Abstract
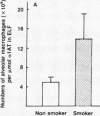
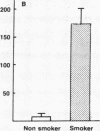
Current concepts relating to the pathogenesis of emphysema associated with cigarette smoking is that an imbalance exists within the lower respiratory tract between neutrophil elastase and the local anti-neutrophil elastase screen, enabling uninhibited neutrophil elastase to destroy the alveolar structures over time. The possible role of alveolar macrophages in contributing to this imbalance was investigated by evaluating the ability of cigarette smokers' alveolar macrophages to inactivate alpha 1-antitrypsin (alpha 1AT), the major anti-neutrophil elastase of the human lower respiratory tract. In vitro, alveolar macrophages of smokers spontaneously released 2.5-fold more superoxide anion and eightfold more H2O2 than macrophages of nonsmokers (P less than 0.01, both comparisons). Using a model system that reproduced the relative amounts of alveolar macrophages and alpha 1AT found in the epithelial lining fluid of the lower respiratory tract, we observed that smokers' macrophages caused a 60 +/- 5% reduction in the ability of alpha 1AT to inhibit neutrophil elastase. In marked contrast, under the same conditions, nonsmokers' macrophages had no effect upon the anti-neutrophil elastase function of alpha 1AT. Addition of superoxide dismutase, catalase, mannitol, and methionine prevented inactivation of alpha 1AT by smokers' macrophages, implying that the release of oxidants mediated the inactivation of alpha 1AT. In addition, by utilizing a recombinant DNA produced modified form of alpha 1AT containing an active site substitution (met358----val), the inactivation of alpha 1AT by smokers' alveolar macrophages was prevented, suggesting that the smokers' macrophages inactivate alpha 1AT by oxidizing the active site of the alpha 1AT molecule. These results suggest that in cigarette smokers, the alveolar macrophage can modulate the activity of alpha 1AT as an inhibitor of neutrophil elastase and thus play a role in the pathogenesis of emphysema associated with cigarette smoking.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud R. T., Fera T., Richter A., Tabona M. Z., Johal S. Acute effect of smoking on the functional activity of alpha1-protease inhibitor in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1985 Jan;131(1):79–85. doi: 10.1164/arrd.1985.131.1.79. [DOI] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Bieth J. G. In vivo significance of kinetic constants of protein proteinase inhibitors. Biochem Med. 1984 Dec;32(3):387–397. doi: 10.1016/0006-2944(84)90046-2. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Saltzman L. E., Adelberg S., Ferrans V. J., Crystal R. G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984 Aug;74(2):460–469. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudier C., Pelletier A., Pauli G., Bieth J. G. The functional activity of alpha 1-proteinase inhibitor in bronchoalveolar lavage fluids from healthy human smokers and non-smokers. Clin Chim Acta. 1983 Aug 31;132(3):309–315. doi: 10.1016/0009-8981(83)90009-8. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Wald M. S. Fate of human neutrophil elastase following receptor-mediated endocytosis by human alveolar macrophages. Implications for connective tissue injury. J Lab Clin Med. 1983 Apr;101(4):527–536. [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Miller F., Hoidal J. R., Janoff A. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2041–2045. doi: 10.1073/pnas.79.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Cohen A. B., James H. L. Reduction of the elastase inhibitory capacity of alpha 1-antitrypsin by peroxides in cigarette smoke: an analysis of brands and filters. Am Rev Respir Dis. 1982 Jul;126(1):25–30. doi: 10.1164/arrd.1982.126.1.25. [DOI] [PubMed] [Google Scholar]
- Cohen A. B. The effects in vivo and in vitro of oxidative damage to purified alpha1-antitrypsin and to the enzyme-inhibiting activity of plasma. Am Rev Respir Dis. 1979 Jun;119(6):953–960. doi: 10.1164/arrd.1979.119.6.953. [DOI] [PubMed] [Google Scholar]
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M., Jallat S., Tessier L. H., Benavente A., Crystal R. G., Lecocq J. P. Synthesis in E. coli of alpha 1-antitrypsin variants of therapeutic potential for emphysema and thrombosis. Nature. 1985 Jan 10;313(5998):149–151. doi: 10.1038/313149a0. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Cohn Z. A. The alveolar macrophage. J Appl Physiol (1985) 1986 Feb;60(2):353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- Fulmer J. D., Roberts W. C., von Gal E. R., Grystal R. G. Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations. J Clin Invest. 1977 Sep;60(3):595–610. doi: 10.1172/JCI108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979 Dec 14;206(4424):1315–1316. doi: 10.1126/science.316188. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P. M., Vissers M. C., Travis J., Winterbourn C. C., Carrell R. W. A genetically engineered mutant of alpha 1-antitrypsin protects connective tissue from neutrophil damage and may be useful in lung disease. Lancet. 1984 Dec 22;2(8417-8418):1426–1428. doi: 10.1016/s0140-6736(84)91623-4. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Cigarette smoking and lung destruction. Accumulation of neutrophils in the lungs of cigarette smokers. Am Rev Respir Dis. 1983 Nov;128(5):833–838. doi: 10.1164/arrd.1983.128.5.833. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Carp H. Possible mechanisms of emphysema in smokers: cigarette smoke condensate suppresses protease inhibition in vitro. Am Rev Respir Dis. 1977 Jul;116(1):65–72. doi: 10.1164/arrd.1977.116.1.65. [DOI] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Janoff A., George-Nascimento C., Rosenberg S. A genetically engineered, mutant human alpha-1-proteinase inhibitor is more resistant than the normal inhibitor to oxidative inactivation by chemicals, enzymes, cells, and cigarette smoke. Am Rev Respir Dis. 1986 Mar;133(3):353–356. doi: 10.1164/arrd.1986.133.3.353. [DOI] [PubMed] [Google Scholar]
- Janoff A., White R., Carp H., Harel S., Dearing R., Lee D. Lung injury induced by leukocytic proteases. Am J Pathol. 1979 Oct;97(1):111–136. [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Kueppers F., Black L. F. Alpha1-antitrypsin and its deficiency. Am Rev Respir Dis. 1974 Aug;110(2):176–194. doi: 10.1164/arrd.1974.110.2.176. [DOI] [PubMed] [Google Scholar]
- McGowan S. E., Arbeit R. D., Stone P. J., Snider G. L. A comparison of the binding and fate of internalized neutrophil elastase in human monocytes and alveolar macrophages. Am Rev Respir Dis. 1983 Oct;128(4):688–694. doi: 10.1164/arrd.1983.128.4.688. [DOI] [PubMed] [Google Scholar]
- Morse J. O. alpha1-antitrypsin deficiency (first of two parts). N Engl J Med. 1978 Nov 9;299(19):1045–1048. doi: 10.1056/NEJM197811092991905. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner D. E., Kleinerman J., Rice D. B. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974 Oct 10;291(15):755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- Nukiwa T., Satoh K., Brantly M. L., Ogushi F., Fells G. A., Courtney M., Crystal R. G. Identification of a second mutation in the protein-coding sequence of the Z type alpha 1-antitrypsin gene. J Biol Chem. 1986 Dec 5;261(34):15989–15994. [PubMed] [Google Scholar]
- Olsen G. N., Harris J. O., Castle J. R., Waldman R. H., Karmgard H. J. Alpha-1-antitrypsin content in the serum, alveolar macrophages, and alveolar lavage fluid of smoking and nonsmoking normal subjects. J Clin Invest. 1975 Feb;55(2):427–430. doi: 10.1172/JCI107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Richter A. M., Abboud R. T., Johal S. S., Fera T. A. Acute effect of smoking on superoxide production by pulmonary alveolar macrophages. Lung. 1986;164(4):233–242. doi: 10.1007/BF02713647. [DOI] [PubMed] [Google Scholar]
- Rosenberg S., Barr P. J., Najarian R. C., Hallewell R. A. Synthesis in yeast of a functional oxidation-resistant mutant of human alpha-antitrypsin. Nature. 1984 Nov 1;312(5989):77–80. doi: 10.1038/312077a0. [DOI] [PubMed] [Google Scholar]
- Saltini C., Hance A. J., Ferrans V. J., Basset F., Bitterman P. B., Crystal R. G. Accurate quantification of cells recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1984 Oct;130(4):650–658. doi: 10.1164/arrd.1984.130.4.650. [DOI] [PubMed] [Google Scholar]
- Snider G. L. The pathogenesis of emphysema--twenty years of progress. Am Rev Respir Dis. 1981 Sep;124(3):321–324. doi: 10.1164/arrd.1981.124.3.321. [DOI] [PubMed] [Google Scholar]
- Stone P. J., Calore J. D., McGowan S. E., Bernardo J., Snider G. L., Franzblau C. Functional alpha 1-protease inhibitor in the lower respiratory tract of cigarette smokers is not decreased. Science. 1983 Sep 16;221(4616):1187–1189. doi: 10.1126/science.6612333. [DOI] [PubMed] [Google Scholar]
- Stone P. J. The elastase-antielastase hypothesis of the pathogenesis of emphysema. Clin Chest Med. 1983 Sep;4(3):405–412. [PubMed] [Google Scholar]
- Straus S. D., Fells G. A., Wewers M. D., Courtney M., Tessier L. H., Tolstoshev P., Lecocq J. P., Crystal R. G. Evaluation of recombinant DNA-directed E.coli produced alpha 1-antitrypsin as an anti-neutrophil elastase for potential use as replacement therapy of alpha 1-antitrypsin deficiency. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1177–1184. doi: 10.1016/0006-291x(85)91739-5. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Hayakawa S., Adachi T., Ito Y., Hirano K., Sawaki S. A simple one-step purification of human alpha 1-proteinase inhibitor by immunoadsorbent column chromatography. J Biochem Biophys Methods. 1981 Dec;5(5):243–249. doi: 10.1016/0165-022x(81)90034-8. [DOI] [PubMed] [Google Scholar]
- Travis J., Owen M., George P., Carrell R., Rosenberg S., Hallewell R. A., Barr P. J. Isolation and properties of recombinant DNA produced variants of human alpha 1-proteinase inhibitor. J Biol Chem. 1985 Apr 10;260(7):4384–4389. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]