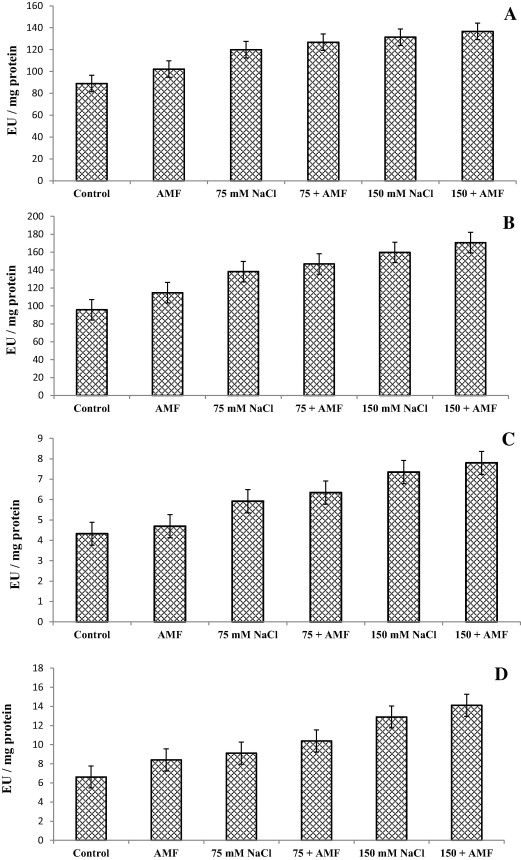
Effect of salinity in presence and absence of AM fungi on (A) superoxide dismutase (SOD), (B) catalase (CAT), (C) ascorbate peroxidase (APX) and (D) glutathione reductase (GR) activity of Sesbania sesban (L.) Merr. Data presented are the means ± SE (n = 5).
3.5. Non-enzymatic antioxidants
Results pertaining to the combined effect of salinity and AMF on ASA, GSSG and GSH are depicted in Fig. 2. Salinity stress reduced ASA content by 36% and 48.3% at 75 mM and 150 mM respectively while as GSSG and GSH was increased by 60.4% and 62.3% at lower concentrations (75 mM) and 151.2% and 123.6% at higher concentrations (150 mM). However inoculation of AMF caused considerable increase in these attributes. Percent increase in ASA, GSSG and GSH due AMF was 12.4%, 15% and 2.4% respectively. Decline in ASA in AMF inoculated salinity stressed plants was only% (75 mM + AMF) and% (150 mM + AMF). AMF inoculation in salinity stressed plants further enhanced the contents of GSSG and GSH (Fig. 2).
Figure 2.
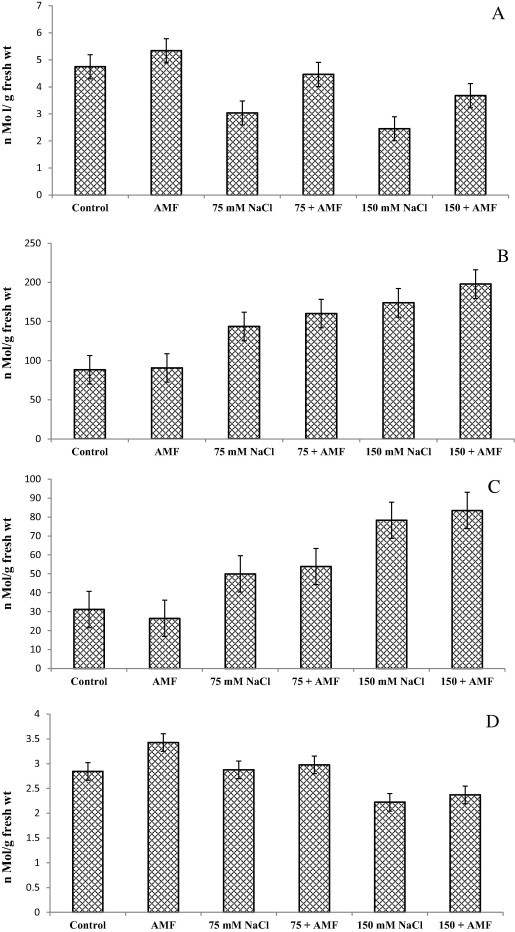
Effect of salinity in presence and absence of AM fungi on (A) ascorbic acid (ASA), (B) reduced glutathione (GSH), (C) oxidized glutathione (GSSG) and (D) GSH/GSSG ratio of Sesbania sesban (L.) Merr. Data presented are the means ± SE (n = 5).
3.6. Endogenous levels of plant growth hormones
Drastic decline was observed in endogenous levels of IAA, IBA and GA3 due to salinity stress. Inoculation of AMF not only increased the growth hormone levels but also ameliorated the salinity induced deleterious effects (Table 4). Percent increase in IAA, IBA and GA due to AMF inoculation was 9.4%, 1.9% and 153.1% respectively. Decline in the above pointed hormones due to salinity was 33%, 54% and 43.8% at 75 mM NaCl and 70.3%, 74.9% and 89.2% at 150 mM NaCl. However ABA levels decreased due to AMF inoculation while increased under salinity stress conditions (Table 4). ABA increased by 125.8% and 236.4% at 75 mM NaCl and 150 mM NaCl respectively.
Table 4.
Effect of salinity in presence and absence of AM fungi on growth hormones (nM g fresh weight−1) of Sesbania sesban (L.) Merr.
| Treatment | Growth hormones (nM g fresh weight−1) |
||||
|---|---|---|---|---|---|
| IAA | IBA | IAA/IBA | GA | ABA | |
| Control | 433.26 | 250.56 | 1.72 | 131.66 | 110.40 |
| AMF | 474.36 | 255.43 | 1.85 | 215.33 | 71.33 |
| 75 mM NaCl | 290.03 | 115.10 | 2.52 | 73.96 | 249.33 |
| 75 + AMF | 332.93 | 188.03 | 1.77 | 120.83 | 145.10 |
| 150 mM NaCl | 128.26 | 62.73 | 2.04 | 14.13 | 371.46 |
| 150 + AMF | 277.96 | 139.80 | 1.98 | 63.33 | 209.80 |
| LSD at: 0.05 | 12.74 | 3.46 | 0.18 | 8.42 | 9.23 |
Indole acetic acid (IAA), indole butyric acid (IBA), gibberellic acid (GA), abscisic acid (ABA).
4. Discussion
Salinity stress reduced the biomass and inoculation of AMF mitigated the stress induced changes in biomass. Reduced rates of cell division and cell elongation due to stress are the main causes of reduced growth of plants under stress (Yeseen et al., 1987). Salinity induced reduction in growth attributes has been reported by several workers (Perveen et al., 2011; Ahmad et al., 2012; Padder et al., 2012). In Vigna radiata L, Padder et al. (2012) and tomato, Babu et al. (2012) demonstrated that length as well as fresh and dry biomass of shoot and root declined with the increasing salinity. Exposure to stress reduces hydraulic conductivity and disturbs extension of cell wall causing considerable decline in morphological attributes of plants (Ehlert et al., 2009). AMF enhances stress resistance and adaptability of plants by mediating maintained uptake and assimilation of important mineral nutrients. Usha et al. (2005) demonstrated that AMF inoculated plants showed maintained growth due to efficient uptake of mineral elements. Our results of AMF induced increase in morphological attributes and subsequent mitigation of salinity stress support the findings of Tang et al. (2009) for maize and Shekoofeh et al. (2012) for Ocimum basilicum. In wheat subjected to salinity, Bheemareddy and Lakshman (2011) demonstrated that reduction in plant height, biomass, leaf area and number of leaves per plants was observed and inoculation of AMF increased these attributes and also mitigated the salinity induced deleterious effects.
Salinity induced reduction in nitrogenase activity reported in our results is corroboration with the findings of Severin et al. (2012). Our results of AMF induced enhancement in nodule growth and nitrogenase activity support the findings of Zarea et al. (2011). In Trifolium alexandrinum L. and Trifolium resupinatum L., Zarea et al. (2011) demonstrated that inoculation of AMF enhanced growth and nitrogenase activity significantly. Reduced nodule weight, nodule number and nitrogenase activity has also been reported in salt stressed soybean plants (Qifu and Murray, 1993). AMF colonization stimulates nodulation, nodule growth and nitrogen fixation in legume crops (Abbott and Robson, 1977). In phosphorous deficient soils, Powell (1976) observed that nitrogen fixation mainly depends on the availability of phosphorous provided by inhabiting AMF.
Reduction in chlorophyll pigment content observed in our study is in corroboration with the findings of Doganlar et al. (2010) Azooz et al. (2011) and Alqarawi et al. (2014) for tomato, faba bean and Ephedra alata respectively. In faba bean, Azooz et al. (2011) demonstrated that increased use of saline water irrigation caused considerable decline in photosynthetic pigment content and hence reduced growth. Reduced synthesis of chlorophyll pigments during stress is due to enhanced activity of chlorophyllase. Essa and Al-Ani (2001) also reported reduction in chlorophyll content due to salinity stress in soybean. Salinity alters synthesis of pigment protein complex and associated proteins hence resulting in altered carbon metabolism (El-Tayeb, 2005). Under normal as well as salinity stress conditions enhanced synthesis of chlorophyll pigments due to inoculation of AMF has earlier been reported by in Solanum lycopersicum L. (Hajiboland et al., 2010) and lettuce (Aroca et al., 2013). Aroca et al. (2013) observed enhanced uptake of essential mineral nutrients in AMF inoculated plants. Enhanced chlorophyll synthesis in AMF inoculated plants and subsequent amelioration of salinity stress induced deleterious effect may be due to the increased uptake of magnesium which forms an important part of chlorophyll pigment molecule (Aroca et al., 2013).
Antioxidant enzymes play an important role in scavenging of reactive oxygen species and hence averting the oxidative stress induced damaging effects on several sensitive molecules like proteins nucleic acids and lipids. In our results increase in activities of SOD, CAT, GR and APX due to salt stress is in concurrence with the findings of Chen et al. (2007) for Vigna unguiculata Koca et al. (2007) for Sesamum indicum and Mittal et al. (2012) for Brassica juncea. Increased activity of antioxidant enzymes help plants to maintain the ROS levels well below to their deleterious levels. SOD is involved in scavenging of superoxide radicals into water and hydrogen peroxide (Mittler, 2002). H2O2 produced is converted into water and oxygen either by CAT and APX (Mittler, 2002). In our results AMF inoculation increased the activity of the antioxidant enzymes studied. Salt stressed AMF inoculated S. sesban plants maintained higher activities of antioxidant enzymes as compared to their uninoculated stressed counterparts. Increased antioxidant activities help plants to mediate quick removal of toxic ROS so that metabolism remains stable. Increased activities of antioxidant enzymes in AMF plants support the findings of Ghorbanli et al. (2004) for soybean, Tang et al. (2009) for Zea mays and Latef and Chaoxing (2011) for tomato. GR, APX, reduced glutathione (GSH), oxidized glutathione (GSSG) and ascorbic acid (ASA) are the important components of ascorbate–glutathione pathway which is actively involved in scavenging of ROS (Mittler, 2002). Ascorbate–glutathione cycle involves a series of redox reactions where the net electron flow is from NADPH to H2O2 resulting in the conversion of H2O2 into water. Increased activity of GR helps in enhanced production of reduced glutathione. Reduced glutathione produced from the reduction of oxidized glutathione acts as electro donor during the conversion of dehydroascorbate (DHA) into ASA and ASA acts a electron donor in conversion of H2O2 into water and oxygen (Noctor and Foyer, 1998; Mittler, 2002). Higher activity of glutathione reductase results in maintaining higher ratio of NADP+/NADPH so that photosynthetic electron transport and flow of electrons to molecular oxygen is maintained resulting in reduced formation of superoxide radicals (Noctor and Foyer, 1998; Mittler, 2002). Increased activity of GR also keeps GSH/GSSG ratio well maintained which is important for several physiological processes (Noctor and Foyer, 1998; Mittler, 2002). Decrease in content of ASA and increase in GSH found in our study is in concurrence with the findings of Umar et al. (2011) for Brassica juncea. Glutathione and ASA are the important non enzymatic antioxidants protecting cells from oxidative stress through their active roles in scavenging of ROS (Umar et al., 2011; Shan et al., 2011). ASA and glutathione are the potential scavengers of toxic ROS like superoxide and hydroxyl radicals (Briviba et al., 1997).
Salinity stressed S. sesban plants showed drastic decline in the endogenous synthesis of IAA, IBA and GA3 while as AMF inoculated plants showed higher contents of these growth regulators. Endophytic fungi cause increase in endogenous levels of IAA and GA (Waqas et al., 2012). Abscisic acid acts as an endogenous anti-transpirant reducing water loss through stomata. Enhanced biosynthesis of ABA in stressed and AMF inoculated plants causes redistribution and accumulation of ABA in guard cells resulting in the release of water, ion flux and turgor loss of guard cells and hence mediating stomatal closure (Bray, 1997). Babu et al. (2012) demonstrated that salinity stressed tomato plants showed increment in the concentration of ABA and IAA leading to better adaptation of tomato to salt stress. Hamayun et al. (2010) observed that soybean cultivars subjected to salt stress exhibited increase in ABA and decrease in GA3 synthesis. ABA is among the different signaling components involved in regulation of stomatal movements (Kuppusamy et al., 2009). ABA is a ubiquitous phytohormone concerned with mediating stomatal closure, regulating plant growth and development under stress conditions (Shinozaki and Yamaguchi-Shinozaki, 2007; Wasilewska et al., 2008). It has been reported that stress resistance is usually acquired by slowing the biosynthesis of gibberellins and while as increasing ABA (Xiong et al., 2002). In our results it was observed that salt stress reduced the content of auxin and GA in S. sesban plants. AMF fetch increased nutrient uptake including phosphorus, sulfur, calcium, magnesium and potassium. AMF induced enhancement in nutrient uptake promotes various biologically important metabolites and enzymes (Yuan et al., 2010). Among essential metabolites plant hormones including GA and auxin have an irreplaceable role in plant growth regulation under normal as well as stress conditions.
5. Conclusion
Salinity stress reduced growth, physiological and nitrogen metabolizing attributes considerably. However inoculation of AMF mitigated the salinity induced deleterious effects on plant growth and physiochemical attributes. Increase in activity of antioxidant enzymes was evident in S. sesban plants subjected to salinity stress which were further increased by AMF inoculation thereby mediating the fast scavenging of ROS and reducing oxidative damage. Increase in levels of growth hormones and non enzymatic antioxidants due to AMF infer the beneficial role of AMF in enhancing growth of S. sesban under salinity stress.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research group No. (RG 1435-014).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbott L.K., Robson A.D. Growth stimulation of subterranean clover with vesicular arbuscular mycorrhizas. Aust. J. Agric. Res. 1977;28:639–649. [Google Scholar]
- Ahanger M.A., Hashem Abeer, Abd Allah E.F., Ahmad P. Arbuscular Mycorrhiza in crop improvement under environmental stress. In: Ahmad P., Rasool S., editors. vol. 2. Academic Press; USA: 2014. pp. 69–95. (Emerging Technologies and Management of Crop Stress Tolerance). ISBN-13: 978-0128008751. [Google Scholar]
- Ahmad P., Hakeem K.R., Kumar A., Ashraf M., Akram N.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.) Afr. J. Biotechnol. 2012;11:2694–2703. [Google Scholar]
- Ahmad P., Jaleel C.A., Salem M.A., Nabi G., Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010;30:161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- Alexander, M., 1982. Most probable number method for microbial populations. In: Page, A.C., Miller, R.H., Keeney, D.R. (Eds.), Methods of Soil Analysis, second ed., Chemical and Microbial Properties, Madison, USA., pp: 815–820.
- Azooz M.M., Youssef A.M., Ahmad P. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int. J. Plant Physiol. Biochem. 2011;3:253–264. [Google Scholar]
- Alqarawi A.A., Abeer Hashem., Abd-Allah E.F., Alshahrani T.S., Huqail A.A. Effect of salinity on moisture content, pigment system and lipid composition in Ephedra alata Decne. Acta Biol. Hung. 2014;65:61–71. doi: 10.1556/ABiol.65.2014.1.6. [DOI] [PubMed] [Google Scholar]
- Anderson M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Aroca R., Ruiz-Lozano J.M., Zamarreno A.M., Paz J.A., Garcia-Mina J.M., Pozo J.M., Lopez-Raez J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013;170:47–55. doi: 10.1016/j.jplph.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Asghari H.R., Marschner P., Smith S.E., Smith F.A. Growth response of Atriplex nummularia to inoculation with arbuscular mycorrhizal fungi at different salinity levels. Plant Soil. 2005;273:245–256. [Google Scholar]
- Babu M.A., Singh D., Gothandam K.M. The effect of salinity on growth, hormones and mineral elements in leaf and fruit of tomato cultivar PKM1. J. Anim. Plant Sci. 2012;22:159–164. [Google Scholar]
- Barnawal D., Bharti N., Maji D., Chanotiya C.S., Kalra A. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J. Plant Physiol. 2014;171:884–894. doi: 10.1016/j.jplph.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Bergersen F.J., Turner G.L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1967;141:507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Bergersen F.J. Measurement of nitrogen fixation by direct means. In: Bergersen F.J., editor. Methods of Evaluating Biological Nitrogen Fixation. John Wile and Sons; New York, USA: 1980. [Google Scholar]
- Bheemareddy V.S., Lakshman H.C. Effect of salt and acid stress on Triticum aestivum inoculated with Glomus fasciculatum. Int. J. Agric. Technol. 2011;7:945–956. [Google Scholar]
- Bray E.A. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. [Google Scholar]
- Briviba K., Klotz L.O., Sies H. Toxic and signaling effects of photochemically or chemically generated singlet oxygen in biological systems. J. Biol. Chem. 1997;378:1259–1265. [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Chen C., Tao C., Peng C., Ding Y. Genetic analysis of salt stress responses in asparagus bean (Vigna unguiculata L. ssp. sesquipedalis Verdc) J. Hered. 2007;98:655–665. doi: 10.1093/jhered/esm084. [DOI] [PubMed] [Google Scholar]
- Cho K., Toler H., Lee J., Ownley B., Stutz J.C., Moore J.L., Auge R.M. Mycorrhizal symbiosis and response of sorghum plants to combined drought and salinity stresses. J. Plant Physiol. 2006;163:517–528. doi: 10.1016/j.jplph.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Doganlar Z.B., Demir K., Basak H., Gul I. Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afr. J. Agric. Res. 2010;5:2056–2065. [Google Scholar]
- Ehlert C., Maurel C., Tardieu F., Simonneau T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol. 2009;150:1093–1104. doi: 10.1104/pp.108.131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tayeb M.A. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul. 2005;45:215–224. [Google Scholar]
- Essa T.A., Al-Ani D.H. Effect of salt stress on the performance of six soybean genotypes. Pak. J. Biol. Sci. 2001;4:175–177. [Google Scholar]
- Ghorbanli M., Ebrahimzadeh H., Sharifi M. Effects of NaCl and mycorrhizal fungi on antioxidative enzymes in soybean. Biol. Plant. 2004;48:575–581. [Google Scholar]
- Gomase P., Gomase P., Anjum S., Shakil S., Shahnavaj K.M. Sesbania sesban Linn: a review on its ethnobotany, phytochemical and pharmacological profile. Asian J. Biomed. Pharm. Sci. 2012;2:11–14. [Google Scholar]
- Hajiboland R., Aliasgharzadeh N., Laiegh S.F., Poschenrieder C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil. 2010;331:313–327. [Google Scholar]
- Hamayun M., Khan S.A., Khan A.L., Shinwari Z.K., Hussain J., Sohn E.Y., Kang S.M., Kim Y.H., Khan M.A., Lee I.J. Effect of salt stress on growth attributes and endogenous growth hormones of Soybean cultivar Hwangkeumkong. Pak. J. Bot. 2010;42:3103–3112. [Google Scholar]
- Hart M.M., Forsythe J.A. Using arbuscular mycorrhizal fungi to improve the nutrient quality of crops; nutritional benefits in addition to phosphorus. Sci. Hortic. 2012;148:206–214. [Google Scholar]
- Hashem Abeer., Abd_Allah E.F., Alqarawi A.A., El-Didamony G., Alwhibi M., Egamberdieva D., Ahmad P. Alleviation of adverse impact of salinity on faba bean (Vicia faba L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2014;46:2003–2013. [Google Scholar]
- Hiscox J.D., Israelstam G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979;57:1332–1334. [Google Scholar]
- Hoagland D.R., Arnon D.I. The water-culture method for growing plants without soil. California Agric. Exp. Station Circ. 1950;347:1–32. [Google Scholar]
- Keilin D., Wang Y.L. Hemoglobin in the root nodules of leguminous plants. Nature. 1945;155:227–229. doi: 10.1038/159692a0. [DOI] [PubMed] [Google Scholar]
- Koca H., Bor M., Ozdemir F., Turkan I. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007;60:344–351. [Google Scholar]
- Kuppusamy K.T., Walcher C.L., Nemhauser J.L. Cross-regulatory mechanisms in hormone signaling. Plant Mol. Biol. 2009;69:375–381. doi: 10.1007/s11103-008-9389-2. [DOI] [PubMed] [Google Scholar]
- Kusaba S., Kano-Murakami Y., Matsuoka M., Tamaoki M., Sakamoto T., Yamaguchi I., Fukumoto M. Alteration of hormone levels in transgenic tobacco plants over expressing a rice homeobox gene OSH1. Plant Physiol. 1998;116:471–476. doi: 10.1104/pp.116.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latef A.A.H.A., Chaoxing H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011;127:228–233. [Google Scholar]
- Law M.Y., Charles S.A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts: the effect of hydrogen peroxide and of Paraquat. Biochem. J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.J., Foster K.R., Morgan P.W. Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 1998;116:1003–1010. doi: 10.1104/pp.116.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Sun H., Chen J., Zhang Y., Li D., Li C. Effects of cadmium (Cd) on seedling growth traits and photosynthesis parameters in cotton (Gossypium hirsutum L.) Plant Omics J. 2014;7:284–290. [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luck H. Catalases. In: Bregmeyer H.U., editor. Methods of Enzymatic Analysis. Academic Press; New York, USA: 1974. [Google Scholar]
- Minchin F.R., Witty J.F., Sheehy J.E., Muller M. A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J. Exp. Bot. 1983;34:641–649. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittal S., Kumari N., Sharma V. Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol. Biochem. 2012;54:17–26. doi: 10.1016/j.plaphy.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Mythili T., Ravindhran R. Pharmacognostical and physico-chemical studies of Sesbania sesban (L.) Merr. Stem. Asian J. Plant Sci. Res. 2012;2:659–663. [Google Scholar]
- Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Navarro J.M., Perez-Tornero O., Morte A. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 2013;171:76–85. doi: 10.1016/j.jplph.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Neumann E., George E. Does the presence of arbuscular mycorrhizal fungi influence growth and nutrient uptake of a wild-type tomato cultivar and a mycorrhiza-defective mutant, cultivated with roots sharing the same soil volume? New Phytol. 2005;166:601–609. doi: 10.1111/j.1469-8137.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- Noctor G., Foyer C.H. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Mol. Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Padder B.M., Agarwal R.M., Kaloo Z.A., Singh S. Nitrate reductase activity decreases due to salinity in mungbean (Vigna radiate L) wilczek var. hum-1. Int. J. Curr. Res. Rev. 2012;4:117–123. [Google Scholar]
- Parvaiz A., Satyawati S. Salt stress and phyto-biochemical responses of plants: a review. Plant Soil Environ. 2008;54:89–99. [Google Scholar]
- Perveen S., Shahbaz M., Ashraf M. Modulation in activities of antioxidant enzymes in salt stressed and non-stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pak. J. Bot. 2011;43:2463–2468. [Google Scholar]
- Porcel R., Aroca R., Ruiz-Lozano J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi: a review. Agron. Sustainable Dev. 2012;32:181–200. [Google Scholar]
- Powell C.L. Mycorrhizal fungi stimulate clover growth in New Zealand hill country soil. Nature. 1976;264:436–438. [Google Scholar]
- Qifu M., Murray F. Effects of SO2 and salinity on nitrogenase activity, nitrogen concentration and growth of young soybean plants. Environ. Exp. Bot. 1993;33:529–537. [Google Scholar]
- Rabie G.H., Almadini A.M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotechnol. 2005;4:210–222. [Google Scholar]
- Ruiz-Lozano J.M., Porcel R., Azcon R., Aroca R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J. Exp. Bot. 2012;63:4033–4044. doi: 10.1093/jxb/ers126. [DOI] [PubMed] [Google Scholar]
- Severin I., Confurius-Guns V., Stal L.J. Effect of salinity on nitrogenase activity and composition of the active diazotrophic community in intertidal microbial mats. Arch. Microbiol. 2012;194:483–491. doi: 10.1007/s00203-011-0787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K., Kumar R.G., Verma S., Dubey R.S. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001;161:1135–1144. [Google Scholar]
- Shan C.J., Zhang S.L., Li D.F., Zhao Y.Z., Tian X.L., Zhao X.L., Wu Y.X., Wei X.Y., Liu R.Q. Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiol. Plant. 2011;33:2533–2540. [Google Scholar]
- Shekoofeh E., Sepideh H., Roya R. Role of mycorrhizal fungi and salicylic acid in salinity tolerance of Ocimum basilicum resistance to salinity. J. Biot. 2012;11:2223–2235. [Google Scholar]
- Shinozaki K., Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Tang M., Chen H., Huang J.C., Tian Z.Q. AM fungi effects on the growth and physiology of Zea mays seedlings under diesel stress. Soil Biol. Biochem. 2009;41:936–940. [Google Scholar]
- Tejera N.A., Campos R., Sanjuan J., Lluch C. Nitrogenase and antioxidant enzyme activities in Phaseolus vulgaris nodules formed by Rhizobium tropici isogenic strains with varying tolerance to salt stress. J. Plant Physiol. 2004;161:329–338. doi: 10.1078/0176-1617-01050. [DOI] [PubMed] [Google Scholar]
- Tong Y.P., Kneer R., Zhu Y.G. Vacuolar compartmentalization: a second-generation approach to engineering plants for phytoremediation. Trends Plant Sci. 2004;9:7–9. doi: 10.1016/j.tplants.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Umar S., Diva I., Anjum N.A., Iqbal M., Ahmad I., Pereira E. Potassium-induced alleviation of salinity stress in Brassica campestris L. Central Eur. J. Biol. 2011;6:1054–1063. [Google Scholar]
- Usha K., Mathew R., Singh B. Effect of three species of arbuscular mycorrhiza on bud sprout and ripening in grapevine (Vitis vinifera L.) cv. Perlette. Biol. Agric. Hortic.: Int. J. Sustainable Prod. Syst. 2005;23:73–83. [Google Scholar]
- Van Rossum M.W.P.C., Alberda M., van der Plas L.H.W. Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci. 1997;130:207–216. [Google Scholar]
- Waqas M., Khan A.L., Kamran M., Hamayun M., Kang S.M., Kim Y.H., Lee I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 2012;17:10754–10773. doi: 10.3390/molecules170910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewska A., Vlad F., Sirichandra C., Redko Y., Jammes F., Valon C., Frey N.F., Leung J. An update on abscisic acid signaling in plants and more. Mol. Plant. 2008;1:198–217. doi: 10.1093/mp/ssm022. [DOI] [PubMed] [Google Scholar]
- Xiong L., Schumaker K.S., Zhu J. Cell signalling during cold, drought and salt stresses. Plant Cell. 2002;14:163–183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeseen B.T., Jurjees J.A., Sofajy S.A. Changes in some growth processes induced by NaCl in individual leaves of two barley cultivars. Indian J. Plant Physiol. 1987;30:1–6. [Google Scholar]
- Yuan Z.L., Zhang C.L., Lin F.C. Role of diverse non-systemic fungal endophytes in plant performance and response to stress: progress and approaches. J. Plant Growth Regul. 2010;29:116–126. [Google Scholar]
- Zarea M.J., Karimi N., Goltapeh E.M., Ghalavand A. Effect of cropping systems and arbuscular mycorrhizal fungi on soil microbial activity and root nodule nitrogenase. J. Saudi Soc. Agric. Sci. 2011;10:109–120. [Google Scholar]


