Abstract
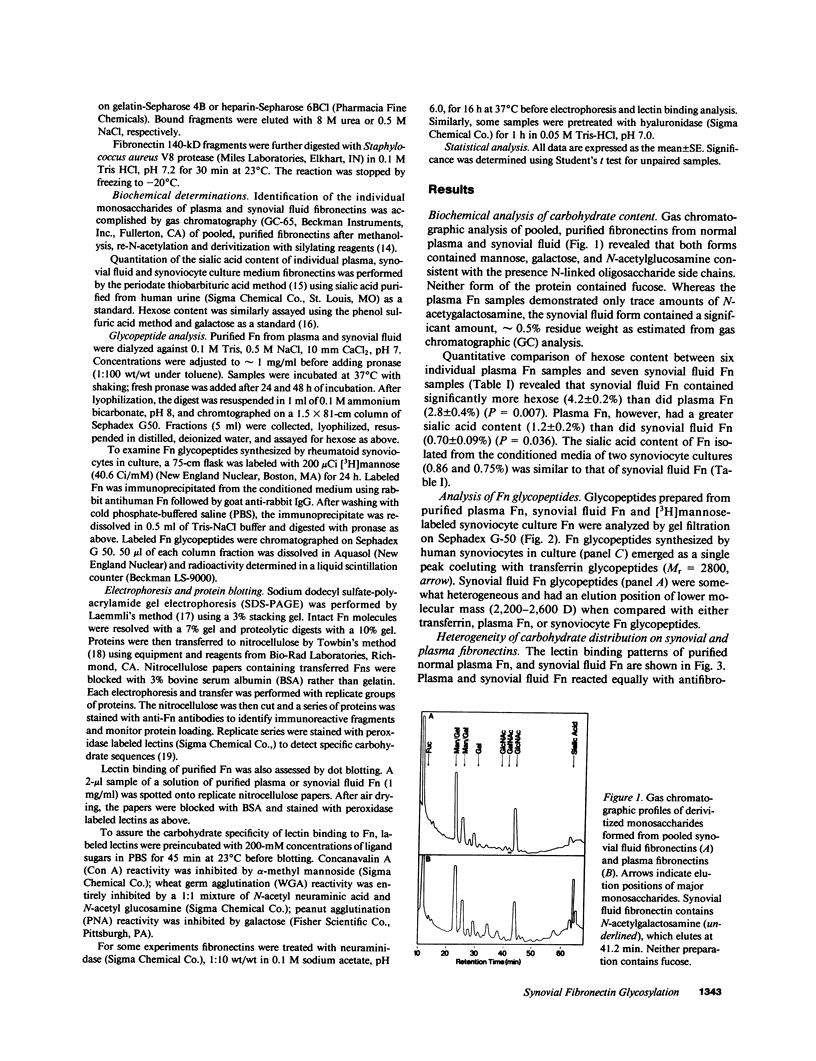
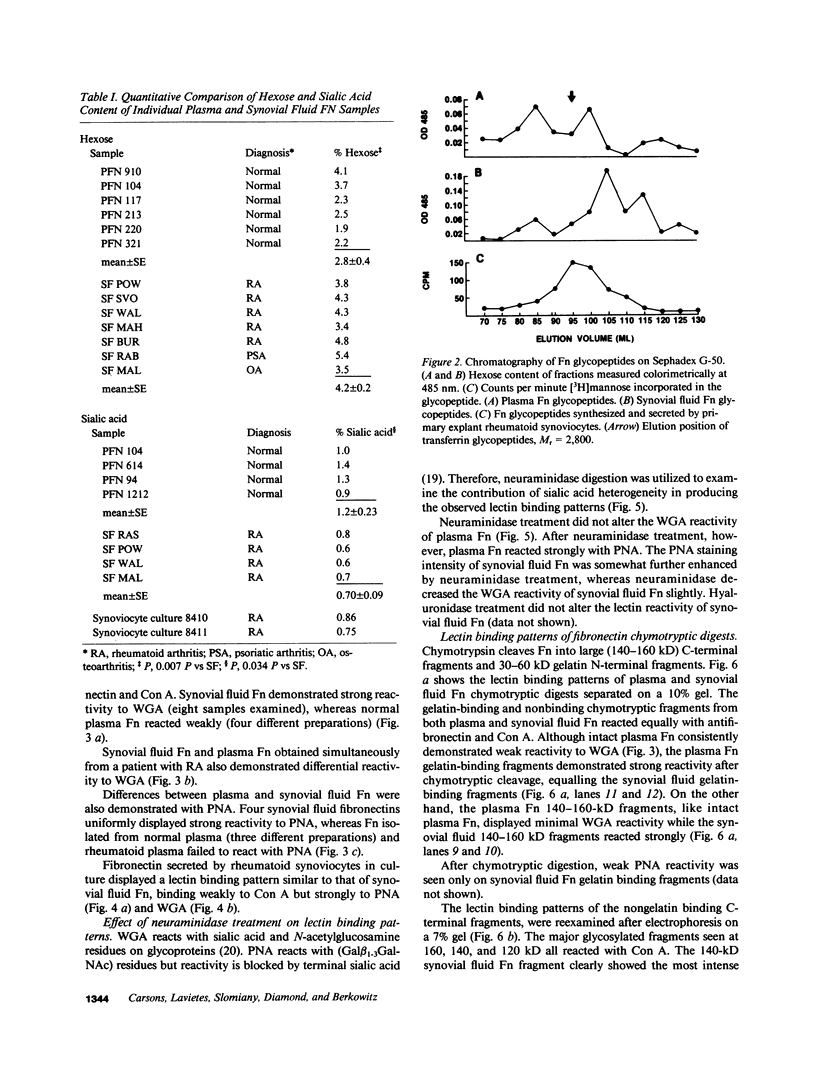
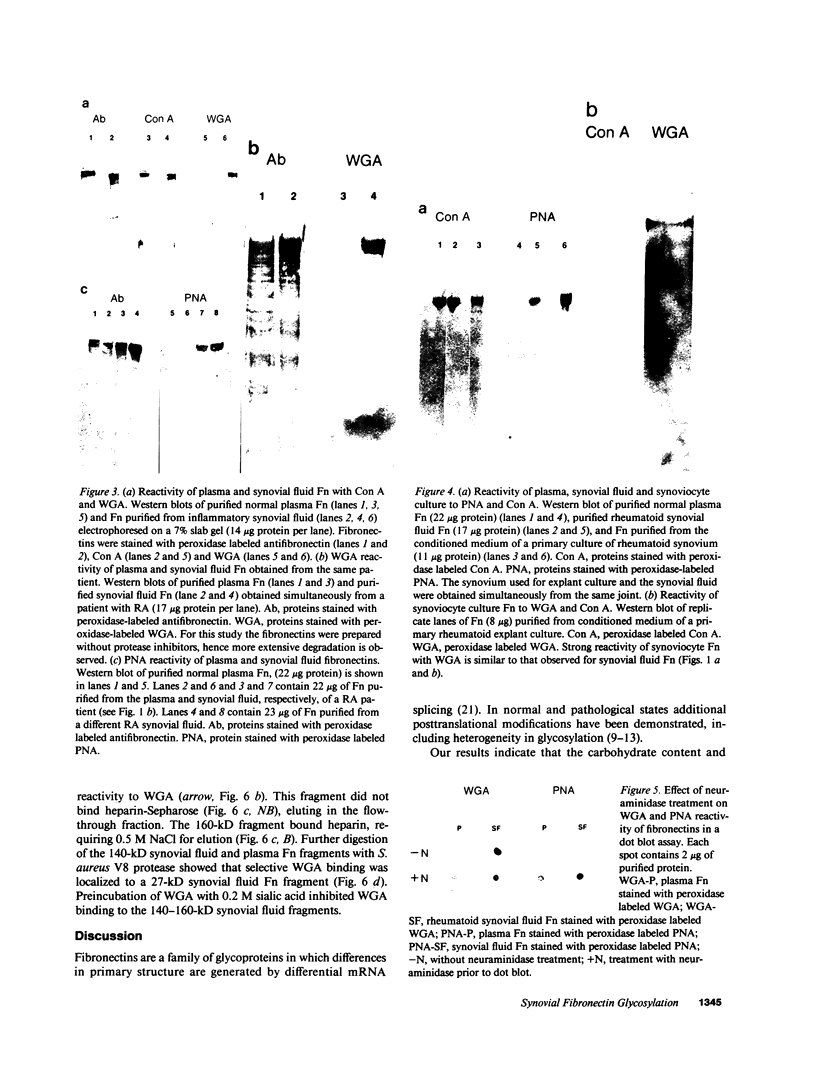
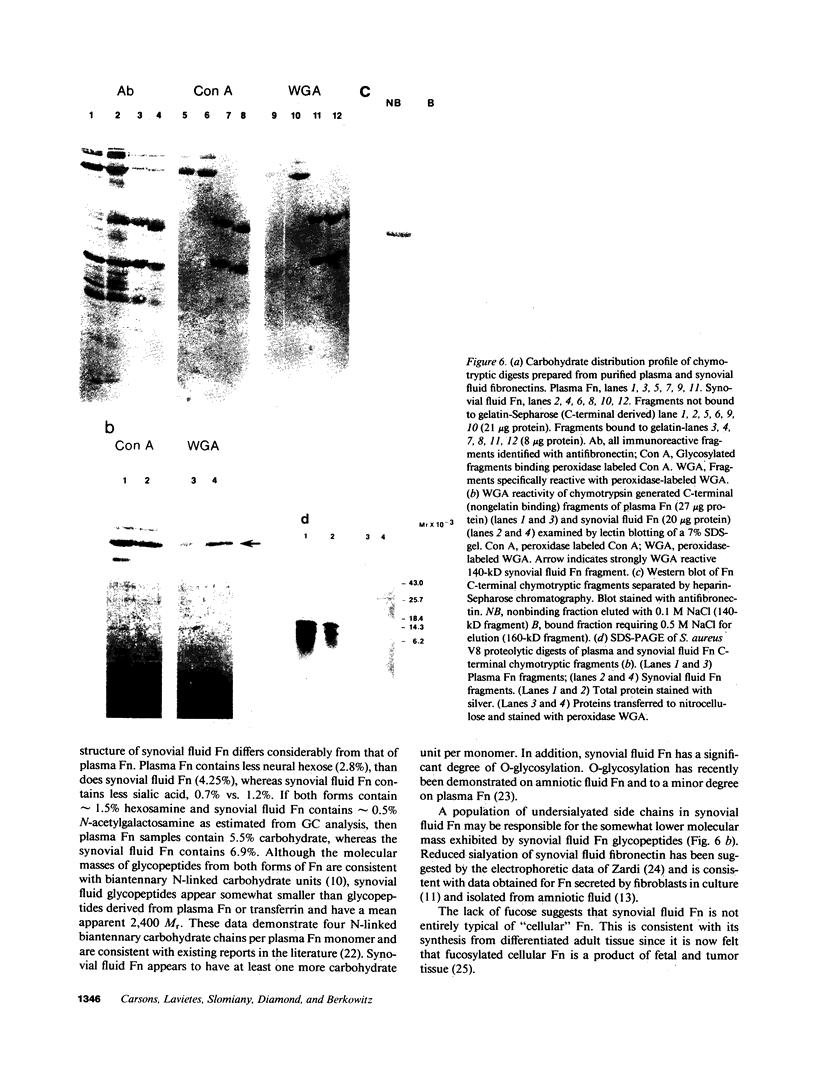
Large quantities of fibronectin (Fn) are present in inflammatory synovial fluid. Inflammatory synovial fluid Fn, while indistinguishable from plasma Fn on the basis of reactivity to polyclonal antibodies, displays alterations in molecular size and charge. Since biochemical differences between plasma and synovial fluid fibronectins might be in part due to differences in glycosylation we have compared the carbohydrate composition of plasma Fn, synovial fluid Fn, and Fn from synoviocyte conditioned medium by biochemical assay, glycopeptide analysis, and binding to a series of lectins. Synovial fluid Fn has a greater carbohydrate content but contains less sialic acid when compared with plasma Fn. Glycopeptides formed from synovial fluid Fn are smaller than plasma Fn glycopeptides. These data suggest the presence of an additional N-linked oligosaccharide chain on synovial fluid Fn. In addition, synovial fluid Fn contains N-acetyl galactosamine indicating the presence of O-linked oligosaccharides. Synovial fluid Fn and Fn isolated from rheumatoid synoviocyte-conditioned medium display strong reactivity with the lectins wheat germ agglutinin (WGA) and peanut agglutinin (PNA), whereas normal and rheumatoid plasma Fn react weakly. The PNA reactivity of synovial fluid Fn is mediated by terminal beta-galactose residues on the gelatin-binding domain, whereas the enhanced WGA reactivity of synovial Fn is mediated by a sialic acid containing oligosaccharide located on a 27-kD C-terminal fragment. These data demonstrate domain-specific biochemical differences between plasma and synovial fluid fibronectins. These differences suggest a local origin for synovial fluid Fn and may contribute to functional differences between these forms of the protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balian G., Crouch E., Click E. M., Carter W. G., Bornstein P. Comparison of the structures of human fibronectin and plasma cold-insoluble globulin. J Supramol Struct. 1979;12(4):505–516. doi: 10.1002/jss.400120410. [DOI] [PubMed] [Google Scholar]
- Bernard B. A., Yamada K. M., Olden K. Carbohydrates selectively protect a specific domain of fibronectin against proteases. J Biol Chem. 1982 Jul 25;257(14):8549–8554. [PubMed] [Google Scholar]
- Carnemolla B., Castellani P., Cutolo M., Borsi L., Zardi L. Lack of sialic acid in synovial fluid fibronectin. FEBS Lett. 1984 Jun 11;171(2):285–288. doi: 10.1016/0014-5793(84)80505-0. [DOI] [PubMed] [Google Scholar]
- Carnemolla B., Cutolo M., Castellani P., Balza E., Raffanti S., Zardi L. Characterization of synovial fluid fibronectin from patients with rheumatic inflammatory diseases and healthy subjects. Arthritis Rheum. 1984 Aug;27(8):913–921. doi: 10.1002/art.1780270811. [DOI] [PubMed] [Google Scholar]
- Carsons S., Lavietes B. B., Diamond H. S., Kinney S. G. The immunoreactivity, ligand, and cell binding characteristics of rheumatoid synovial fluid fibronectin. Arthritis Rheum. 1985 Jun;28(6):601–612. doi: 10.1002/art.1780280602. [DOI] [PubMed] [Google Scholar]
- Carsons S., Mosesson M. W., Diamond H. S. Detection and quantitation of fibronectin in synovial fluid from patients with rheumatic disease. Arthritis Rheum. 1981 Oct;24(10):1261–1267. [PubMed] [Google Scholar]
- Clark R. A., Quinn J. H., Winn H. J., Lanigan J. M., Dellepella P., Colvin R. B. Fibronectin is produced by blood vessels in response to injury. J Exp Med. 1982 Aug 1;156(2):646–651. doi: 10.1084/jem.156.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen I., Andersen R. B. Different molecular forms of fibronectin in rheumatoid synovial fluid. Arthritis Rheum. 1982 Jan;25(1):25–31. doi: 10.1002/art.1780250104. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Kadish J. L., Zepf D. M., Austen K. F. Identification with monoclonal antibodies of different regions of human plasma fibronectin, including that which interacts with human monocyte fibronectin receptors. Immunology. 1985 Mar;54(3):407–417. [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Levery S. B., Hakomori S. Carbohydrate structure of hamster plasma fibronectin. Evidence for chemical diversity between cellular and plasma fibronectins. J Biol Chem. 1982 Jun 25;257(12):6856–6860. [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Domain structure of the carboxyl-terminal half of human plasma fibronectin. J Biol Chem. 1983 Mar 10;258(5):3332–3340. [PubMed] [Google Scholar]
- Hörmann H., Richter H., Jelinić V. Evidence for a cryptic lectin site in the cell-binding domain of plasma fibronectin. Hoppe Seylers Z Physiol Chem. 1984 May;365(5):517–524. doi: 10.1515/bchm2.1984.365.1.517. [DOI] [PubMed] [Google Scholar]
- Krusius T., Fukuda M., Dell A., Ruoslahti E. Structure of the carbohydrate units of human amniotic fluid fibronectin. J Biol Chem. 1985 Apr 10;260(7):4110–4116. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavietes B. B., Carsons S., Diamond H. S., Laskin R. S. Synthesis, secretion, and deposition of fibronectin in cultured human synovium. Arthritis Rheum. 1985 Sep;28(9):1016–1026. doi: 10.1002/art.1780280909. [DOI] [PubMed] [Google Scholar]
- Lu-Steffes M., Iammartino A. J., Schmid F. R., Castor C. W., Davis L., Entwistle R., Anderson B. Fibronectin in rheumatoid and non-rheumatoid arthritic synovial fluids and in synovial fluid cryoproteins. Ann Clin Lab Sci. 1982 May-Jun;12(3):178–185. [PubMed] [Google Scholar]
- Matsuura H., Hakomori S. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6517–6521. doi: 10.1073/pnas.82.19.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C., Beaulieu A. D., Audette M., Corbeil J., Latulippe L. Studies on fibronectin in inflammatory vs non-inflammatory polymorphonuclear leucocytes of patients with rheumatoid arthritis. II. Synthesis and release of fibronectin in vitro. Clin Exp Immunol. 1985 May;60(2):347–354. [PMC free article] [PubMed] [Google Scholar]
- Nichols E. J., Fenderson B. A., Carter W. G., Hakomori S. Domain-specific distribution of carbohydrates in human fibronectins and the transformation-dependent translocation of branched type 2 chain defined by monoclonal antibody C6. J Biol Chem. 1986 Aug 25;261(24):11295–11301. [PubMed] [Google Scholar]
- Nicolson G. L., Irimura T. Estimating glycoprotein carbohydrate chain structures by lectin reactivities in polyacrylamide gels. Biol Cell. 1984;51(2):157–164. doi: 10.1111/j.1768-322x.1984.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Olden K., Bernard B. A., White S. L., Parent J. B. Function of the carbohydrate moieties of glycoproteins. J Cell Biochem. 1982;18(3):313–335. doi: 10.1002/jcb.1982.240180306. [DOI] [PubMed] [Google Scholar]
- Pande H., Corkill J., Sailor R., Shively J. E. Comparative structural studies of human plasma and amniotic fluid fibronectins. Biochem Biophys Res Commun. 1981 Jul 16;101(1):265–272. doi: 10.1016/s0006-291x(81)80040-x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Jalanko H., Comings D. E., Neville A. M., Raghavan D. Fibronectin from human germ-cell tumors resembles amniotic fluid fibronectin. Int J Cancer. 1981 Jun 15;27(6):763–767. doi: 10.1002/ijc.2910270606. [DOI] [PubMed] [Google Scholar]
- Schauer R., Wember M., Tschesche H. Isolation and characterization of an oligosaccharide- and glycoprotein-specific sialidase from human leucocytes. Hoppe Seylers Z Physiol Chem. 1984 Apr;365(4):419–426. doi: 10.1515/bchm2.1984.365.1.419. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Carter S. D., Coppock J. S., Robinson M., Walton K. W. Differences between plasma and synovial fluid fibronectin. Rheumatol Int. 1985;5(2):49–54. doi: 10.1007/BF00270296. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Delamere J. P., Walton K. W. The distribution of fibronectin in the pannus in rheumatoid arthritis. Br J Exp Pathol. 1981 Aug;62(4):362–368. [PMC free article] [PubMed] [Google Scholar]
- Scott D. L., Farr M., Crockson A. P., Walton K. W. Synovial fluid and plasma fibronectin levels in rheumatoid arthritis. Clin Sci (Lond) 1982 Jan;62(1):71–76. doi: 10.1042/cs0620071. [DOI] [PubMed] [Google Scholar]
- Skorstengaard K., Jensen M. S., Sahl P., Petersen T. E., Magnusson S. Complete primary structure of bovine plasma fibronectin. Eur J Biochem. 1986 Dec 1;161(2):441–453. doi: 10.1111/j.1432-1033.1986.tb10464.x. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Zdebska E., Slomiany B. L. Structures of the neutral oligosaccharides isolated from A-active human gastric mucin. J Biol Chem. 1984 Dec 10;259(23):14743–14749. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T. Fibronectin: multiple interactions assigned to structural domains. Med Biol. 1983;61(6):283–295. [PubMed] [Google Scholar]
- Vartio T., Vaheri A., Von Essen R., Isomäki H., Stenman S. Fibronectin in synovial fluid and tissue in rheumatoid arthritis. Eur J Clin Invest. 1981 Jun;11(3):207–212. doi: 10.1111/j.1365-2362.1981.tb01842.x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wagner D. D., Ivatt R., Destree A. T., Hynes R. O. Similarities and differences between the fibronectins of normal and transformed hamster cells. J Biol Chem. 1981 Nov 25;256(22):11708–11715. [PubMed] [Google Scholar]
- Zhu B. C., Laine R. A. Polylactosamine glycosylation on human fetal placental fibronectin weakens the binding affinity of fibronectin to gelatin. J Biol Chem. 1985 Apr 10;260(7):4041–4045. [PubMed] [Google Scholar]
- Zucker M. B., Mosesson M. W., Broekman M. J., Kaplan K. L. Release of platelet fibronectin (cold-insoluble globulin) from alpha granules induced by thrombin or collagen; lack of requirement for plasma fibronectin in ADP-induced platelet aggregation. Blood. 1979 Jul;54(1):8–12. [PubMed] [Google Scholar]