Abstract
The purpose of this research was to investigate rates of residual limb fluid volume change within a day on people with transtibial limb loss. Rates of fluid volume change during 30-minute test sessions of sitting, standing, and walking activities were measured twice a day on twelve regular prosthesis users, once in the morning and once in the afternoon, using bioimpedance analysis. Between test sessions all subjects consumed food and drink, and subject activity ranged from low to high. The rate of fluid volume change within sessions ranged from −8.5%/h to +5.9%/h with a median of −2.3%/h. The rate of fluid volume change between sessions ranged from −2.6%/h to 1.2%/h with a median of −1.0%/h. The between-session rate of fluid volume change was highly correlated with afternoon within-session rates of change (r=0.9) but not well-correlated with morning within-session rates of change (r=0.8). Subjects with peripheral arterial complications showed greater fluid volume loss rates during test sessions than between sessions. Rate of fluid volume change may be affected by sitting, standing, and walking activities; presence of peripheral arterial complications; being a female; time since amputation; and maintaining the socket without doffing for extended periods.
INTRODUCTION
Some individuals with limb loss experience large changes in residual limb volume during a day. The change may detrimentally affect the quality of fit of the prosthesis and the prosthesis user's skin health. Patients are advised to be mindful of their skin health and to add socks when the prosthesis feels loose [1,2]. New ways to control residual limb volume change are being encouraged [3]. The presence of commercial volume accommodation technologies (e.g., elevated vacuum, fluid inserts [4,5,6,7]) suggests a need to meet the clinical demand for overcoming the detrimental impact of residual limb volume fluctuation.
There is a single report in the literature about residual limb volume changes measured from morning to afternoon in the same day [8]. Limb volume changes measured five hours apart every five weeks for six months on eight subjects, all of whom had their limb amputation more than two years prior as a result of traumatic injury, ranged from a 1.5% volume loss to a 2.0% volume gain with an absolute mean of 0.4%. This result suggests much variability in the data and, consistent with clinical experience, that limb volume change may be strongly subject-dependent and/or day-dependent.
Recently, we developed a technique to measure limb fluid volume change continuously while a subject wears their prosthetic limb [9,10,11,12]. This method potentially allows us to gain further insight into within-day limb fluid volume change and variables that affect it. The purpose of this research was to assess limb fluid volume in sessions of sitting, standing, and walking activities 3 to 5 h apart within the same day, to investigate how the volume change rate (%volume change/h) varied among subjects, and to explore whether the variation was related to subject health. We also compared between-session rates of change to within-session rates of change. This observational effort is an attempt to gain insight into how time, activity, subject health, and other subject characteristics affected limb fluid volume, helping to identify potential variables to study in more depth in larger research studies.
METHODS
Subjects
Volunteers were eligible for inclusion in the study if they had a transtibial limb amputation at least 12 months prior, and were a limited community-level ambulator or more active (≥K-2 on the Medicare Function Classification Level scale [13]). Other inclusion criteria were the capability to walk on a treadmill for at least 2 minutes at a self-selected walking speed, and the capability to negotiate a 10 cm high step (to step onto the treadmill). Exclusion criteria included current skin breakdown, and a residual limb length that did not allow at least 5.5 cm distance between voltage-sensing electrodes (described below). Human subject's approval was received from a University of Washington Internal Review Board, and informed consent was obtained from subjects before test procedures were initiated.
Apparatus
Residual limb fluid volume was measured using a multi-frequency bioimpedance analyzer (Hydra 4200, Xitron, San Diego, California) that we modified for measurement of extracellular fluid volume change on amputee subject residual limbs. We prepared custom electrodes using conductive tape (ARCare 8881, Adhesives Research Inc., Glen Rock, Pennsylvania) (0.09mm thickness) and custom multi-stranded silver-plated copper wire (32 AWG) with an Aramid core strand and PVC insulation (New England Wire, Lisbon, New Hampshire) (0.76mm o.d.). We attached the wire to the electrode by splaying its ends and then sandwiching it between two pieces of the conductive tape. We covered the underside of the conductive tape with a hydrogel (KM10B, Katecho, Des Moines, Iowa) to ensure good electrical coupling with the skin. We applied a very thin layer of ultrasonic coupling gel (Couplant D, GE Panametrics, West Chester, Ohio) between the hydrogel and skin. We covered the outside of the conductive tape with Tegaderm™ (Transparent Film Dressing, 3M, St. Paul, Minnesota) (0.03mm thickness) such that the edges of the Tegaderm extended over the edges of the electrodes, preventing the electrode edges from peeling up during strenuous activity. Different electrode dimensions were used depending on their function and position on the residual limb. The proximal current injecting electrode was of dimension 15.0 cm × 2.0 cm while the distal current injecting electrode was of dimension 3.5 cm diameter. The voltage sensing electrodes were both of dimension 7.5 cm × 2.0 cm. To reduce signal noise due to mechanical movement of the wires, we created a custom, four-pin, Delrin, flat connector (9.0 mm × 11.5 mm, 2.5 mm thickness) that accommodated gold-plated pins (WPI, Viking Electronics, Inc. (division of Cooper Interconnect), Moorpark, California) to attach the four insulated lead wires from the Xitron instrument cable to the electrodes. The Xitron cable was modified to include a robust cable connector (MS3116F106S, Burndy, Manchester, New Hampshire) at the unit so as to minimize noise at this connection from cable motion. These enhancements ensured a stable and consistent signal was recorded while the subject walked on the treadmill wearing the electrodes. The peak-to-peak fluctuation in signal while the subject stood bearing weight was typically less than 0.1% of the limb fluid volume.
We plotted the bioimpedance data in approximately real time (3 s delay) at a 0.5 Hz sampling rateusing custom Matlab (v. 7.10, MathWorks, Natick, Massachusetts) code implemented on a PC (Latitude D620, Dell, Round Rock, Texas). The custom Matlab code implemented a Cole model [14], similar to that used in the Xitron post-processing program [15]. Visualization of the data during the test session helped us to identify any set up problems if they existed.
Procedures
On a separate day before bioimpedance testing, but not more than 12 months prior, we conducted a series of vascular tests. Subjects were asked to refrain from consuming alcohol or caffeine during the morning of the test day before arriving at the lab. To test for presence of high blood pressure, we conducted orthostatic blood pressure (OBP) assessment. We measured the subject's systolic and diastolic blood pressures and heart rate during sitting, resting supine, and standing using an electronic blood pressure measurement unit (HEM-775, Omron, Kyoto, Japan). To test for presence of arterial disease, we assessed ankle brachial index (ABI) and segmental limb pressures (SLP) on the contralateral limb using a commercial system (TD312 Cuff Inflator, MV10 Manifold Selector, and SC12 and SC10 cuffs, Hokanson, Bellevue, Washington) and a Doppler flow meter (MD6 Doppler, Hokanson, Bellevue, Washington). We did not conduct ABI testing on subjects with bilateral amputation. Collected data were interpreted for presence of high blood pressure and peripheral arterial complications by a practicing endocrinologist using standard clinical procedures [16,17,18]. Subject health records were consulted to identify presence of a major medical condition (e.g., congestive heart failure, kidney failure, diabetes, cancer).
On the day of bioimpedance testing, subjects were asked to refrain from consuming alcohol or caffeine during the morning of the test day before arriving at the lab. After arriving for testing, the subject continued to wear his or her prosthesis while mass and height were recorded. The research practitioner assessed socket fit, ensuring pistoning was within clinically-acceptable limits. If socket fit was deemed unacceptable then the subject was referred to his or her regular practitioner for modification. Afterwards, the subject sat with the prosthesis supported on the floor. The research prosthetist recorded medical and prosthetic history. The interview lasted approximately 10 minutes.
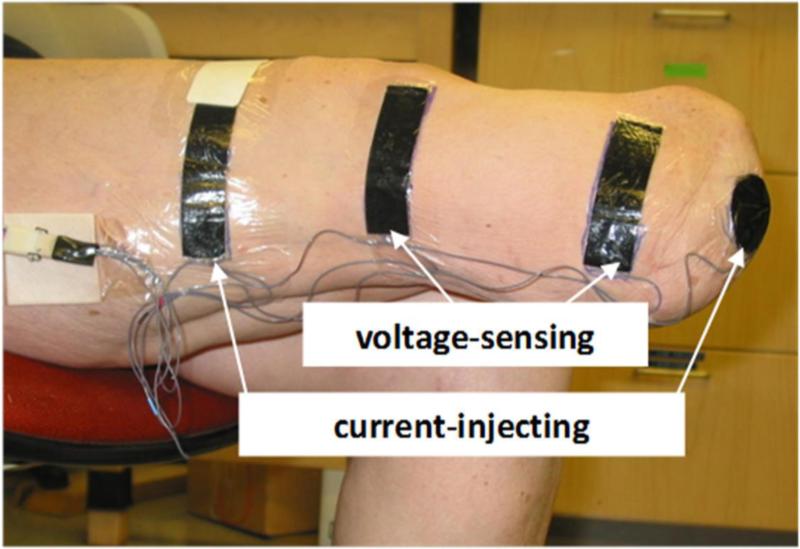
We then conducted OBP assessment. If the results indicated instability relative to the OBP test results recorded during the vascular tests then the vascular tests were repeated, and bioimpedance testing was scheduled for a different day. The subject doffed the prosthesis. We rubbed the skin gently with sandpaper (Red Dot™ Trace Prep 2236, 3M, St. Paul, Minnesota) at sites electrodes were to be placed so as to achieve good electrical coupling [19]. Four electrodes were placed on the residual limb. The outer pair injected current while the inner pair sensed voltage (FIGURE 1). We positioned the proximal voltage-sensing electrode at the level of the patellar tendon proximal to the fibular head. This position maximized the length over which we monitored and thus ensured a clinically relevant measurement, while at the same time avoided error to the volume change measurement induced by knee flexion. By avoiding bony prominences, we minimized stress concentrations in the electrode and thus minimized risk of electrode mechanical failure. We placed the distal current injecting electrode on the bottom of the residual limb. We used a circular electrode for the distal current-injecting electrode instead of a rectangular one positioned more proximally, as done previously [9,10,11,12], so as to allow a longer portion of the residual limb to be monitored. The distal voltage-sensing electrode was positioned at least 3.5 cm proximal to the distal current-injecting electrode and always proximal to the distal end of the tibia. The proximal current-injecting electrode was placed 7 cm to 12 cm proximal of the proximal voltage-sensing electrode such that it was outside of the socket brim but under the liner or suspension sleeve. To ensure no loss of suction from air escaping along the lead wires extending out at the thigh from under the liner or sleeve, we placed Tegaderm over the four wires from the electrodes, making sure the wires were not bundled under the Tegaderm which could have created channels for air to escape. The Xitron instrument applied current at between 50 μ and 700 μ across 50 frequencies (5 kHz to 1 MHz) each second, and measured amplitude and phase differences between the injected and sensed signals at a 1Hz sampling rate.
FIGURE 1.
Electrode configuration for bioimpedance analysis.
Data were collected during two 30-minute test sessions spaced 3 to 5 hours apart, with the first session starting during morning hours (between 8:30AM and 10:30 AM) and the second session starting during the afternoon (between 12:30 PM and 2:30 PM). These times were selected because they were the longest intervals participants’ schedules allowed. The test protocol was the same for both sessions. After we started collecting data with the bioimpedance analyzer, the subject donned the prosthesis and sat without talking for 2 minutes with the foot supported by the floor. Care was taken to ensure good sitting posture, since too much knee flexion occludes blood flow, and too much extension causes a slouching posture. The subject underwent five repeated cycles of: sitting (90 s); standing with equal weight-bearing (90 s); walking on a treadmill at their self-selected walking speed (90 s); and standing with equal weight-bearing (10 s). Subjects were asked not to talk because we found in pilot studies that some subjects got excited while talking, which caused them to move their residual limb and affected the limb fluid volume measurement. The total time of bioimpedance analysis sampling during a session averaged 38 minutes (s.d.=1).
The electrodes were left on the residual limb between sessions. We put the lead wires and thin custom connector under the proximal portion of the elastomeric liner or suspension sleeve so that they were not within the socket and were flush on the skin (no instrumentation was exposed). Because the electrodes were low profile, they did not cause skin irritation and were well tolerated by the subjects. For the first six subjects tested, at the end of the morning session we instrumented the subject's prosthesis with a gait monitor (StepWatch, Orthocare Innovations, Oklahoma City, Oklahoma). However, because of battery deterioration as a result of many years of disuse and thus performance problems, the gait monitors were not used on the remaining subjects.
We instructed the subject to conduct activities between sessions consistent with his or her normal lifestyle. Subjects were allowed to leave the lab for 3 to 5 h, and they were permitted to add socks between sessions if they considered it necessary but otherwise they were asked not to doff their prosthesis. They were asked not to consume alcohol or caffeine between sessions. Upon returning to the lab in the afternoon, the subject sat with the prosthesis doffed for 10 minutes so as to mimic the doffing period during the morning session when electrodes were put on the limb. The electrodes were inspected to make sure they were intact and functioned properly. The research practitioner assessed socket fit, inspected the residual limb for injury, and queried the subject about sock changes, activity, and food and liquid consumption since the morning session. Based on the subject's description, the subject's between session activity was rated as low, medium, or high, where high was considered standing or walking for at least half of the time between sessions. We noted that the activity within a test session was more intense than any subject's between-session activity. Low activity between sessions indicated that the subject sat in a lobby near the lab for the time between sessions. The gait monitor was removed and the data downloaded. We started collecting data with the bioimpedance analyzer, the subject donned the prosthesis, and the same test protocol as described above for the morning session was conducted.
Analysis
Body mass index (BMI) was calculated as the quotient of mass (kg) and the square of height (m2)[20]. Because subjects wore their prosthesis while we measured mass, no correction was made to BMI for the lack of an intact limb.
Bioimpedance data were processed using custom code that implemented a Cole model to calculate extracellular fluid resistance [14]. Our algorithm was similar to that used by the commercial instrument manufacturer (v.2.2, Xitron, San Diego, California). We developed our own code because of performance and processing speed problems encountered using the commercial software. We then converted the data to extracellular fluid volume using limb circumference and segment length measurements in a well accepted geometric limb model [21].
Residual limb fluid volume measured during equal weight bearing within the 10 s standing periods after the 90 s walking intervals and the time of the measurement were used in analysis. These were the only data used for the quantitative results presented below. The fluid volume (in mL) measured after the 1st walk cycle was considered the reference volume for each subject. All fluid volume data were expressed as a percentage of that reference (V%(t) = 100% × (VmL(t)-VmL,ref)/VmL,ref). We used percent change instead of an absolute measure because bioimpedance measures fluid volume only within the region between voltage-sensing electrodes. The size of the region varied among subjects and was dependent on their residual limb size and shape. Thus we needed to normalize the data to a consistent reference for each subject. To determine the rate of fluid volume change within each session (AM%/h, PM%/h), we used the slope of a linear fit (lowest root-mean-square error) of the five data points ((V%(i),ti), i=1 to 5, where i's are the data point during the 10 s standing periods) within each session. We calculated the rate of fluid volume change between sessions (Between%/h) using the difference in limb fluid volumes after the first cycle in the PM and AM sessions and dividing by the reference volume and the time between the two measurements ((VmL, 1st cycle PM – VmL, 1st cycle AM)/VmL, ref)/tbetween sessions.
Descriptive analyses (summary statistics and visual displays) were performed for all variables. The linear association between variables was assessed by Pearson correlation. Due to the exploratory nature of the study and the small sample size, the data analysis focused on exploratory and descriptive methods.
RESULTS
A total of 12 subjects (9 male, 3 female) with lower-limb amputation (11 unilateral, 1 bilateral), ranging in age from 25 to 65 years and averaging 54 years (s.d.=11) with a median of 55 years, participated in this research. Eight subjects had their amputation as a result of traumatic injury, and one subject each had their amputation as a result of: thrombosis subsequent to trauma; arterial disease subsequent to diabetes; MRSA (Methicillin-resistant Staphylococcus aureus) infection; and osteomyelitis. Subject mass averaged 97 kg (s.d.=26) with a median of 100 kg. Time since amputation averaged 11 years (s.d.=13) with a median of 6 years with six of the subjects between 1 and 3 years post-amputation. Subjects used their regular prosthesis in the study, which was deemed in the morning session by the research prosthetist to be of acceptable fit for regular use. Eight subjects used an elastomeric liner with lock and pin suspension, one used a Pelite liner with neoprene sleeve suspension, two used a suction socket with a gel liner, and one used a gel liner with no pin. All subjects used dynamic response prosthetic feet.
All electrodes functioned properly, and none needed to be replaced during or between tests on any subject. No subjects complained of discomfort or skin irritation from the instrumentation. The temperature in the room during testing was approximately 23°C.
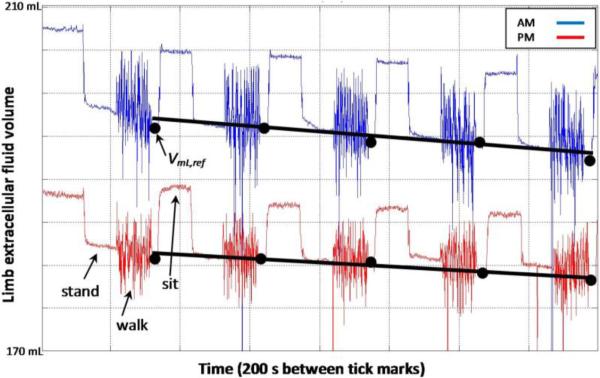
Relationships between percent residual limb fluid volume vs. time were approximately linear (FIGURE 2). Root-mean-square errors in linear fits to the percent volume vs. time curves for within-session data averaged 0.15% (s.d.=0.06%) for morning sessions and 0.16% (s.d.=0.08%) for afternoon sessions.
FIGURE 2. Exemplary bioimpedance data collected during test sessions.
The black dots indicate points during the 10 s stand interval after each 90 s walk that were used in analysis. The rate of fluid volume change was the slope of a linear fit to those five data points for each session.
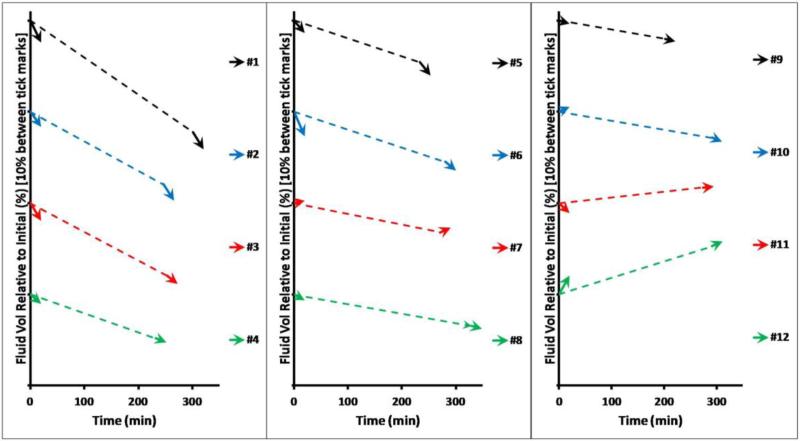
Of the twelve subjects tested, fluid volume losses over time (apparent as a negative rate of fluid volume change) were demonstrated by nine subjects for AM%/h, nine subjects for PM%/h, and ten subjects for Between%/h. The direction of fluid volume change (loss or gain) for Between%/h was the same as the direction of fluid volume change for AM%/h for nine subjects, and the same as the direction of fluid volume change for PM%/h for eleven subjects. Data illustrating fluid volumes over time as a percentage of the reference volume for all subjects are illustrated in FIGURE 3. The range, median, and mean (s.d.) fluid volume changes for each test condition are listed in TABLE 1.
FIGURE 3. Percent residual limb fluid volume change vs. time for all subjects tested.
The arrowed lines illustrate the within-session fluid volume changes (AM and PM), and the dashed liner represents the between-session changes. Subjects are ordered from highest to lowest between-session rate of change (Between%/h).
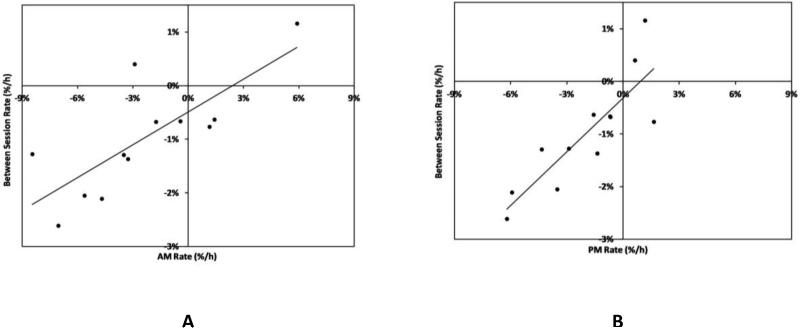
We found a strong Pearson correlation (r=0.9) between PM%/h and Between%/h (FIGURE 4B). There was a moderate correlation (r=0.8) between AM%/h and Between%/h (FIGURE 4A).
FIGURE 4. Relationships between rates of fluid volume change.
(A) Morning session (AM) data correlated moderately well with between-session data (r=0.8). (B) Afternoon session (PM) data strongly correlated with between-session data (r=0.9).
We conducted an exploratory analysis to investigate relationships between rate of fluid volume change and aspects of subject health. Subjects with peripheral arterial complications and females tended to have greater between-session (Between%/h) and within-PM session (PM%/h) loss rates than subjects without peripheral arterial complications and males (TABLE 2). Participants who had their limb amputation more than five years prior tended to have greater between-session (Between%/h) and within-PM session (PM%/h) loss rates than those less than five years prior though we expect this result may reflect, in part, their less favorable health condition. Activity between sessions, K-level, time current socket used, presence of high blood pressure, and presence of obesity or being overweight did not appear to show a trend with AM%/h, PM%/h, or Between%/h in this pilot study.
All five of the subjects who had arterial disease (#1,#2,#3,#5,#6) and only those five subjects, demonstrated faster rates of fluid volume loss within sessions than between sessions (FIGURE 5). Two subjects (#7,#12) demonstrated fluid volume gains during sessions greater than fluid volume gains between sessions.
FIGURE 5. Rates of fluid volume change.
Subjects with peripheral arterial complications (indicated with a *) showed greater fluid volume loss rates within sessions than between sessions. Subject identification #'s are the same as listed in TABLE 1.
Only two of the twelve subjects added socks between sessions (#9,#10). These two subjects showed low rates of fluid volume change compared with most of the other subjects. Interestingly, the research prosthetist's clinical inspection of socket fit during the afternoon session revealed that most of the subjects should have added socks but chose not to do so.
DISCUSSION
This preliminary investigation represents an extension from previous work quantifying limb volume change on people with transtibial amputation [22]. We used a very sensitive in-socket measurement method, bioimpedance analysis, to quantify fluid volume changes within and between sessions conducted on the same day.
We considered several sources of error in our measurement and their impact on results and interpretation. In the presented analysis we only used bioimpedance data collected while the subjects were in a consistent position, standing with equal weight-bearing. We used this strategy to help ensure other potentially influential variables, for example different limb-socket interface stress distributions from different postures, did not distort the data of interest. We expect that bioimpedance data, presented here as percentage limb fluid volume change per hour, were minimally sensitive to the anthropometric model used to convert extracellular fluid resistance to limb fluid volume [21] since fluid volume is proportional to extracellular fluid resistance. Since most subjects decreased in limb fluid volume within and between test sessions, it is unlikely that sweating affected instrument performance. If subjects sweated, conductivity between the skin and electrodes would be enhanced, reducing extracellular fluid resistance and increasing limb fluid volume, opposite of the within-session or between-session trends seen here.
The median rate of fluid volume change between sessions measured here, −1.0%/h, is larger than the rate of limb volume change measured in a previous study using an optical scanner, −0.3%/h to 0.4%/h [8]. However, a different modality was used in that study (optical imaging) and out-of-socket data were collected not in-socket data as collected here.
Fluid volume gain (edema) over the day, as occurred for two subjects in the present study (#11,#12), might initially seem counter-intuitive. However, this trend was demonstrated in a related study investigating effects of sock addition and removal on limb fluid volume in five of 28 amputee subjects tested [12]. Also, limb fluid volume gain over the day occurs in able-bodied individuals [23,24,25] and was demonstrated in the contralateral limb of a person with unilateral amputation [10]. In able-bodied people this increase is thought to result from gravity pulling fluid distally into the limb during standing and walking.
The result that the slope of the plot relating between-session rate of fluid volume change (Between%/h) and afternoon within-session rate of change (PM%/h) was less than 1.0 (i.e. 0.3) and that subject activity within sessions was typically greater than that between sessions suggests that the high activity within test sessions increased fluid volume change. This result is consistent with clinical experience. However, because subjects still lost fluid volume between sessions when they were minimally active, the result also suggests that factors other than activity induced between-session fluid volume losses. It may be that wearing the socket without doffing for extended periods contributed to the limb fluid volume decrease that occurred between sessions. With the socket donned, interstitial pressures will be elevated, reducing arterial to interstitial fluid transport and increasing interstitial to venous fluid transport. The net result is a fluid volume loss. A subject's posture while sitting might also reduce limb fluid volume if a major vessel were restricted for a prolonged interval. Noteworthy in the present study, and potentially relevant to the development of new volume management strategies, is that the magnitude of between-session change was relatively high even for subjects with low activity between sessions.
In the present study given the wide range of rate of fluid volume changes measured among subjects, we cannot state that there is a typical within-session rate and a typical between-session rate. However, the correlation between the rate of within-session change and that between sessions was strong for the afternoon session (r=0.9). We do not know from the present investigation how consistent the rate of change between sessions relative to the rate of change within sessions is from day to day for a person though the strong correlation across subjects here suggests that it may be day-independent. A study collecting data from subjects on multiple days is needed to address this issue.
The magnitude of the ratio between Between%/h and PM%/h, 0.3, may reflect the controlled study conditions under which we evaluated our test subjects and might not occur when test subjects are outside of the lab conducting their normal routines. Future research studies investigating the influence of one variable at a time determined in the present study to potentially have impact on the rate of fluid volume change would help to clarify this issue: amount and nature of activity, food and liquid intake, presence of peripheral arterial complications, female gender, time since amputation, and presence and durations of periods of prosthesis doffing.
In the present study the absolute rate of limb fluid volume change tended to be larger in the morning than in the afternoon, though this pattern did not happen in all subjects. Our study design did not control subject activity before the morning session, though from verbal input some subjects told us that they rose for the day less than an hour prior to arriving at the lab while others had been active for several hours. Further investigation is needed to understand the time course of limb fluid volume change over the day and how much it depends upon activity.
The trend of a greater rate of fluid volume loss during periods within sessions of high activity than between sessions with presence of peripheral arterial complications is consistent with physiological changes induced by arterial difficulties. Arterial complications may restrict fluid transport from the arterial vasculature into the interstitial space during activity thus off-balancing it with fluid transport from the interstitial space into the venous system. More fluid may leave than enter the interstitial space because of insufficient arterial drive, unlike unaffected individuals who increase arterial drive during activity. High blood pressure, presence of a major disease, and BMI did not show a relationship with rates of fluid volume change in the present study though the few number of subjects may have limited our capability to identify a trend.
We expect prosthetic suspension to influence the rate of limb fluid volume change. Suspension techniques that apply tension to the distal residual limb during swing phase, for example lock and pin, suction, and vacuum, would be expected to facilitate limb fluid volume recovery. They should offset the fluid volume departure during stance phase. Thus a lower rate of fluid volume loss should occur with these suspension systems than without them. However, in the present study the two subjects who did not use a lock and pin, suction, or vacuum suspension system (#1, #11) did not show consistently less limb fluid volume recovery than the other ten subjects. It is likely that other variables besides suspension, for example subject health characteristics, influenced the results. A study isolating suspension as the controlled variable would need to be conducted to quantify its impact on residual limb fluid volume changes within the day.
An important need in future research is to investigate relationships between volume change and subject outcomes in a large sample of this population. How much less comfortable are subjects with limb loss who experience large volume fluctuations within a day than those without, particularly if they do not accommodate their prosthesis? Do volume change and subject comfort improve when the patient is fit with a new socket or uses a volume accommodation strategy intended to stabilize limb fluid volume over the day [4,5,6,7]? Addressing these questions will help specify design needs of volume accommodation technologies.
CONCLUSION
Percent residual limb fluid volume change during sessions of activity involving cycles of sitting, standing, and walking changed approximately linearly over time. The within-session rate of change ranged from −8.5%/h to +5.9%/h with a median of −2.3%/h. Between-session (3 h to 5 h) rates of change ranged from −2.6%/h to +1.2%/h with a median of −1.0%/h.
Of the twelve transtibial amputee subject participants, ten decreased in limb fluid volume between sessions and two increased. The direction of fluid volume change for the afternoon session was the same as that between sessions for eleven of the twelve participants, but the same as the morning session for only nine of the participants.
There was a strong correlation between the afternoon rate of fluid volume change (PM%/h) and the between-session rate of fluid volume change (Between%/h) (r=0.9). The slope relating Between%/h to PM%/h was 0.3. The correlation between AM%/h and Between%/h was less strong (r=0.8).
Subjects with peripheral arterial complications (n=5) experienced greater fluid volume losses during sessions than between sessions; subjects who did not have peripheral arterial complications (n=7) did not.
Only two subjects added socks between test sessions, but the practitioner deemed by clinical assessment fit that most subjects should have added socks to maintain a proper prosthetic fit.
REFERENCES
- 1.Highsmith J, Kahle JT. Prosthetic socks: simple, low-cost, helpful ways to protect your skin. In Motion. 2006;16(2):1–4. [Google Scholar]
- 2.Uellendahl JE. Prosthetic socks and liners. Military In-Step, Amputee Coalition of America and U.S. Army Amputee Patient Care Program. http://www.amputee-coalition.org/military-instep/prosthetic-socks-liners.html.
- 3.Thomas PW, Shah PB. State-of-the-Art Conference Highlights Federally Funded O&P Research. O&P Business News. May;1:2010. [Google Scholar]
- 4.Caspers CA. Hypobarically-controlled artificial limb for amputees. US Patent Number 5,549,709.
- 5.Dean RC, Mayor MB, Nelson DF, Bradley CS, Blanchard MW. Dynamic variable geometry fitting system for use with a body appliance. US Patent Number 6,585,774.
- 6.Collier MS. Vacuum-assisted prosthetic device. US Patent Number 7,468,079.
- 7.Haines WA, Colvin JM, Haynes ML, Kelley CT, Ford MW, Groves MW, Denune JA. Prosthetic device using electric vacuum pump. US Patent Number 7,947,085.
- 8.Sanders JE, Zachariah SG, Jacobsen AK, Fergason JR. Changes in interface pressures and shear stresses over time on transtibial amputee subjects ambulating with prosthetic limbs: comparison of diurnal and six-month differences. J Biomech. 2005;38:1566–1573. doi: 10.1016/j.jbiomech.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Sanders JE, Rogers EL, Abrahamson DC. Assessment of residual limb volume change using bioimpedance. J Rehabil Res Dev. 2007;44(4):525–536. doi: 10.1682/jrrd.2006.08.0096. [DOI] [PubMed] [Google Scholar]
- 10.Sanders JE, Harrison DS, Allyn KJ, Myers TR. Clinical utility of in-socket residual limb volume change measurement: Case study results. Prosthet Orthot Int. 2009;33(4):378–390. doi: 10.3109/03093640903214067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders JE, Harrison DS, Myers TR, Allyn KJ. Effects of elevated vacuum on in-socket residual limb fluid volume: Case study results using bioimpedance analysis. J Rehabil Res Dev. 2011;48(10) doi: 10.1682/jrrd.2010.11.0219. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders JE, Harrison DS, Allyn KJ, Myers TR, Ciol MA, Tsai EC. How do sock ply changes affect residual limb fluid volume in people with transtibial amputation? J Rehabil Res Dev. 2012;49(2) doi: 10.1682/jrrd.2011.02.0022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medicare region C durable medical equipment prosthetics orthotic supplier (DMEPOS) manual. Vol. 53. Palmetto GBA; Columbia (SC): 2005. pp. 5–53.6. [Google Scholar]
- 14.De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedence by using theoretical methods: a technological review. J Appl Physiol. 1997;82(5):1542–1558. doi: 10.1152/jappl.1997.82.5.1542. [DOI] [PubMed] [Google Scholar]
- 15.Hydra ECF/ICFModel 4200 Bio-impedance spectrum analyzer, Operating Manual (v. 1.02) Xitron Technologies Inc.; San Diego, CA: Aug, 2001. [Google Scholar]
- 16.Adragao T, Pires A, Branco P, Castro R, Oliveira A, Nogueira C, Bordalo J, Curto JD, Prata MM. Ankle-brachial index, vascular calcifications and mortality in dialysis patients. Nephrol Dial Transplant. 2012;27(1):318–325. doi: 10.1093/ndt/gfr233. [DOI] [PubMed] [Google Scholar]
- 17.Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg. 2011;41:110–116. doi: 10.1016/j.ejvs.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaides AN. Investigation of chronic venous insufficiency. A consensus statement. Circulation. 2000;102(20):E126–E163. doi: 10.1161/01.cir.102.20.e126. [DOI] [PubMed] [Google Scholar]
- 19.Grimnes S, Martinsen OG. Bioimpedance and bioelectricity basics. 2nd ed. Elsevier Ltd; Amsterdam: 2008. [Google Scholar]
- 20.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25(6):329–43. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 21.Fenech M, Jaffrin MY. Extracellular and intracellular volume variations during postural change measured by segmental and wrist-ankle bioimpedance spectroscopy. IEEE Trans Biomed Eng. 2004;51(1):166–175. doi: 10.1109/TBME.2003.820338. [DOI] [PubMed] [Google Scholar]
- 22.Sanders JE, Fatone S. Residual limb volume change: Systematic review of measurement and management. J Rehabil Res Dev. 2011;48(8):949–986. doi: 10.1682/jrrd.2010.09.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludbrook J, Loughlin J. Regulation of volume in postarteriolar vessels of the lower limb. Am Heart J. 1964;67:493–507. doi: 10.1016/0002-8703(64)90096-1. [DOI] [PubMed] [Google Scholar]
- 24.Stick C, Stöfen P, Witzleb E. On physiological edema in man's lower extremity. Eur J Appl Physiol Occup Physiol. 1985;54(4):442–449. doi: 10.1007/BF02337192. [DOI] [PubMed] [Google Scholar]
- 25.Stick C, Jaeger H, Witzleb E. Measurements of volume changes and venous pressure in the human lower leg during walking and running. J Appl Physiol. 1992;72(6):2063–2068. doi: 10.1152/jappl.1992.72.6.2063. [DOI] [PubMed] [Google Scholar]