Abstract
The role of the medial temporal lobes (MTL) in short-term memory (STM) remains a matter of debate. While imaging studies commonly show hippocampal activation during short-delay memory tasks, evidence from amnesic patients with MTL lesions is mixed. It has been argued that apparent STM impairments in amnesia may reflect long-term memory (LTM) contributions to performance. We challenge this conclusion by demonstrating that MTL amnesic patients show impaired delayed matching-to-sample (DMS) for faces in a task that meets both a traditional delay-based and a recently proposed distractor-based criterion for classification as a STM task. In Experiment 1, we demonstrate that our face DMS task meets the proposed distractor-based criterion for STM classification, in that extensive processing of delay-period distractor stimuli disrupts performance of healthy individuals. In Experiment 2, MTL amnesic patients with lesions extending into anterior subhippocampal cortex, but not patients with lesions limited to the hippocampus, show impaired performance on this task without distraction at delays as short as 8s, within temporal range of delay-based STM classification, in the context of intact perceptual matching performance. Experiment 3 provides support for the hypothesis that STM for faces relies on configural processing by showing that the extent to which healthy participants’ performance is disrupted by interference depends on the configural demands of the distractor task. Together, these findings are consistent with the notion that the amnesic impairment in STM for faces reflects a deficit in configural processing associated with subhippocampal cortices and provide novel evidence that the MTL supports cognition beyond the LTM domain.
Keywords: working memory, episodic memory, hippocampus, amnesia
The medial temporal lobes (MTL) have long been recognized as a critical neural substrate for long-term declarative memory. Until recently, however, it was thought that they play little or no role in short-term memory (STM) (Baddeley & Warrington, 1970; Scoville & Milner, 1957). Evidence hinting at a possible contribution of the MTL to STM comes from recent observations of activity in the hippocampus (Axmacher et al., 2007; Hannula & Ranganath, 2008; Luck et al., 2010; Mitchell, Johnson, Raye, & D'Esposito, 2000; Nee & Jonides, 2011; Nichols, Kao, Verfaellie, & Gabrieli, 2006; Olsen et al., 2009; Piekema, Kessels, Mars, Petersson, & Fernandez, 2006; Piekema, Kessels, Rijpkema, & Fernandez, 2009; Ranganath, Cohen, & Brozinsky, 2005; Ranganath & D'Esposito, 2001; Schon, Ross, Hasselmo, & Stern, 2012) as well as activity in subhippocampal MTL cortex (Bergmann, Rijpkema, Fernandez, & Kessels, 2012; Hannula & Ranganath, 2008; Luck et al., 2010; Olsen et al., 2009; Piekema, Kessels, Rijpkema, & Fernandez, 2009; Ranganath & D'Esposito, 2001; Schon, Ross, Hasselmo, & Stern, 2012) when information of various kinds is retained over a period of seconds. For example, recruitment of the anterior hippocampus has been observed when face stimuli are maintained over an interval of just 7s in a delayed matching-to-sample task (Nichols et al., 2006). High-resolution MRI of the MTL has replicated and extended this result, revealing persistent, performance-related activity in the anterior hippocampus, entorhinal cortex, and perirhinal cortex in a delayed face matching-to-sample task for the duration of a 30s delay between study and test faces (Olsen et al., 2009). While such neuroimaging results document MTL activity during STM tasks, they leave open the question as to the necessity of the MTL for STM. Such evidence can only come from lesion studies, but to date, neuropsychological evidence for MTL contributions to STM is mixed.
Initial evidence that the MTL does not participate in STM came from reports of amnesic patients who were able to retain information over brief delays despite severe deficits in long-term memory (Baddeley & Warrington, 1970; Cave & Squire, 1992; Scoville & Milner, 1957; Wickelgren, 1968). This notion has been further supported by more recent reports of intact short-delay memory performance in amnesic patients with lesions primarily limited to the hippocampus (Baddeley, Allen, & Vargha-Khadem, 2010; Jeneson, Mauldin, Hopkins, & Squire, 2011; Jeneson, Mauldin, & Squire, 2010; Jeneson, Wixted, Hopkins, & Squire, 2012; Shrager, Levy, Hopkins, & Squire, 2008). For example, Shrager et al. (2008) found that amnesic patients with lesions primarily restricted to the hippocampus were able to retain words in memory at delays up to 14s and could retain single faces in memory at delays up to 7s. However, other studies have reported deficits in MTL amnesia in similar tasks that require maintaining information in memory over a matter of seconds (Aggleton, Shaw, & Gaffan, 1992; Buffalo, Reber, & Squire, 1998; Ezzyat & Olson, 2008; Hannula, Tranel, & Cohen, 2006; Hartley et al., 2007; Nichols et al., 2006; Olson, Moore, Stark, & Chatterjee, 2006; Olson, Page, Moore, Chatterjee, & Verfaellie, 2006; Owen, Sahakian, Semple, Polkey, & Robbins, 1995; Piekema et al., 2007; Rose, Olsen, Craik, & Rosenbaum, 2012; Ryan & Cohen, 2004; Warrington & Taylor, 1973). For example, Olsen and colleagues (2006; 2008) and Nichols et al. (2006) found that amnesic patients with MTL damage were impaired at remembering single faces over delays of just 4–7s and Ezzyat and Olson (2008) found that MTL amnesics were less accurate than controls at remembering single faces at delays as short as 1s, even though performance on a perceptual control task was intact. Interestingly, many of the reports of impaired STM in amnesia have come from patient populations whose MTL damage either included regions outside the hippocampus or was not quantified (Aggleton, Shaw, & Gaffan, 1992; Buffalo, Reber, & Squire, 1998; Ezzyat & Olson, 2008; Hartley et al., 2007; Nichols et al., 2006; Olson, Moore, Stark, & Chatterjee, 2006; Olson, Page, Moore, Chatterjee, & Verfaellie, 2006; Owen, Sahakian, Semple, Polkey, & Robbins, 1995; Piekema et al., 2007; Ryan & Cohen, 2004; Warrington & Taylor, 1973). However, impaired STM has also been reported in patients whose MTL damage was thought to be primarily limited the hippocampus (e.g., Hannula, Tranel, & Cohen, 2006; Rose, Olsen, Craik, & Rosenbaum, 2012). Thus, the status of STM in amnesia remains an open question and further research is needed to define whether and how the MTL contributes to memory performance on short-delay tasks.
One possibility is that the MTL is only necessary for short-delay memory tasks that draw upon long-term memory (LTM), and that deficits observed in amnesia on short-delay tasks reflect impairments in LTM rather than STM. Although impairments in amnesia have been observed in tasks that fall within the traditional conception of short-term memory (Atkinson & Shiffrin, 1968; Cowan, 1988; Peterson & Peterson, 1959), it has been argued that such delay-based criteria are not sufficient to determine whether a task draws upon STM or LTM, and that memory deficits in amnesia could reflect LTM impairments even at short delays (Buffalo et al., 1998; Jeneson et al., 2011; Jeneson et al., 2010; Shrager et al., 2008; Warrington & Taylor, 1973). Similarly, it has been argued that neuroimaging evidence of neural activity in the MTL during short-delay tasks could reflect LTM encoding-related processes rather than STM per se (Ranganath et al., 2005; Ranganath & D'Esposito, 2001; Schon, Hasselmo, Lopresti, Tricarico, & Stern, 2004).
To determine more clearly whether short-delay tasks rely on STM or LTM, Shrager et al. (2008) proposed an additional distractor-based criterion for STM classification that is independent of retention interval (see also Jeneson & Squire, 2012). Specifically, Shrager et al. proposed susceptibility to interference as such a criterion: They suggested that short-delay tasks can be classified as relying on STM only if delay-period interference disrupts task performance. This proposal is based on the notion that STM entails the active maintenance of information that is susceptible to interference and the assumption that LTM is immune to such interference. When this classification was applied in the context of tasks administered to amnesic patients, Shrager et al. found that MTL amnesic patients were impaired only on short-delay tasks in which delay-period interference did not disrupt control performance (meeting their classification as a LTM task) but showed intact performance on short-delay tasks in which delay-period interference did disrupt control performance (meeting their classification as a STM task). While these results support the notion that amnesic deficits on short-delay tasks reflect impairments in LTM rather than STM, it is important to note that the distractor tasks used by Shrager et al. did not require extensive processing of distractor stimuli (simply requiring counting the number of distractor faces presented during the delay period between study and test faces or deciding whether one of the distractor faces was Bill Clinton). The degree to which distraction disrupts short-delay memory performance may depend on the nature and difficulty of the distractor task.
A second possibility is that MTL contributions to short-delay memory depend on the nature of the information maintained over the delay and the degree to which this information is verbalizable. Indeed, many of the reports of preserved short-delay memory in amnesia have involved maintaining easily verbalizable material (Baddeley et al., 2010; Baddeley & Warrington, 1970; Rose et al., 2012), which may be supported by rehearsal in the phonological loop and processing in extra-MTL regions such as the frontal cortex, parietal cortex, and cerebellum (e.g., Awh, Jonides, Smith, Schumacher, Koeppe, & Katz, 1996; Desmond, Gabrieli, Wagner, Ginier, & Glover, 1997; Jonides, Schumacher, Smith, Koeppe, Awh, et al., 1998; Paulesu, Frith, & Frackowaik, 1993; Schumacher, Lauber, Awh, Jonides, Smith, & Koeppe, 1996; Trost & Gruber, 2012). However, preserved short-delay memory in MTL amnesia has also been reported in tasks involving nonverbalizable material (e.g., Baddeley et al., 2010; Jeneson et al., 2011; Shrager et al., 2008). Non-famous faces are one class of nonverbalizable material that has produced particularly mixed results in amnesia, with reports of both preserved short-delay memory (Shrager et al., 2008; Warrington & Taylor, 1973) and impaired short-delay memory (Ezzyat & Olson, 2008; Nichols et al., 2006; Olson et al., 2006; Olson et al., 2008; Rose et al., 2012). Important questions remain about the status of short-delay memory for faces in amnesia and the factors that determine whether short-delay memory for faces is impaired or preserved following MTL damage.
Faces are an interesting class of stimuli for several reasons. First, our extensive experience with faces results in perceptual expertise, with associated greater STM capacity for faces than for other objects (Curby & Gauthier, 2007). Second, faces are relationally complex but are perceived holistically (i.e., as an integrated entity). The holistic representation of faces results from configural processing in which individual facial features are bound into a single unit or configuration (Maurer, Le Grand, & Mondloch, 2002; Piepers, & Robbins, 2012; Rhodes, 1988; Sergent, 1984). In the LTM domain, configural processing and memory for such unitized representations has been associated with neural substrates in the subhippocampal MTL cortex, such as perirhinal cortex (e.g., Haskins, Yonelinas, Quamme, & Ranganath, 2008; Preston & Gabrieli, 2008). Specifically, subhippocampal regions are thought to support configural processing or intra-item binding (i.e., the binding of information encoded as a single unit, such as faces). In contrast, the hippocampus is thought to support relational processing or inter-item binding (i.e., the binding of information that is not encoded configurally as a single unit, such as object-location associations) (Eichenbaum, Schoenbaum, Young, & Bunsey, 1996; Giovanello, Keane, & Verfaellie, 2006; Preston & Gabrieli, 2008; Quamme, Yonelinas, & Norman, 2007). By extension, an important outstanding question is whether STM for faces depends on the integrity of the subhippocampal cortex rather than the hippocampus proper, and whether STM for faces in amnesia is intact when lesions are restricted to the hippocampus but impaired when lesions extend into MTL cortex.
The current study first investigates whether the MTL plays a critical role in STM for faces by testing the status of short-delay memory for faces in MTL amnesia on a task that meets both the traditional delay-based criterion and the distractor-based criterion for STM classification (Shrager et al., 2008). In Experiment 1, we determine whether a short-delay face matching task is disrupted in healthy individuals by delay-period interference that requires extensive processing of distractor stimuli, such that the task meets the proposed distractor-based criterion for classification as a STM task. In Experiment 2, we test whether MTL amnesic patients are impaired on this task without distraction at delays as short as 8 sec (within the temporal range of delay-based STM classification). If MTL amnesics are impaired on a short-delay memory task that meets both delay-based and distractor-based criteria for classification as a STM task, this would provide novel evidence that the mnemonic contributions of the MTL extend into the STM domain. Furthermore, we investigate whether the status of short-delay memory for faces in amnesia depends on the locus of neural damage within the MTL by comparing the performance of patients with MTL damage limited to the hippocampus to that of patients whose MTL damage includes subhippocampal regions of MTL cortex. In Experiment 3, we investigate one potential underlying mechanism, configural processing, that may support STM for faces, by comparing the effect of configural versus featural interference on performance in the short-delay face memory task. If configural interference disrupts face memory performance to a greater extent than featural interference, this would provide evidence that configural processing supports STM for faces and would point to MTL-mediated configural processing as a possible basis of the observed STM impairment in amnesia.
Experiment 1
In Experiment 1, we examine whether a delayed matching-to-sample task using faces as stimuli meets the proposed distractor-based criterion for STM classification by determining whether performance is disrupted by a distractor task that extensively engages the same domain of working memory and uses the same type of stimuli as the delayed matching-to-sample task. Healthy participants were tested on a delayed face matching-to-sample task and had to make gender discriminations about distractor faces presented during the delay. If delay-period processing of distractor faces disrupts participants’ ability to make match/mismatch decisions about study and test faces, this would qualify the delayed face matching-to-sample task as a STM task according to the proposed distractor-based criterion (Shrager et al., 2008).
Method
Participants
Sixteen healthy participants took part (mean age = 61 years, mean education = 14 years, mean verbal IQ = 105; healthy participants were matched in terms of mean age, education, and verbal IQ to the patients in Experiment 2, all t's < .84). All participants were paid for their participation and provided informed consent in accordance with the procedures of the Institutional Review Boards at Boston University and the VA Boston Healthcare System.
Stimuli
Face stimuli (512 study-test faces and 320 distracter faces) were gathered from various face databases, including tarrlab.com (Images courtesy of Michael J. Tarr, Brown University, http://www.tarrlab.org/), AT&T database of faces (AT&T Laboratories, Cambridge, http://www.cl.cam.ac.uk/research/dtg/attarchive/facedatabase.html), Yale Face Database B (Gerghiades, Belhumeur, & Kriegman, 2001), Georgia Tech Face Database (www.anefian.com/face_reco.htm), the grayscale NIST FERET database (http://www.nist.gov/humanid/colorferet/home.html), NIST Mugshot Identification Database (MID) (http://www.nist.gov/srd/nistsd18.cfm), and the Psychological Image Collection at Stirling (PICS) (http://pics.psych.stir.ac.uk/). All faces were Caucasian, forward facing, and included hair, but were free of jewelry or facial hair (see Figure 1A for example stimuli). The 512 study-test faces were all male, whereas the distractor faces included 160 female faces and 160 male faces.
Figure 1.
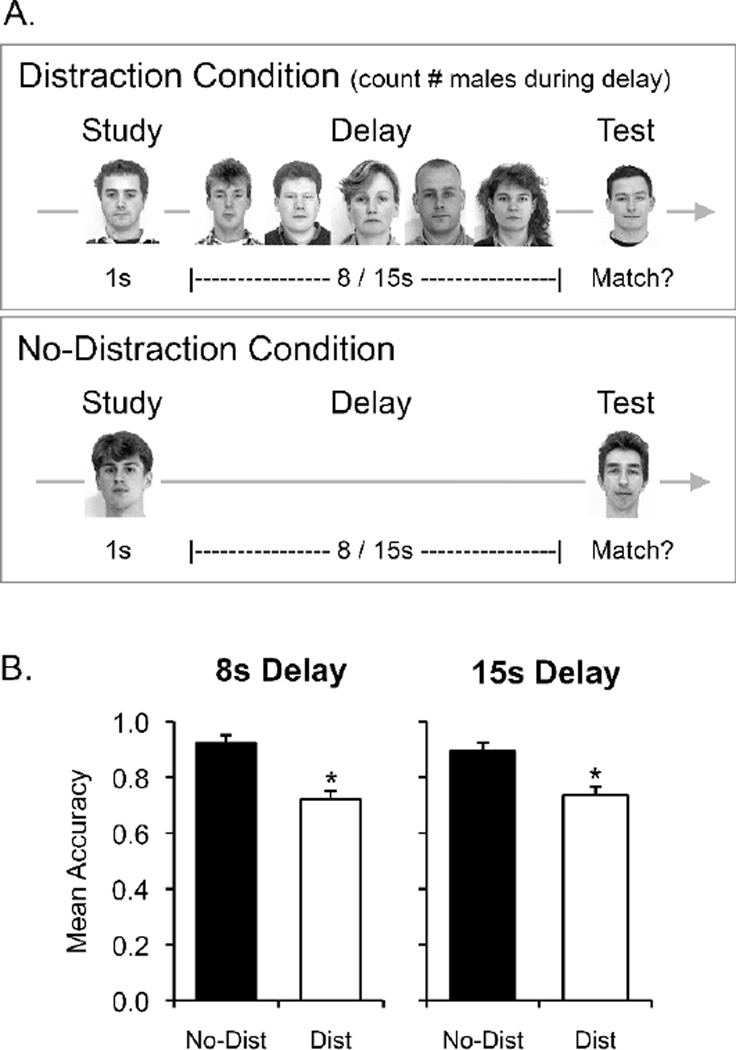
(A) Experiment 1 task design. (B) Results from Experiment 1. Mean accuracy (hits – false alarms) for healthy participants on the delayed face matching-to-sample task at 8s and 15s delays without distraction (black bars) and with distraction (white bars). Error bars represent within-subject standard error. * p < .005 compared to performance without distraction.
Study-test faces were divided into eight sets of 32 pairs. One of the faces in each pair was designated as a test face. The study face was designated as the other face in the pair (mismatch) for half of the pairs, and the same face as the test face (match) for the other half of the pairs. The assignment of each pair as a match or mismatch pair was counterbalanced across subjects to ensure that each test face appeared equally as often as a match or a mismatch face and each set of faces rotated through each condition (see below) across subjects.
Subjects performed eight study-test blocks: four blocks with an 8s delay between study and test faces, and four blocks with a 15s delay between study and test faces. Two blocks within each delay condition contained distraction between study and test, and two blocks within each delay condition did not contain distraction between study and test, resulting in four main conditions: 8s distraction, 8s no-distraction, 15s distraction, and 15s no-distraction.
Distractor faces were divided into two sets of 160 faces (80 male, 80 female), containing 32 trials of five faces. The number of male faces in each trial ranged from one to four and was evenly distributed across the trials (i.e., eight trials had one male face, eight trials had two male faces, eight trials had three male faces, eight trials had four male faces). Each set of distractor faces was viewed twice during the experiment (with a different, randomized order of presentation) but was not repeated within the same condition for any one subject to ensure that each distraction condition (8s or 15s) was paired once with each set of distractor faces for each subject. The pairing of distractor sets to conditions was counterbalanced across subjects.
Procedure
Each subject was tested in two sessions. Each session included one block of each condition, with the order of conditions counterbalanced across subjects and the specification that subjects always alternated between distraction and no-distraction conditions. During each session, subjects were given practice trials before starting the task. Subjects were first given a practice block of two 8s no-distraction trials. They were also given a practice block of two 8s distraction trials immediately before their first distraction block. If the first block was a no-distraction block, subjects were given this block before the practice distraction trials. If the first block was a distraction block, they were given the practice distraction trials immediately following the no-distraction practice trials.
The experimental task design is presented in Figure 1A. On all trials, subjects were first shown a blank screen for 1000ms, followed by a central fixation cross for 1000ms, another blank screen for 1000ms, and a study face for 1000ms. Subjects were told to remember the identity of the study face over the subsequent delay. For the no-distraction trials, a blank screen followed the study face for either a 7983ms delay (8s delay condition) or a 14983ms delay (15s delay condition)1, followed by the test face in which the subjects reported whether the test face did or did not match the study face. For the distraction trials, an ISI of 4003ms followed the study face, and was followed by presentation of the five distractor faces, one at a time, for 579ms each with a 17ms ISI between each distractor face. Following the distractor faces, there was an additional ISI of 1000 ms (8s delay condition) or 8000 ms (15s delay condition). This timing ensured that the same amount of time passed between the study and test faces in the no-distraction and the distraction conditions. Subjects were told to remember the identity of the study face over the delay and also (in the distraction condition) to count the number of male faces presented in the intervening period. Test faces in both conditions remained on the screen until subjects made a verbal response. In the distraction condition, subjects first reported the number of male faces presented during the distractor period and then reported whether or not the test face matched the study face.
Results
The results from Experiment 1 are presented in Figure 1B. Compared to the no-distraction condition, distraction reduced subjects’ mean accuracy (hits minus false alarms) at both the 8s delay (92% to 72%) and the 15s delay (89% to 73%). To verify the reliability of these effects, mean accuracy in each condition was entered into a 2 × 2 repeated-measures ANOVA with factors of delay (8s, 15s) and distraction (no-distraction, distraction). ANOVA confirmed that performance was disrupted with distraction (main effect of distraction, F(1,15) = 37.51, p < .001, ηp2 = .71). There was no difference in performance on the 8s and 15s trials (main effect of delay, F(1,15) = 0.19, p > .50, ηp2 = .01) and distraction had a similar effect on performance at both delays (distraction x delay, F(1,15) = 0.83, p > .30, ηp2 = .05).
Discussion
Healthy participants were presented with pictures of individual faces and their memory for each face was tested after a short (8s) or longer (15s) delay. During the delay period, subjects either viewed a blank screen (no-distraction condition) or made gender discriminations about five serially presented distractor faces (distraction condition). We found that delayed face matching-to-sample performance decreased following distraction at both the short and longer delays. These results stand in contrast to the lack of an effect of interference on delayed matching-to-sample performance observed by Shrager et al. (2008) when subjects had to either count the number of distractor faces presented during a 14-second delay period or decide whether one of the distractor faces was Bill Clinton.
Our findings further clarify the conditions that are required to test whether performance in short-delay memory tasks is sensitive to interference. Logie, Zucco and Baddeley (Logie, Zucco, & Baddeley, 1990) highlighted the importance of a match between the processing resources required by the distractor task and those needed to maintain the to-be-remembered information. More specifically, they showed that the maintenance of nonverbalizable stimuli was disrupted by a distractor task that required visual imagery but not one that required mental arithmetic. Conversely, maintenance of verbalizable stimuli was disrupted by a distractor task that required mental arithmetic, but not one that required visual imagery. Shrager et al. (2008) acknowledged the importance of engaging the same domain of working memory in the memory task and the distractor task, and noted that their first distractor task (counting faces) may not have achieved this aim. Thus, they included a second distractor task that required processing of face identity (identifying the presence of Bill Clinton’s face among a set of distractor faces), with the assumption that this task would provide the requisite load to reveal any potential interference effect. The absence of an interference effect with their distractor task, however, contrasts with our finding of an interference effect when the distractor task required gender judgments. Presumably, the gender task, which required processing of every distractor face, imposed a greater load than did the face identity task in Shrager et al. (2008), in that processing in their task could be aborted once the target face had been encountered. These findings highlight the fact that a demand on similar processing resources in the memory and distractor task may not, by itself, be adequate to induce interference, but that the distractor task must engage those processing resources to a sufficient degree. This notion is consistent with the view that there is a necessary trade-off between maintenance and processing in working memory that is mediated by a common demand on attention, and that memory traces decay only when processing activities sufficiently drain attentional resources so as to disrupt maintenance (Barrouillet & Camos, 2012; Cowan, 1999).
Returning to the primary aim of Experiment 1, we found that performance in the delayed face matching-to-sample task was disrupted by interference, thus qualifying this task as a STM task according to the proposed distractor-based criterion (Shrager et al., 2008). As such, the task is appropriate for our examination of the role of the MTL in STM.
Experiment 2
In Experiment 2, we aim to examine whether MTL amnesic patients show impaired performance on the delayed face matching-to-sample task used in Experiment 1. The face delayed matching-to-sample task was administered to amnesic and control participants without distraction in the 8s and 15s delay conditions. Critically, not only does this task meet the proposed distractor-based criterion as a STM task (Experiment 1), but the inclusion of an 8s delay condition also clearly qualifies it as a STM task according to the traditional delay-based criterion (Atkinson & Shiffrin, 1968; Cowan, 1988; Peterson & Peterson, 1959).
In Experiment 2a, MTL amnesic patients and control subjects were tested on the task from Experiment 1 (without distraction). Experiment 2b constituted a control condition aimed at ruling out perceptual impairments at the level of face discrimination as the possible cause of any observed STM impairment in the MTL amnesic patients. Here, participants completed a face-matching task in which pairs of faces were presented simultaneously and same/different judgments were required.
Experiment 2a: Face Memory without Distraction
Method
Participants
Nine amnesic patients with MTL lesions participated in the study (Table 1). Eleven healthy controls also participated and were matched to the patient group in terms of mean age (59.18 years, SD = 10.94), education (16.09 years, SD = 3.18), and verbal IQ (106.36, SD = 12.94) (all t’s < .69). All participants were paid for their participation and provided informed consent in accordance with the procedures of the Institutional Review Boards at Boston University and the VA Boston Healthcare System.
Table 1.
Patient Demographic, Neuropsychological and Neurological Characteristics
| Patient | Etiology | Age | Edu | WAIS,III |
WMS,III |
Hipp Vol Loss |
Subhipp Vol Loss |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| VIQ | GM | VD | AD | WM | ||||||
| P01 | Encephalitis | 55 | 14 | 92 | 45 | 56 | 55 | 85 | 73% | 78%* |
| P02 | Encephalitis | 66 | 12 | 106 | 69 | 68 | 77 | 111 | 66% | 72%+ |
| P03 | Anoxia | 60 | 12 | 83 | 52 | 56 | 55 | 91 | N/A | N/A |
| P04 | Anoxia + left temporal lobectomy |
46 | 16 | 86 | 49 | 53 | 52 | 93 | 63% | 60%^ |
| P05 | Anoxia | 54 | 14 | 111 | 59 | 72 | 52 | 96 | 22% | - |
| P06 | Encephalitis | 82 | 18 | 135 | 45 | 53 | 58 | 141 | N/A | N/A |
| P07 | Anoxia | 58 | 17 | 134 | 70 | 75 | 67 | 126 | N/A | N/A |
| P08 | Anoxia | 60 | 16 | 110 | 62 | 68 | 61 | 92 | N/A | N/A |
| P09 | Anoxia | 55 | 18 | 119 | 58% | - | ||||
Note. Age = Age (years); Edu = Education (years); WA1S, III = Wcchslcr Adult Intelligence Scale. III; VIQ = Verbal IQ; WMS, III = Wcchslcr Memory Scale, III; GM = General Memory; VD = Visual Delayed; AD = Auditory Delayed; WM = Working Memory; Hipp Vol Loss = Bilateral Hippocampal Volume Loss; Subhipp Vol Loss = Parahippocampal Gyrus Volume Loss;
= volume loss in bilateral anterior parahippocampal gyrus and left posterior parahippocampal gyrus.
= volume loss in bilateral anterior parahippocampal gyrus and right posterior parahippocampal gyrus.
= volume loss in left anterior parahippocampal gyrus.
To assess the extent of patients’ neural damage, structural magnetic resonance imaging (MRI) scans were collected for five of the patients. (MRI could not be obtained for the remaining patients because of medical contraindications, but MTL pathology can be inferred on the basis of etiology and neuropsychological profile.) Information about the acquisition and analysis of MRI scans and lesion volumetrics has been previously reported for patients P01, P02, P04, P05 (Kan, Giovanello, Schnyer, Makris, & Verfaellie, 2007). For P09, lesion volumetric analysis of medial temporal lobe regions was performed in a semi-automated fashion using ITK-SNAP (www.itksnap.org) (Yushkevich et al., 2006) following the same segmentation parameters. Quantitative analysis compared patients’ regional brain volumes (corrected for intracranial volume) to volumes from eight age- and gender-matched control subjects. Two of the anoxic patients (P05 and P09) had damage limited to the hippocampus and two of the encephalitic patients (P01 and P02) and one of the anoxic patients (P04) had damage to the hippocampus and surrounding parahippocampal gyrus (volume reductions >2 SDs from the control mean; see Table 1). For the encephalitic patient P06, a computerized tomography (CT) scan was available and visual inspection indicated extensive hippocampal and parahippocampal gyrus damage. Measurements of frontal, parietal, occipital, and lateral temporal cortex were also made to assess the possibility of additional damage outside the MTL in patients for whom whole-brain volumetrics were available (P01, P02, P04, P05). No common volume reductions were found outside the MTL. We were particularly interested in the possibility of neural damage in the fusiform cortex, given the importance of this area for face processing. The only volume reduction in fusiform cortex was found in the left fusiform gyrus of the patient whose etiology included left temporal lobectomy (P04), and inclusion or exclusion of this patient did not affect results. The neuropsychological profiles of all patients indicate impairments isolated to the domain of memory with profound impairments in new learning (Table 1).
Stimuli
Experiment 2a used a subset of face stimuli used in Experiment 1 (256 study-test faces, divided into four sets of 32 pairs). As in Experiment 1, one of the faces in each pair was designated as a test face. The study face was designated as the other face in the pair (mismatch) for half of the pairs, and the same face as the test face (match) for the other half of the pairs. The assignment of each pair as a match or mismatch pair was counterbalanced across subjects, to ensure that each test face appeared equally often as a match or a mismatch face, and each set of faces appeared equally often in the 8s and 15s conditions across subjects.
Procedure
Patients and controls performed two sessions of the task, with each session containing two study-test blocks (one 8s delay block and one 15s delay block). The order of the 8s and 15s delay blocks for each session was counterbalanced within and across subjects, resulting in each condition appearing an equal number of times as the first block and the second block. During each session, subjects were given a practice block of two 8s delay trials before starting the task.
As in Experiment 1, subjects were first shown a blank screen for 1000ms, followed by a central fixation cross for 1000ms, another blank screen for 1000ms, and a study face for 1000ms. Subjects were told to remember the identity of the study face over the subsequent delay. A blank screen followed the study face for either a 7983ms delay (8s delay condition) or a 14983ms delay (15s delay condition), followed by the test face in which the subjects reported whether or not the test face matched the study face (see Figure 2A). The test face remained on the screen until subjects made a verbal response.
Figure 2.
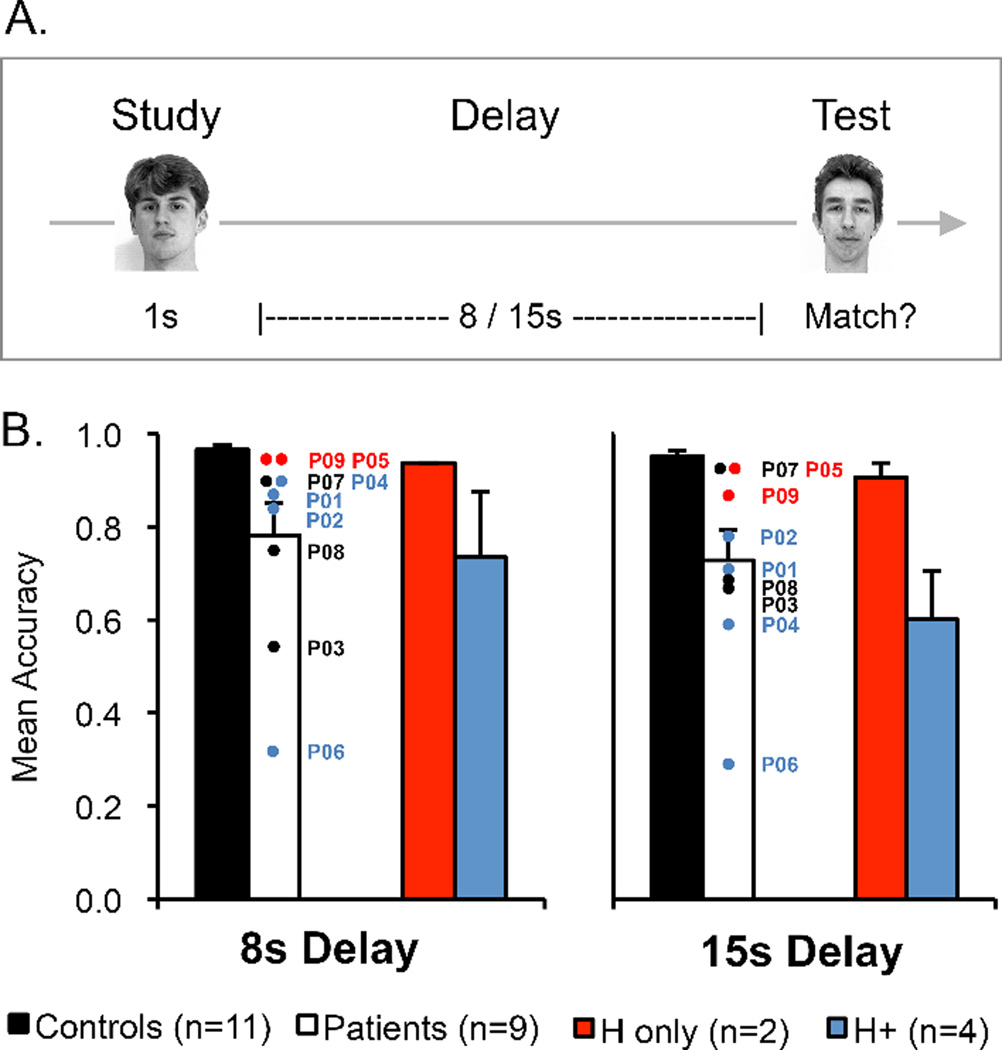
(A) Experiment 2a task design. (B) Results from Experiment 2a. Mean accuracy (hits – false alarms) for controls (black bars), the whole amnesic patient group (white bars), amnesic patients with confirmed MTL damage limited to the hippocampus (H-only; red bars), and amnesic patients with MTL damage that included the hippocampus and subhippocampal cortex (H+; blue bars) on the 8s and 15s delayed face matching-to-sample task without distraction. Error bars indicate SEM.
Results
Results from Experiment 2a are presented in Figure 2B. Patients’ mean accuracy (hits minus false alarms) was reduced compared to controls’ both in the 8s delay condition (78% vs. 96%) and the 15s delay condition (72% to 95%). To verify the reliability of these effects, mean accuracy in each condition was entered into a 2 × 2 mixed model ANOVA with factors of delay (8s, 15s) and group (patients, controls). ANOVA confirmed that patients’ performance was impaired compared to controls’ (main effect of group, F(1,18) = 11.74, p = .003, ηp2 = .40). There was no difference in performance in the 8s versus the 15s condition (main effect of delay, F(1,18) = 2.96, p > .10, ηp2 = .14) and the performance difference between groups was similar in both delay conditions (group x delay, F(1,18) = 1.04, p > .30, ηp2 = .06).
In order to investigate more precisely the anatomical basis of the delayed matching-to-sample impairment in amnesia, performance was separately analyzed for the patients with volumetrically confirmed damage limited to the hippocampus (P05, P09; H-only group) and for the patients with volumetrically or visually confirmed MTL damage that included the hippocampus and subhippocampal cortex (P01, P02, P04, P06; H+ group). A 3 × 2 ANOVA with factors of group (controls, H+ patients, H-only patients) and delay (8s, 15s) revealed that performance differed across groups (main effect of group, F(2,14) = 9.71, p = .002, ηp2 = .58) and that this difference in group performance varied according to delay (group x delay, F(2,14) = 4.59 p = .03, ηp2 = .40). Follow-up analysis revealed that the performance of the H+ patients was impaired compared to controls, whereas performance of the H-only patients was intact. Specifically, when data from the H+ patients were entered into 2 × 2 ANOVA with factors of delay (8s, 15s) and group (H+ patients, controls), there was a main effect of group, F(1,13) = 17.85, p < .001, ηp2 = .58) and a group x delay interaction (F(1,13) = 8.67, p = .01, ηp2 = .40). Although H+ patients demonstrated greater impairment in the 15s delay condition (mean accuracy = 60%) than in the 8s delay condition (mean accuracy = 73%), H+ patients’ memory impairment was significant at both delays (t(13) = 5.71 p < .001, d = 3.17 and t(13) = 2.85, p = .01, d = 1.58, respectively). In contrast, when data from the H-only group were entered into a 2 × 2 ANOVA with factors of delay (8s, 15s) and group (H-only patients, controls), there was no main effect of group, F(1,11) = 2.06, p > .10, ηp2 = .16. Further follow-up analyses using a modified t-test for single cases (Crawford & Howell, 1998) confirmed that even at the shortest delay (8s) there was no evidence that either of the patients with damage limited to the hippocampus performed differently than controls (both ts(10) = 0.83, ps > .20, zCCs = .87), whereas memory performance of each of the patients in the H+ group was reduced compared to controls (ts(10) > 1.74, ps ≤ .056, zCCs > 1.82). The single case analyses for the H+ group indicate that the impairment for the H+ group as a whole was not driven solely by the low performance of P06.
Experiment 2b: Face Matching Task
Method
Participants
The same groups of patients and controls tested in Experiment 2a were tested in Experiment 2b, with the exception of one control subject who was lost to follow up.
Stimuli
The same face stimuli used in Experiment 2a were used in Experiment 2b (256 study-test faces, divided into four sets of 32 pairs). For each pair, one of the faces was designated as the target face. Half of the pairs were used in match trials, in which two identical target faces were presented. The other half of the pairs were used in mismatch trials, in which the target face was presented with its mate. The assignment of each pair to the match or mismatch conditions was counterbalanced across subjects, to ensure that each target face appeared equally often in the two conditions. Critically, the two faces were presented simultaneously in Experiment 2b, with no intervening delay.
Procedure
Each subject performed one session of the matching task, consisting of 32 trials (Figure 3A). Prior to the start of the matching task, subjects were given two practice trials. On each trial, subjects were first shown a blank screen for 1000ms, followed by a central fixation cross for 1000ms. After the fixation-cross disappeared, two faces simultaneously appeared on the screen side-by-side for 2000ms. Subjects were asked to determine whether the two faces matched, meaning that they were the same picture, or did not match, meaning that they were different pictures. A blank screen followed presentation of the faces and remained present until subjects made a verbal decision about whether the two faces did or did not match.
Figure 3.
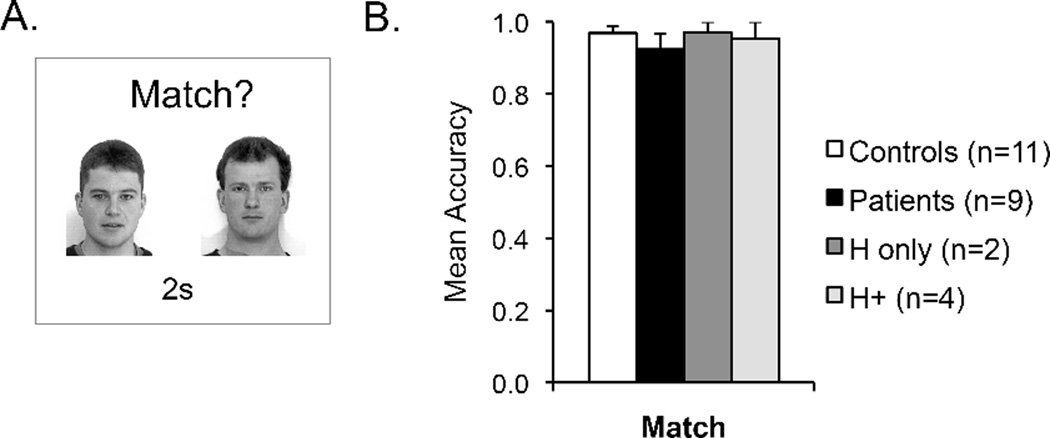
(A) Experiment 2b task design. (B) Results from Experiment 2b. Mean accuracy (hits – false alarms) for healthy controls (white bar), the whole amnesic patient group (black bar), amnesic patients with confirmed MTL damage limited to the hippocampus (H only; dark grey bars), and amnesic patients with MTL damage that included the hippocampus and subhippocampal cortex (H+ group; light grey bars) on the face matching task. Error bars indicate SEM.
Results
Performance on the face matching task is presented in Figure 3B. Mean accuracy (hits minus false alarms) on the face matching task did not differ between patients (92%) and controls (97%) (t(17) = 0.99, p > .30, d = 0.48), indicating that patients could discriminate the faces used in this experiment as well as controls. Follow-up analyses confirmed that face matching performance did not differ from controls for both the patients with confirmed damage limited to the hippocampus (t(10) = 0.02, p > .50, d = 0.01) and for the patients with confirmed MTL damage that included the hippocampus and subhippocampal cortex (t(12) = 0.39, p > .50, d = 0.23). Finally, when data across Experiment 2a and 2b were entered into ANOVA with factors of group (control, H+ patients, H-only patients) and delay (8s, 15s, match), there was a significant group x delay interaction (F(4,26) = 11.23, p < .001, ηp2 = .63), confirming that the memory impairment in H+ patients depended on the presence of a temporal delay between study and test faces.
Discussion: Experiment 2a and Experiment 2b
In Experiment 2a, we demonstrated that amnesic patients with MTL damage were impaired at a delayed face matching-to-sample task that met both the traditional delay-based criterion and the recently proposed distractor-based criterion for classification as a STM task. In Experiment 2b, with stimuli that were identical to those used in Experiment 2a, we found that face matching performance did not differ between patients and controls. The finding that patients could discriminate between the same faces used in Experiment 2a as well as controls argues against the possibility that the STM impairment observed in Experiment 2a was simply due to patients’ inability to visually discriminate between study and test faces. While it is possible that patients may demonstrate deficits in other perceptual tasks, for example tasks that use stimuli with higher feature ambiguity, the results of Experiment 2b confirm that our patients do not have perceptual deficits at the level of face stimulus matching that could account for the deficits observed in the present STM task.
Our findings also elucidate the neural basis of this STM impairment in MTL amnesia. Amnesic patients’ performance differed as a function of the extent of MTL lesion. Patients with a volumetrically documented lesion restricted to the hippocampus performed as well as controls, suggesting that STM for faces does not depend on the hippocampus. In contrast, four patients with documented lesions extending into anterior subhippocampal cortices showed impaired performance relative to control subjects. These findings suggest that the face STM impairment observed in amnesia may be linked specifically to lesions of the subhippocampal cortex rather than the hippocampus proper. Taken alone these findings must be interpreted with caution, given that the patients with H+ lesions also had greater volume reduction in the hippocampus than did the H-only patients. It is notable, however, that our interpretation is consistent with converging evidence for a role of anterior subhippocampal cortices, and in particular the perirhinal cortex, in both short-delay and long-delay memory for faces (e.g., Preston et al., 2010; Schultz, Sommer, & Peters, 2012).
Experiment 3
In Experiment 3, we test a hypothesis about the mechanism underlying the STM impairment in the H+ amnesic group in Experiment 2a. In light of the evidence that subhippocampal cortices play a critical role in configural processing in the service of LTM (Haskins, Yonelinas, Quamme, & Ranganath, 2008; Giovanello et al., 2006; Preston & Gabrieli, 2008; Quamme et al., 2007) we hypothesize that the this brain region may play a similar role in STM. Specifically, given the extensive evidence that face recognition relies on configural processing and the binding of individual features into a unitized representation (Maurer, Grand, & Mondloch, 2002; Rhodes, 1988; Sergent, 1984), it seems likely that STM for faces relies on the encoding and maintenance of such bound representations such that a failure in configural binding might underlie the STM impairment that we observed in the H+ amnesic group.
To test the hypothesis that STM for faces depends on configural processing, we examined, in healthy individuals, whether two distractor tasks that differ in their demands on configural processing would differentially disrupt delayed face matching-to-sample performance. As a “configural” distractor, we used the same gender discrimination task used in Experiment 1, given that this task has been shown to rely on configural processing (Zhao & Hayward, 2010). As a “featural” distractor, we used a task that required subjects to judge whether faces had visible teeth, thus requiring part-based processing of only a single feature. Although face processing is inherently configural in nature, we reasoned that the teeth discrimination task would impose a lower configural demand than the gender discrimination task. If STM performance is worse in the context of the gender discrimination task than the teeth discrimination task, this would suggest that configural processing plays an important role in supporting STM for faces.
Materials and Methods
Participants
Eighteen healthy participants took part (mean age = 65 years, mean education = 16 years, mean verbal IQ = 104; healthy participants were matched in terms of mean age, education, and verbal IQ to the patients in Experiment 2; all t’s < 1.85). All participants were paid for their participation and provided informed consent in accordance with the procedures of the Institutional Review Boards at Boston University and the VA Boston Healthcare System.
Stimuli
Stimuli consisted of six sets of 32 pairs taken from the stimuli used in Experiment 1. As in Experiment 1, one of the faces in each pair was designated as a test face. The study face was designated as the other face in the pair (mismatch) for half of the pairs, and the same face as the test face (match) for the other half of the pairs. The assignment of each pair as a match or mismatch pair was counterbalanced across subjects, to ensure that each test face appeared equally often as a match or a mismatch face. Each participant received three of the six stimulus sets. The assignment of each set of faces was counterbalanced across the three main conditions (no distraction, configural distraction, and featural distraction) to ensure that each test face appeared equally often as a match or a mismatch face and each set of faces rotated through each condition across subjects.
Distractor faces consisted of 320 face stimuli gathered from various face databases (see Experiment 1 and http://www.face-rec.org/databases/). All faces were forward facing, and included hair, but were free of jewelry or facial hair. To accommodate the two distractor tasks, half of the stimuli were male faces and half were female faces. Additionally, half of the male and female faces had visible teeth whereas half of the faces did not have visible teeth. Faces were organized into 64 sets of five faces, of which 16 sets contained one male face, 16 sets contained two male faces, 16 sets contained three male faces, and 16 sets contained 4 male faces. Within each of the 16 sets of faces, four subsets contained one face with teeth, four subsets contained two faces with teeth, four subsets contained three faces with teeth, and four subsets contained four faces with teeth. The 64 sets of faces were divided into two lists that contained an equal number of sets with 1–4 male faces and an equal number of sets with 1–4 faces with teeth. The assignment of distractor list to configural or featural distraction was counterbalanced across subjects.
Procedure
Participants performed three blocks of delayed matching-to-sample trials with an 8s delay. One block contained no distraction during the delay between study and test faces, one block contained configural distraction during the delay, and one block contained featural distraction during the delay, with the order of blocks counterbalanced across participants. For the distraction blocks, subjects were told to remember the identity of the study face over the delay and to count either the number of male faces presented in the intervening distractor period (configural distraction) or the number of faces displaying teeth in the intervening distractor period (featural distraction). Test faces remained on the screen until subjects made a verbal response. Subjects first responded to the distractor question and then reported whether or not the test face matched the study face.
Pilot data were collected to establish that the configural and featural distractor tasks were equated for difficulty. The 64 sets of distractor faces were presented to thirty undergraduate participants, who performed the configural distractor task with half of the faces and the featural distractor task with the other half. Participants reported the number of male faces (configural distraction) or the number of faces displaying teeth (featural distraction) in each set by typing their response into a numeric keypad as quickly and accurately as possible. Subjects’ performance did not differ across the two distractor tasks in terms of mean accuracy (configural distractor mean = 91%; featural distractor mean = 89%; t(58) = 1.43, p > .10, d = 0.38) or mean reaction time (configural distractor mean (correct trials only) = 546.91ms; featural distractor mean (correct trials only) = 516.02ms; t(58) = 0.88, p > .30, d = 0.23), confirming that the configural and featural distractor tasks were equated for difficulty.
Results
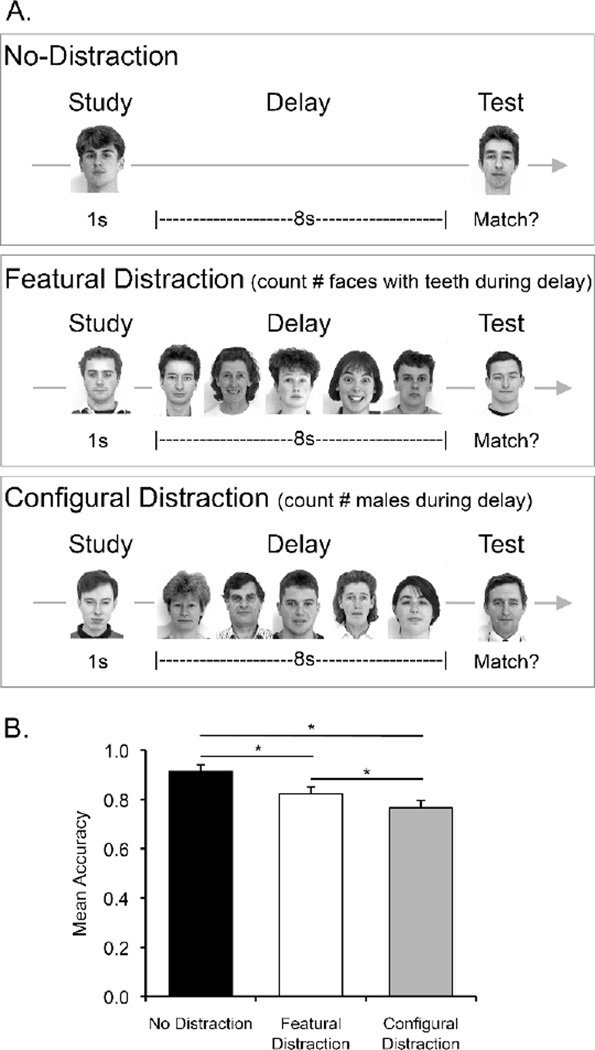
We first examined performance in the configural and featural discrimination distractor tasks. Importantly, there was no difference in performance accuracy in the two tasks (configural distractor mean = 82%; featural distractor mean = 85%; t(17) = .079, p > .40, d =0.20), ensuring that any differential effect of the two distractor tasks on STM performance could not be attributed to differential engagement in the two distractor tasks. The results for the STM task are presented in Figure 4B. Mean accuracy (hits minus false alarms) was reduced both in the featural distraction condition (mean = 82%) and in the configural distraction condition (mean = 77%) compared to the no distraction condition (mean = 91%), and the performance reduction was numerically greater in the configural than in the featural condition. ANOVA confirmed the performance disruption with distraction (main effect of distraction, F(2,34) = 7.48, p = .003, ηp2 = .31). Pairwise comparisons confirmed that this performance disruption was significant for both featural distraction (t(17) = 2.05, p = .03, d = 0.56 , 1-tailed) and configural distraction (t(17) = 3.63, p = .001, d = 0.98, 1-tailed), and that configural distraction had a greater impact on delayed matching-to-sample performance than featural distraction (t(17) = 1.97, p = .03, d = 0.29, 1-tailed).
Figure 4.
(A) Experiment 3 task design. (B) Results from Experiment 3. Mean accuracy (hits – false alarms) for healthy controls on a 8s delayed face matching-to-sample task with no-distraction (black bar), featural distraction (white bar), and configural distraction (grey bar). Error bars represent within-subject standard error. * p < .05 (1-tailed).
Discussion
In Experiment 3, we demonstrated that the degree to which performance in the delayed face matching-to-sample task was disrupted by a distractor task depends on the configural processing load of the distractor task: Performance was worse with the distractor task that made greater demands on configural processing (gender discrimination). We note that even in the teeth discrimination task some degree of whole-face processing likely occurred (cf. the composite face effect; Hole, 1994; Richler, Tanaka, Brown, & Gauthier, 2008; Young, Hellawell, & Hay, 1987) which may explain in part the decline in STM performance in that condition compared to the no-distractor condition. Importantly, however, the larger disruption in STM in the condition with greater configural demands provides support for the notion that STM for faces requires the maintenance of configural information. These findings are consistent with the hypothesis that the observed STM impairment for faces in amnesic patients with damage to subhippocampal MTL cortex reflects a disruption of configural processing.
General Discussion
The current study found that amnesic patients with MTL damage are impaired at remembering single faces over delays as short as 8 seconds (Experiment 2a), an impairment that occurred in the context of intact perceptual matching of the same stimuli (Experiment 2b). Notably, the study-test delay in this task falls within traditional delay-based limits of STM and the task meets a recently proposed distractor-based criterion for STM classification (Experiment 1). Thus, we interpret the impairment in amnesia on this task as reflecting a deficit in STM rather than being an artifact of the well-documented LTM impairment in amnesia. These findings add to a growing body of literature indicating a role for the MTL in short-delay memory tasks and clarify that this role is specifically related to STM.
The observation that MTL lesions impair STM for faces sheds new light on current controversies about the nature and necessity of MTL processes for STM. While STM has traditionally been regarded as independent of the MTL, accumulating evidence from neuroimaging studies has challenged this notion by demonstrating hippocampal activity (Axmacher et al., 2007; Hannula & Ranganath, 2008; Nichols et al., 2006; Olsen et al., 2009; Piekema et al., 2009; Ranganath et al., 2005; Ranganath & D'Esposito, 2001; Schon et al., 2012) as well as subhippocampal activity (Ranganath & D'Esposito, 2001; Schon et al., 2012) when nonverbal information is maintained over short delays. However, neuroimaging evidence cannot demonstrate whether MTL activity is necessary for short-delay memory and it has been unclear whether the MTL activity observed in short-delay tasks specifically relates to STM function. It has been suggested alternatively that MTL activity during short-delay tasks may simply reflect feed-forward projections from extra-MTL cortices that are responsible for the encoding and short-term maintenance of stimulus representations (Olsen et al., 2009). Another suggestion is that this MTL activity reflects stimulus novelty or LTM encoding signals that are not directly related to the short-term maintenance of information (Ranganath et al., 2005; Ranganath & D'Esposito, 2001; Schon et al., 2004). Both of these possibilities suggest that MTL processes are not necessary for STM and make the prediction that MTL lesions should leave STM performance intact. By demonstrating that MTL amnesics are impaired on a STM task, the current study provides evidence against this notion. Without negating the possibility that some of the MTL activity observed during short-delay tasks may reflect processes not specifically related to STM, the current results provide compelling evidence that the MTL does play a critical role in STM.
Our results support and extend prior reports of impaired short-delay memory in MTL amnesia (Aggleton et al., 1992; Buffalo et al., 1998; Ezzyat & Olson, 2008; Hannula et al., 2006; Hartley et al., 2007; Nichols et al., 2006; Olson, Moore, et al., 2006; Olson, Page, et al., 2006; Owen et al., 1995; Piekema et al., 2007; Rose et al., 2012; Ryan & Cohen, 2004; Warrington & Taylor, 1973) by demonstrating that STM for faces is impaired in patients with subhippocampal lesions but is intact in patients with lesions limited to the hippocampus. It is noteworthy that a majority of the MTL amnesic patients in the Shrager et al (2008) study, which demonstrated intact short-delay memory for faces, had lesions limited to the hippocampus. Our findings help explain the variability across studies concerning the status of short-delay memory for faces in MTL amnesia (Ezzyat & Olson, 2008; Nichols et al., 2006; Olson, Moore, et al., 2006; Rose et al., 2012; Shrager et al., 2008; Warrington & Taylor, 1973), and suggest that STM for faces may be impaired only in patients with subhippocampal lesions. The one apparent contradiction to this pattern comes from the developmental amnesic HC (Rose et al., 2012), described to have damage limited to the hippocampus, who showed impaired STM for non-famous faces. It should be noted, however, that the absence of extrahippocampal MTL damage was inferred on the basis of visual inspection and was not volumetrically confirmed.
The results of Experiment 3 suggest that the impairment in patients with subhippocampal lesions may be due to the configural processing demands of the task. In particular, Experiment 3 demonstrated that the degree to which STM for faces is disrupted by a distractor task in healthy participants depends on the degree to which the distractor task requires configural processing. This finding suggests that the maintenance of configural information is critical to successful performance on this task, and is consistent with the notion that impaired performance in amnesia is due to a failure of MTL-mediated configural processing mechanisms. Demands on a similar configural memory mechanism may explain deficits in MTL amnesia in short-delay memory tasks for other complex novel stimuli that are processed as single units, such as fractals (Holdstock, Gutnikov, Gaffan, & Mayes, 2000) and visual patterns (Owen et al., 1995; Sidman, Stoddard, & Mohr, 1968).
The fact that the STM impairment for faces was present only in patients with subhippocampal lesions is consistent with the proposed specialization within the MTL in service of LTM, whereby subhippocampal MTL cortex supports configural (intra-item) binding and memory for unitized associations (Eichenbaum et al., 1996; Norman & O’Reilly, 2003; Haskins et al., 2008; O’Reilly and Rudy, 2001; Preston & Gabrieli, 2008). Indeed, neuroimaging data suggest a role for subhippocampal cortices, and specifically perirhinal cortex, in face encoding and retrieval (Preston et al., 2010; Schultz et al., 2012) and more generally, in the memorial binding of unitized associations (Haskins, Yonelinas, Quamme, & Ranganath, 2008) and item-related elements, such as object-color associations (Diana et al., 2010; Staresina & Davachi, 2008). Further support comes from neuropsychological findings demonstrating that memory for unitized associations is relatively preserved following hippocampal damage but is impaired following MTL damage that extends into MTL cortex (Giovanello et al., 2006; Quamme et al., 2007). The current findings extend this functional specialization to the STM domain by demonstrating that STM for faces, a task that requires configural processing, was impaired selectively in patients with subhippocampal lesions.
The intact performance in the present study of the two patients with lesions limited to the hippocampus is consistent with the notion that the kind of mnemonic binding mediated by the hippocampus is distinct from that mediated by subhippocampal cortices. More specifically, it has been proposed that the hippocampus supports relational binding and memory for inter-item associations that are not unitized (Cohen et al., 1999; Cohen & Eichenbaum, 1993; Diana, Yonelinas, & Ranganath, 2007; Konkel & Cohen, 2009). Further, such hippocampally-mediated relational processing is thought to be important for binding novel relations in memory both in the short and long term (Cashdollar et al., 2009; Finke et al., 2008; Hannula et al., 2006; Jonides et al., 2008; Olsen et al., 2009; Olson, Page, et al., 2006; Rose et al., 2012). This proposal provides a straightforward account of impaired short-delay memory performance in MTL amnesia on tasks that require maintenance of the association between items, the association between an item and its location (Finke et al., 2008; Olson, Moore, et al., 2006; Olson, Page, et al., 2006), or the location of objects within a scene (Cashdollar et al., 2009; Hannula et al., 2006). It also accounts for the preservation of STM on tasks that require only intra-item binding (as is the case in the present study) in patients with lesions limited to the hippocampus.
The above considerations highlight that understanding MTL contributions to STM, and resolving discrepancies in the literature about the status of STM in MTL amnesia, requires consideration both of the nature of the binding processes required by a task and the precise MTL region that is implicated in that type of binding. In this context, it is worth emphasizing that some STM tasks make no demands on either configural or relational binding and, as such, their preservation in MTL amnesia is explicable in the present framework. Prominent among those are tasks that entail maintenance of easily verbalizable material. Indeed, MTL amnesics show spared STM for words (Baddeley et al., 2010; Baddeley & Warrington, 1970), nameable shapes (Baddeley et al., 2010), and famous faces (Rose et al., 2012). Intact performance in amnesia in these instances may be due to the fact that maintenance can be supported by rehearsal in the phonological loop (e.g., Rose et al., 2012), which is thought to depend on a network of frontal, parietal and cerebellar regions (Awh et al., 1996; Desmond, Gabrieli, Wagner, Ginier, & Glover, 1997; Jonides et al., 1998; Paulesu, Frith, & Frackowiak, 1993; Schumacher et al., 1996; Trost & Gruber, 2012). Many standard neuropsychological tests of STM, such as Corsi Blocks and Letter-Number Sequencing, similarly may make limited demands on the integration and/or maintenance of bound information, which may explain why performance on these tasks is unaffected by MTL lesions.
Finally, on a different note, findings of impaired STM in amnesia raise questions regarding the potential contribution of perceptual impairments to deficits in STM. This issue is particularly compelling in light of our finding that the STM impairment was specifically linked to damage to anterior subhippocampal cortices. Anterior subhippocampal cortex, and specifically perirhinal cortex, has been implicated, not only in mnemonic processing, but also in perceptual processing of complex visuospatial stimuli including faces (Barense, Henson, Lee, & Graham, 2010; Iidaka, Harada, Eifuku, Nakata, & Sadato, 2012; Lee, Scahill, & Graham, 2008; Preston et al., 2010; Schultz et al., 2012). Some of this evidence has come from studies showing that patients with MTL lesions involving perirhinal cortex show impaired performance on perceptual tasks that involve discrimination of stimuli with high feature overlap, which therefore require integration of features (Graham, Barense, & Lee, 2010). It has been argued that apparent STM deficits for complex visual stimuli in amnesia may actually be the consequence of perceptual impairments (Graham et al., 2010). The finding of preserved face matching in the current study provides evidence against this interpretation of our results (see also Ezzyat & Olson, 2008). One could argue however, that we cannot exclude the possibility of subtle perceptual impairment in our patients, which might manifest only in face matching tasks that use stimuli with higher feature ambiguity than did ours. By this view, it is possible that the face representations formed by the amnesic patients in our study were less robust than those of healthy individuals. As a consequence, amnesics’ face representations may have been susceptible to accelerated degradation, leading to impaired short-delay matching performance (Olsen et al., 2012; Warren, Duff, & Trannel, 2011). This argument, however, begs the question as to whether accelerated degradation is better characterized as a deficit in perception or STM. The question as to how best characterize the impairment in short-delay visuospatial memory tasks in amnesia is likely to continue to generate vigorous debate, but it is noteworthy that MTL-mediated configural-relational processing is a central tenet on both sides of the debate.
Conclusions
In summary, our findings demonstrate impaired performance in amnesia on a short-delay face matching task that meets both a traditional time-based as well as an interference-based criterion for classification as a STM task. By demonstrating in healthy individuals that the extent to which performance is disrupted by interference depends on the configural processing demands of the distractor task, our findings point to impaired MTL-mediated configural processing as a possible source of the observed STM impairment in the present study. Finally, the absence of impairment in patients with documented lesion limited to the hippocampus suggests that the STM impairment for faces may be specifically linked to subhippocampal cortices. These findings point to the necessary role of MTL structures in STM and highlight that their contribution can best be understood with reference to the types of binding operations required.
Acknowledgments
This research was supported by the National Institute of Neurological Disorders and Stroke (NINDS) Grant No. F32NS073212 and the Clinical Science Research and Development Service, Department of Veterans Affairs. The authors thank Margaret Cadden, Michelle Lee, Katie Sheahon, and Charleen Wilder for help with data collection.
Footnotes
These delay intervals were chosen to approximate those used in Shrager et al. (2008) based on calculation of the interval needed to present their maximum number of distractors, but Shrager et al. (2008) refer to these as 7s and 14s delay conditions.
Contributor Information
Elizabeth Race, Memory Disorders Research Center, VA Boston Healthcare System and Boston University School of Medicine.
Karen F. LaRocque, Memory Disorders Research Center, VA Boston Healthcare System and Boston University School of Medicine
Margaret M. Keane, Department of Psychology, Wellesley College and VA Boston Healthcare System
Mieke Verfaellie, Memory Disorders Research Center, VA Boston Healthcare System and Boston University School of Medicine.
References
- Aggleton JP, Shaw C, Gaffan EA. The performance of postencephalitic amnesic subjects on two behavioural tests of memory: concurrent discrimination learning and delayed matching-to-sample. Cortex. 1992;28(3):359–372. doi: 10.1016/s0010-9452(13)80146-3. [DOI] [PubMed] [Google Scholar]
- Atkinson RC, Shiffrin RM. Human memory: A proposed system and its control processes. In: Spence KW, Spence JT, editors. The psychology of learning and motivation. Vol. 2. New York: Academic Press; 1968. pp. 89–195. [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychological Science. 1996;7(1):25–31. [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. Journal of Neuroscience. 2007;27:7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Allen R, Vargha-Khadem F. Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia. 2010;48:1089–1095. doi: 10.1016/j.neuropsychologia.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Warrington EK. Amnesia and the distinction between long- and short-term memory. Journal of Verbal Learning and Verbal Behavior. 1970;9:176–189. [Google Scholar]
- Barense MD, Henson RN, Lee AC, Graham KS. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: Effects of viewpoint. Hippocampus. 2010;20:389–401. doi: 10.1002/hipo.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrouillet P, Camos V. As time goes by: Temporal constraints in Working Memory. Current Directions in Psychological Science. 2012;21:413–419. [Google Scholar]
- Bergmann HC, Rijpkema M, Fernandez G, Kessels RP. Distinct neural correlates of associative working memory and long-term memory encoding in the medial temporal lobe. Neuroimage. 2012;63:989–997. doi: 10.1016/j.neuroimage.2012.03.047. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8(4):330–339. doi: 10.1002/(SICI)1098-1063(1998)8:4<330::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Cashdollar N, Malecki U, Rugg-Gunn FJ, Duncan JS, Lavie N, Duzel E. Hippocampus-dependent and -independent theta-networks of active maintenance. Proceedings of the National Academy of Sciences U S A. 2009;106:20493–20498. doi: 10.1073/pnas.0904823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2:151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum HE. Memory, amnesia, and the hippocampal system. Cambridge: MIT Press; 1993. [Google Scholar]
- Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychological Bulletin. 1988;104(2):163–191. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- Cowan N. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of active maintenance and executive control. Cambridge, U.K: Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- Crawford JR, Howell DC. Comparing and individual's test score against norms derived from small samples. Clinical Neuropsychology. 1998;12:482–486. [Google Scholar]
- Curby KM, Gauthier I. A visual short-term memory advantage for faces. Psychonomic Bulletin and Review. 2007;14(4):620–628. doi: 10.3758/bf03196811. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. Journal of Neuroscience. 1997;17(24):9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. Journal of Cognitive Neuroscience. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Schoenbaum G, Young B, Bunsey M. Functional organization of the hippocampal memory system. Proclamation of the National Academy of Sciences. 1996;93(24):13500–13507. doi: 10.1073/pnas.93.24.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Olson IR. The medial temporal lobe and visual working memory: comparisons across tasks, delays, and visual similarity. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(1):32–40. doi: 10.3758/cabn.8.1.32. [DOI] [PubMed] [Google Scholar]
- Finke C, Braun M, Ostendorf F, Lehmann TN, Hoffmann KT, Kopp U, Ploner CJ. The human hippocampal formation mediates short-term memory of colour-location associations. Neuropsychologia. 2008;46(2):614–623. doi: 10.1016/j.neuropsychologia.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Gerghiades AS, Belhumeur PN, Kriegman DJ. From few to many: Illumination cone models for face recognition under variable lighting and pose. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2001;23(6):643–660. [Google Scholar]
- Giovanello KW, Keane MM, Verfaellie M. The contribution of familiarity to associativ memory in amnesia. Neuropsychologia. 2006;44(10):1859–1865. doi: 10.1016/j.neuropsychologia.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M, Keane MM. Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(3):186–194. doi: 10.3758/cabn.3.3.186. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recgontition of novel associations. Neuron. 2008;59:554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. Journal of Neuroscience. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. Journal of Neuroscience. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hole G. Configural factors in the perception of unfamiliar faces. Perception. 1994;23(1):65–74. doi: 10.1068/p230065. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Gutnikov SA, Gaffan D, Mayes AR. Perceptual and mnemonic matching-to-sample in humans: contributions of the hippocampus, perirhinal and other medial temporal lobe cortices. Cortex. 2000;36(3):301–322. doi: 10.1016/s0010-9452(08)70843-8. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Harada T, Eifuku S, Nakata R, Sadato N. Distinct human face representations in the perirhinal cortex and fusiform gyrus. Brain Research. 2012;1452:119–129. doi: 10.1016/j.brainres.2012.02.072. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Hopkins RO, Squire LR. The role of the hippocampus in retaining relational information across short delays: the importance of memory load. Learning & Memory. 2011;18:301–305. doi: 10.1101/lm.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Squire LR. Intact working memory for relational information after medial temporal lobe damage. Journal of Neuroscience. 2010;30:13624–13629. doi: 10.1523/JNEUROSCI.2895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learning & Memory. 2012;19:15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Wixted JT, Hopkins RO, Squire LR. Visual working memory capacity and the medial temporal lobe. Journal of Neuroscience. 2012;32:3584–3589. doi: 10.1523/JNEUROSCI.6444-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annual Review of Psychology. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Willis CR. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18(13):5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Giovanello KS, Schnyer DM, Makris N, Verfaellie M. Role of the medial temporal lobes in relational memory: neuropsychological evidence from a cued recognition paradigm. Neuropsychologia. 2007;45:2589–2597. doi: 10.1016/j.neuropsychologia.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Frontiers in Neuroscience. 2009;3:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cerebral Cortex. 2008;18:683–696. doi: 10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Logie RH, Zucco GM, Baddeley AD. Interference with visual short-term memory. Acta Psychologia (Amsterdam) 1990;75(1):55–74. doi: 10.1016/0001-6918(90)90066-o. [DOI] [PubMed] [Google Scholar]
- Luck D, Danion JM, Marrer C, Pham BT, Gounot D, Foucher J. The right parahippocampal gyrus contributes to the formation and maintenance of bound information in working memory. Brain and Cognition. 2010;72:255–263. doi: 10.1016/j.bandc.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Maurer D, Grand RL, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Sciences. 2002;6:255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cognitive Brain Research. 2000;10(1–2):197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Dissociable contributions of prefrontal cortex and the hippocampus to short-term memory: evidence for a 3-state model of memory. Neuroimage. 2011;54:1540–1548. doi: 10.1016/j.neuroimage.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrieli JD. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychological Review. 2003;110(4):611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychological Review. 2001;108(2):311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Frontiers in Human Neuroscience. 2012;6:1–13. doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JD, Wagner AD. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. Journal of Neuroscience. 2009;29:11880–11890. doi: 10.1523/JNEUROSCI.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2006;18:1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. Journal of Neuroscience. 2006;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychological Review. 2001;108(2):311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33(1):1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Peterson MJ. Short-term retention of individual verbal items. Journal of Experimental Psychology. 1959;58(3):193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Piekema C, Fernandez G, Postma A, Hendriks MP, Wester AJ, Kessels RP. Spatial and non-spatial contextual working memory in patients with diencephalic or hippocampal dysfunction. Brain Research. 2007;1172:103–109. doi: 10.1016/j.brainres.2007.07.066. [DOI] [PubMed] [Google Scholar]
- Piekema C, Kessels RP, Mars RB, Petersson KM, Fernandez G. The right hippocampus participates in short-term memory maintenance of object-location associations. Neuroimage. 2006;33:374–382. doi: 10.1016/j.neuroimage.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Piekema C, Kessels RP, Rijpkema M, Fernandez G. The hippocampus supports encoding of between-domain associations within working memory. Learning & Memory. 2009;16:231–234. doi: 10.1101/lm.1283109. [DOI] [PubMed] [Google Scholar]
- Piepers DW, Robbins RA. A Review and Clarification of the Terms “holistic,” “configural,” and “relational” in the Face Perception Literature. Frontiers in Psychology. 2012;3:559. doi: 10.3389/fpsyg.2012.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD. High-resolution fMRI of content-sensitive subsequent memory responses in human medial temporal lobe. Journal of Cognitive Neuroscience. 2010;22:156–173. doi: 10.1162/jocn.2009.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Gabrieli JDE. Dissociation between Explicit Memory and Configural Memory in the Human Medial Temporal Lobe. Cerebral Cortex. 2008;18:2192–2207. doi: 10.1093/cercor/bhm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Norman KA. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends in Cognitive Sciences. 2005;9:374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. Journal of Cognitive Neuroscience. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Rhodes G. Looking at faces: first-order and second-order features as determinants of facial appearance. Perception. 1988;17(1):43–63. doi: 10.1068/p170043. [DOI] [PubMed] [Google Scholar]
- Richler JJ, Tanaka JW, Brown DD, Gauthier I. Why does selective attention to parts fail in face processing? Journal of Experimental Psychology: Learning, Memory and Cognitition. 2008;34:1356–1368. doi: 10.1037/a0013080. [DOI] [PubMed] [Google Scholar]
- Rose NS, Olsen RK, Craik FI, Rosenbaum RS. Working memory and amnesia: the role of stimulus novelty. Neuropsychologia. 2012;50:11–18. doi: 10.1016/j.neuropsychologia.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. The nature of change detection and online representations of scenes. Journal of Experimental Psychology: Human Perception and Performance. 2004;30:988–1015. doi: 10.1037/0096-1523.30.5.988. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. Journal of Neuroscience. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Ross RS, Hasselmo ME, Stern CE. Complementary roles of medial temporal lobes and mid-dorsolateral prefrontal cortex for working memory for novel and familiar trial-unique visual stimuli. European Journal of Neuroscience. 2012 doi: 10.1111/ejn.12062. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Schultz H, Sommer T, Peters J. Direct evidence for domain-sensitive functional subregions in human entorhinal cortex. Journal of Neuroscience. 2012;32:4716–4723. doi: 10.1523/JNEUROSCI.5126-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA. PET evidence for an amodal verbal working memory system. Neuroimage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery & Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J. An investigation into component and configural processes underlying face perception. British Journal of Psychology. 1984;75(Pt 2):221–242. doi: 10.1111/j.2044-8295.1984.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Shrager Y, Levy DA, Hopkins RO, Squire LR. Working memory and the organization of brain systems. Journal of Neuroscencei. 2008;28:4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Stoddard LT, Mohr JP. Some additional quantitative observations of immediate memory in a patient with bilateral hippocampal lesions. Neuropsychologia. 1968;6(3):245–254. [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost S, Gruber O. Evidence for a double dissociation of articulatory rehearsal and non-articulatory maintenance of phonological information in human verbal working memory. Neuropsychobiology. 2012;65:133–140. doi: 10.1159/000332335. [DOI] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Observing degradation of visual representations over short intervals when medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2011;23:3862–3873. doi: 10.1162/jocn_a_00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Taylor AM. Immediate memory for faces: long- or short-term memory? Quarterly Journal of Exerimentap Psychology. 1973;25:316–322. doi: 10.1080/14640747308400352. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Sparing of short-term memory in an amnesic patient: Implications for strengh theory of memory. Neuropsycholgia. 1968;6:235–244. [Google Scholar]
- Young AW, Hellawell D, Hay DC. Configural information in face perception. Perception. 1987;16(6):747–759. doi: 10.1068/p160747. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zhao M, Hayward WG. Holistic processing underlies gender judgments of faces. Attention, Perception & Psychophysics. 2010;72:591–596. doi: 10.3758/APP.72.3.591. [DOI] [PubMed] [Google Scholar]