Abstract
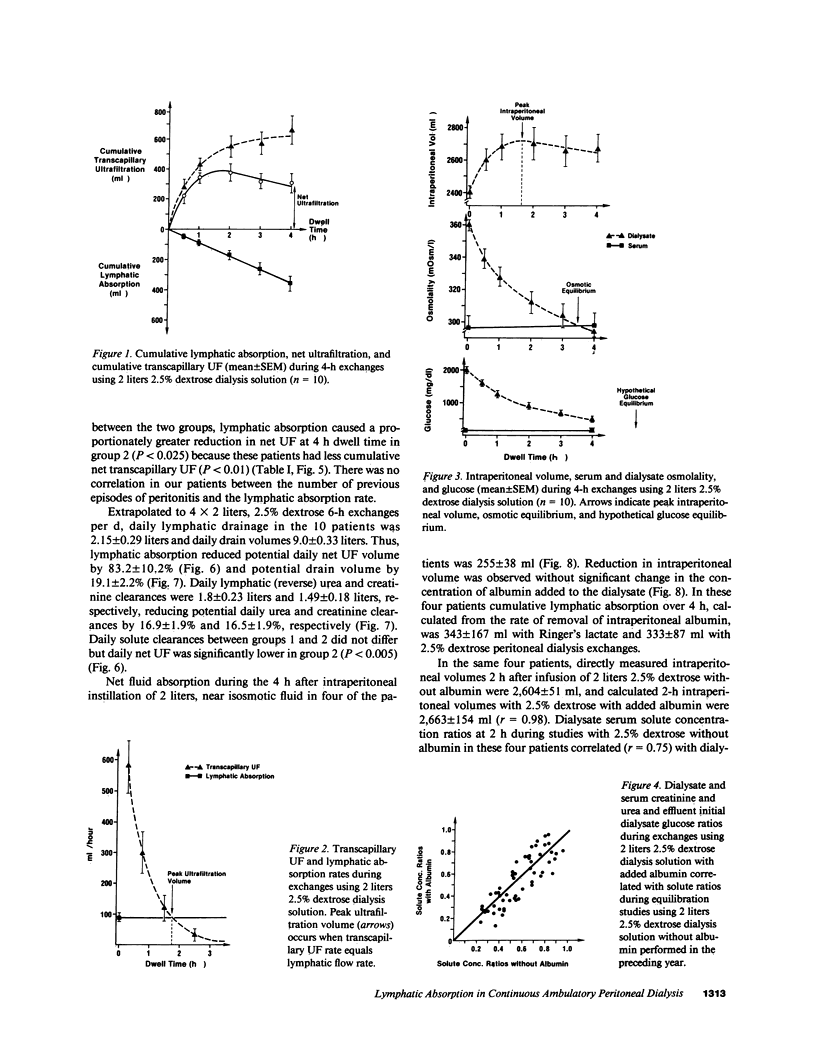
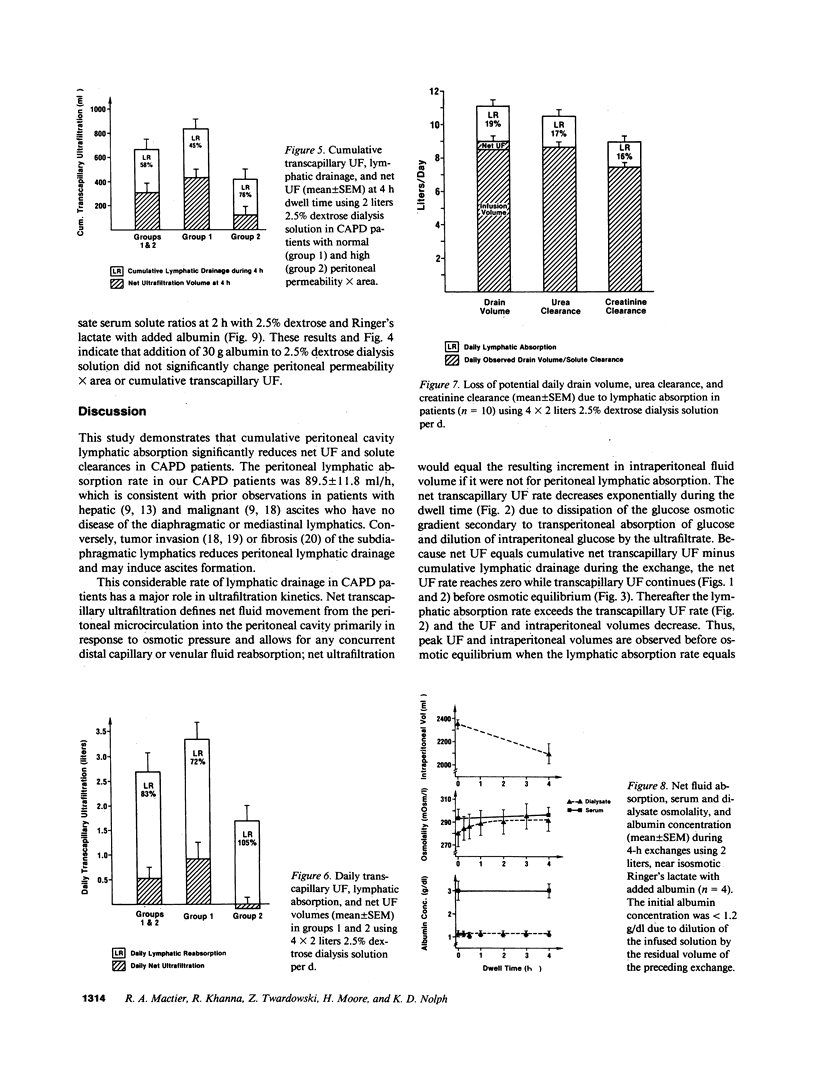
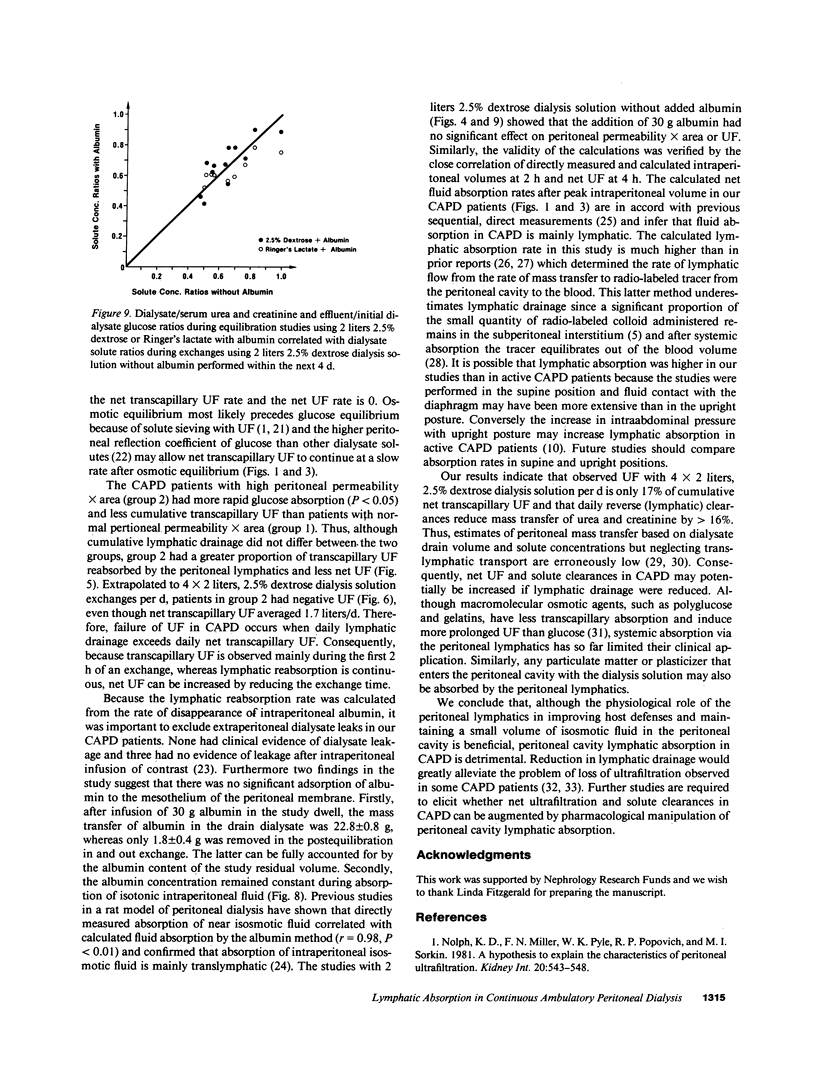
The contribution of peritoneal cavity lymphatic absorption to ultrafiltration kinetics and solute clearances in continuous ambulatory peritoneal dialysis was evaluated in patients with normal (group 1) and high (group 2) peritoneal permeability X area during 4-h exchanges using 2 liters 2.5% dextrose dialysis solution with 30 g added albumin. Cumulative lymphatic drainage in all continuous ambulatory peritoneal dialysis (CAPD) patients averaged 358 +/- 47 ml per 4-h exchange and reduced cumulative net transcapillary ultrafiltration at the end of the exchange by 58 +/- 7.2%. The peak ultrafiltration volume was observed before osmotic equilibrium between serum and dialysate was reached and occurred when the net transcapillary ultrafiltration rate had decreased to equal the lymphatic absorption rate. Thereafter the lymphatic absorption rate exceeded the net transcapillary ultrafiltration rate, and intraperitoneal volume decreased. Extrapolated to 4 X 2 liters, 2.5% dextrose, 6-h exchanges per d, lymphatic drainage reduced potential daily net ultrafiltration by 83.2 +/- 10.2%, daily urea clearance by 16.9 +/- 1.9%, and daily creatinine clearance by 16.5 +/- 1.9%. Although lymphatic absorption did not differ between the two groups, lymphatic drainage caused a proportionately greater reduction in net ultrafiltration in group 2 (P less than 0.025), because these patients had more rapid dialysate glucose absorption (P less than 0.05) and less cumulative transcapillary ultrafiltration (P less than 0.01). These findings indicate that cumulative lymphatic drainage significantly reduces net ultrafiltration and solute clearances in CAPD and that ultrafiltration failure in CAPD occurs when daily lymphatic absorption equals or exceeds daily transcapillary ultrafiltration. Reduction of lymphatic absorption may provide a means for future improvement in the efficiency of CAPD.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune S. Transperitoneal exchange. IV. The effect of transperitoneal fluid transport on the transfer of solutes. Scand J Gastroenterol. 1970;5(4):241–252. [PubMed] [Google Scholar]
- Bronskill M. J., Bush R. S., Ege G. N. A quantitative measurement of peritoneal drainage in malignant ascites. Cancer. 1977 Nov;40(5):2375–2380. doi: 10.1002/1097-0142(197711)40:5<2375::aid-cncr2820400554>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- COURTICE F. C. Lecture II: Ascites: the role of the lymphatice in the accumulation of ascitic fluid. Med J Aust. 1959 Dec 26;46(2):945–951. [PubMed] [Google Scholar]
- COURTICE F. C., SIMMONDS W. J. Physiological significance of lymph drainage of the serous cavities and lungs. Physiol Rev. 1954 Jul;34(3):419–448. doi: 10.1152/physrev.1954.34.3.419. [DOI] [PubMed] [Google Scholar]
- COURTICE F. C., STEINBECK A. W. The lymphatic drainage of plasma from the peritoneal cavity of the cat. Aust J Exp Biol Med Sci. 1950 Mar;28(2):161–169. doi: 10.1038/icb.1950.15. [DOI] [PubMed] [Google Scholar]
- Coates G., Bush R. S., Aspin N. A study of ascites using lymphoscintigraphy with 99m Tc-sulfur colloid. Radiology. 1973 Jun;107(3):577–583. doi: 10.1148/107.3.577. [DOI] [PubMed] [Google Scholar]
- Cook J. G., Association of clinical Biochemists' Scientific and Technica Committee Factors influencing the assay of creatinine. Ann Clin Biochem. 1975 Nov;12(6):219–232. doi: 10.1177/000456327501200162. [DOI] [PubMed] [Google Scholar]
- DUMONT A. E., MULHOLLAND J. H. Flow rate and composition of thoracic-duct lymph in patients with cirrhosis. N Engl J Med. 1960 Sep 8;263:471–474. doi: 10.1056/NEJM196009082631001. [DOI] [PubMed] [Google Scholar]
- Daugirdas J. T., Ing T. S., Gandhi V. C., Hano J. E., Chen W. T., Yuan L. Kinetics of peritoneal fluid absorption in patients with chronic renal failure. J Lab Clin Med. 1980 Mar;95(3):351–361. [PubMed] [Google Scholar]
- Feldman G. B., Knapp R. C. Lymphatic drainage of the peritoneal cavity and its significance in ovarian cancer. Am J Obstet Gynecol. 1974 Aug 1;119(7):991–994. doi: 10.1016/0002-9378(74)90021-0. [DOI] [PubMed] [Google Scholar]
- Flessner M. F., Parker R. J., Sieber S. M. Peritoneal lymphatic uptake of fibrinogen and erythrocytes in the rat. Am J Physiol. 1983 Jan;244(1):H89–H96. doi: 10.1152/ajpheart.1983.244.1.H89. [DOI] [PubMed] [Google Scholar]
- Henriksen J. H., Lassen N. A., Parving H. H., Winkler K. Filtration as the main transport mechanism of protein exchange between plasma and the peritoneal cavity in hepatic cirrhosis. Scand J Clin Lab Invest. 1980 Oct;40(6):503–513. doi: 10.3109/00365518009091957. [DOI] [PubMed] [Google Scholar]
- Ismail A. H., Mohamed F. S. Structural changes of the diaphragmatic peritoneum in patients with schistosomal hepatic fibrosis: its relation to ascites. Lymphology. 1986 Jun;19(2):82–87. [PubMed] [Google Scholar]
- Knochel J. P. Formation of peritoneal fluid hypertonicity during dialysis with isotonic glucose solutions. J Appl Physiol. 1969 Aug;27(2):233–236. doi: 10.1152/jappl.1969.27.2.233. [DOI] [PubMed] [Google Scholar]
- Lill S. R., Parsons R. H., Buhac I. Permeability of the diaphragm and fluid resorption from the peritoneal cavity in the rat. Gastroenterology. 1979 May;76(5 Pt 1):997–1001. [PubMed] [Google Scholar]
- Nicoll P. A., Taylor A. E. Lymph formation and flow. Annu Rev Physiol. 1977;39:73–95. doi: 10.1146/annurev.ph.39.030177.000445. [DOI] [PubMed] [Google Scholar]
- Nolph K. D., Miller F. N., Pyle W. K., Popovich R. P., Sorkin M. I. An hypothesis to explain the ultrafiltration characteristics of peritoneal dialysis. Kidney Int. 1981 Nov;20(5):543–548. doi: 10.1038/ki.1981.175. [DOI] [PubMed] [Google Scholar]
- Nolph K. D., Popovich R. P., Ghods A. J., Twardowski Z. Determinants of low clearances of small solutes during peritoneal dialysis. Kidney Int. 1978 Feb;13(2):117–123. doi: 10.1038/ki.1978.17. [DOI] [PubMed] [Google Scholar]
- OLIN T., SALDEEN T. THE LYMPHATIC PATHWAYS FROM THE PERITONEAL CAVITY: A LYMPHANGIOGRAPHIC STUDY IN THE RAT. Cancer Res. 1964 Nov;24:1700–1711. [PubMed] [Google Scholar]
- Slingeneyer A., Canaud B., Mion C. Permanent loss of ultrafiltration capacity of the peritoneum in long-term peritoneal dialysis: an epidemiological study. Nephron. 1983;33(2):133–138. doi: 10.1159/000182927. [DOI] [PubMed] [Google Scholar]
- Twardowski Z. J., Khanna R., Nolph K. D. Osmotic agents and ultrafiltration in peritoneal dialysis. Nephron. 1986;42(2):93–101. doi: 10.1159/000183645. [DOI] [PubMed] [Google Scholar]
- Twardowski Z., Janicka L. Three exchanges with a 2.5-liter volume for continuous ambulatory peritoneal dialysis. Kidney Int. 1981 Aug;20(2):281–284. doi: 10.1038/ki.1981.132. [DOI] [PubMed] [Google Scholar]
- Wideröe T. E., Smeby L. C., Mjåland S., Dahl K., Berg K. J., Wessel Aas T. Long-term changes in transperitoneal water transport during continuous ambulatory peritoneal dialysis. Nephron. 1984;38(4):238–247. doi: 10.1159/000183316. [DOI] [PubMed] [Google Scholar]
- Zink J., Greenway C. V. Control of ascites absorption in anesthetized cats: effects of intraperitoneal pressure, protein, and furosemide diuresis. Gastroenterology. 1977 Nov;73(5):1119–1124. [PubMed] [Google Scholar]


