Abstract
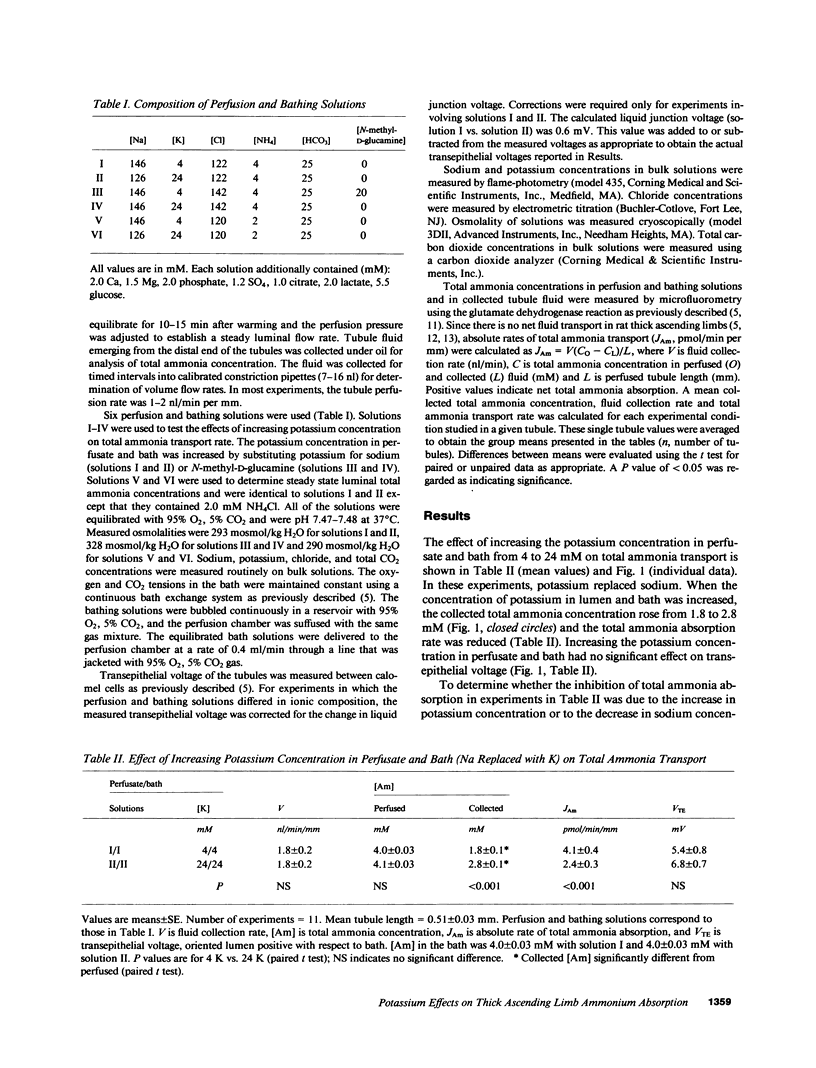
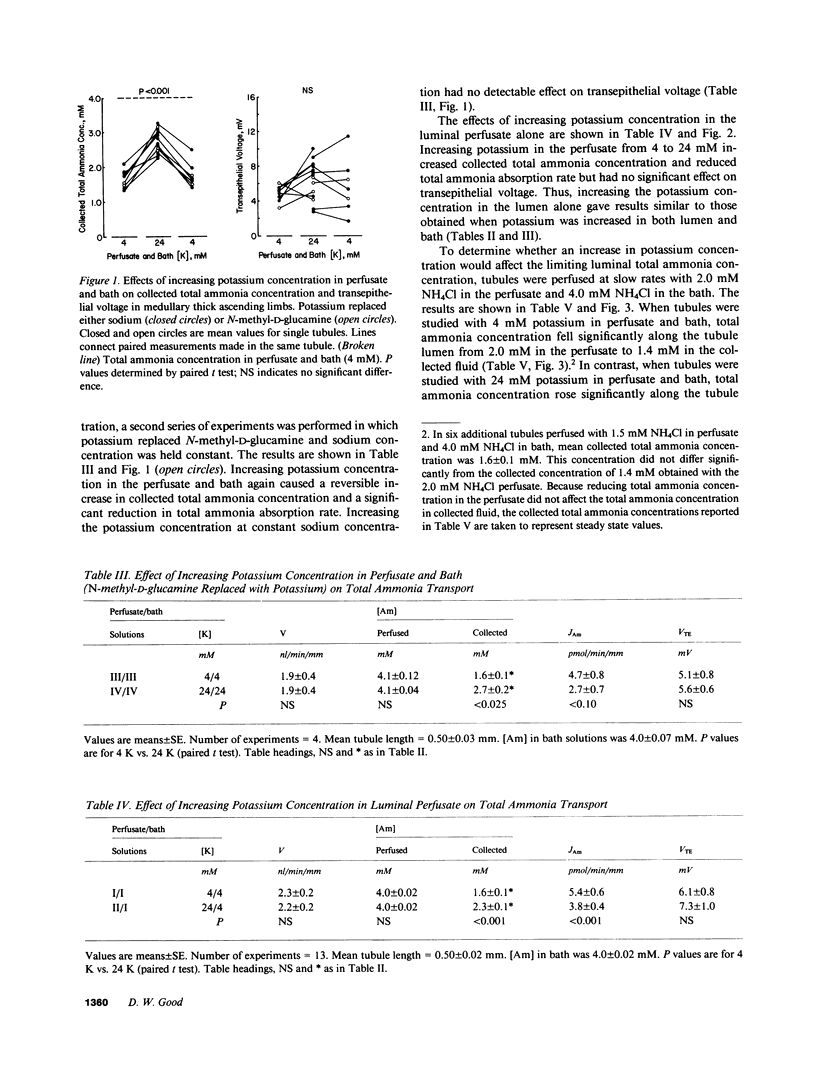
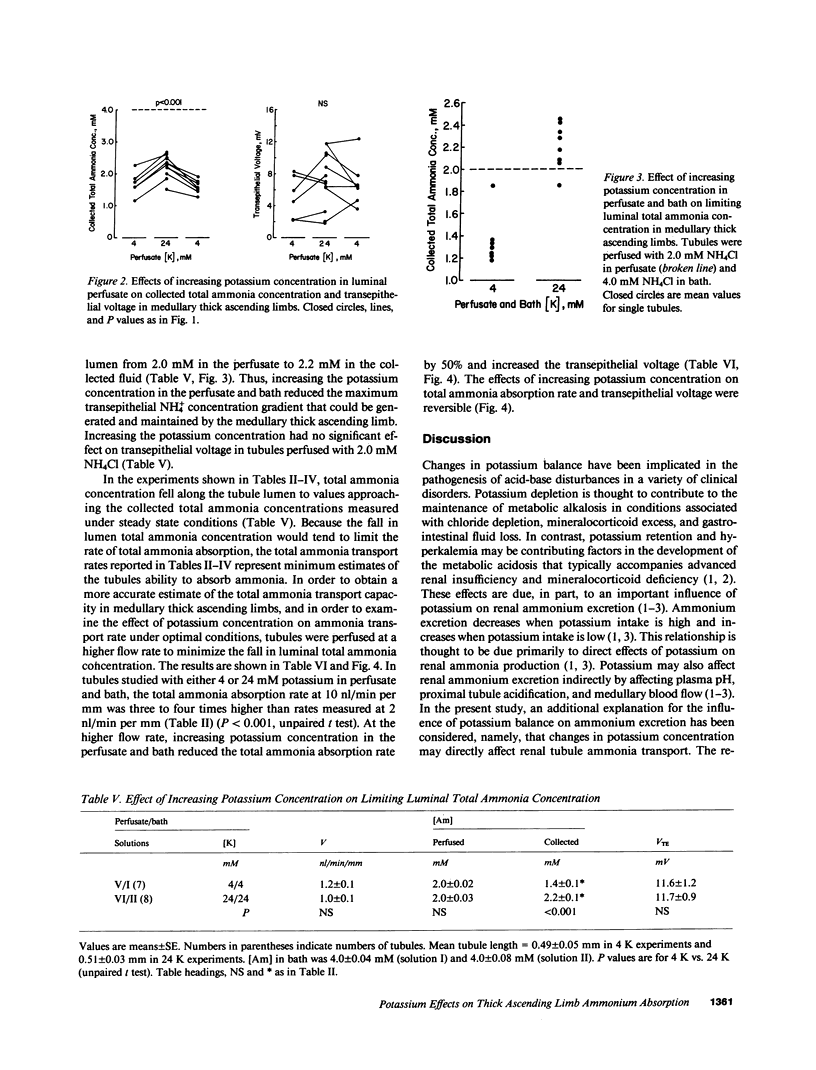
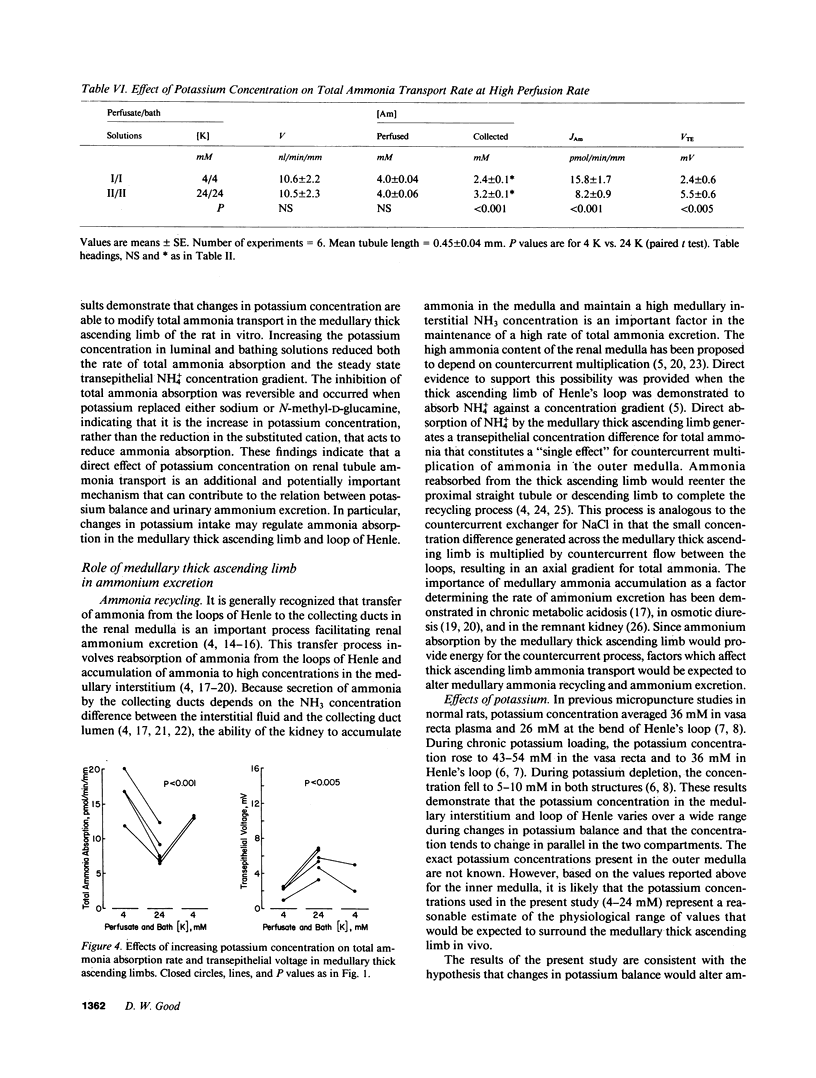
Renal ammonium excretion is increased by potassium depletion and reduced by potassium loading. To determine whether changes in potassium concentration would alter ammonia transport in the medullary thick ascending limb (MAL), tubules from rats were perfused in vitro and effects of changes in K concentration within the physiological range (4-24 mM) were evaluated. Increasing K concentration from 4 to 24 mM in perfusate and bath inhibited total ammonia absorption by 50% and reduced the steady-state transepithelial NH+4 concentration gradient. The inhibition of total ammonia absorption was reversible and occurred when K replaced either Na or N-methyl-D-glucamine. Increasing K concentration in the luminal perfusate alone gave similar inhibition of total ammonia absorption. At 1-2 nl/min per mm perfusion rate, increasing K concentration in perfusion and bathing solutions had no significant effect on transepithelial voltage. With either 4 or 24 mM K in perfusate and bath, an increase in luminal perfusion rate markedly increased total ammonia absorption. Thus, both potassium concentration and luminal flow rate are important factors capable of regulating total ammonia transport by the MAL. Changes in systemic potassium balance may influence renal ammonium excretion by affecting NH+4 absorption in the MAL and altering the transfer of ammonia from loops of Henle to medullary collecting ducts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battilana C. A., Dobyan D. C., Lacy F. B., Bhattacharya J., Johnston P. A., Jamison R. L. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest. 1978 Nov;62(5):1093–1103. doi: 10.1172/JCI109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Ammonium handling by superficial and juxtamedullary nephrons in the rat. Evidence for an ammonia shunt between the loop of Henle and the collecting duct. J Clin Invest. 1982 Jul;70(1):1–12. doi: 10.1172/JCI110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Segmental analysis of the renal tubule in buffer production and net acid formation. Am J Physiol. 1983 Apr;244(4):F442–F454. doi: 10.1152/ajprenal.1983.244.4.F442. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D., Simon E. Effect of reduced renal mass on ammonium handling and net acid formation by the superficial and juxtamedullary nephron of the rat. Evidence for impaired reentrapment rather than decreased production of ammonium in the acidosis of uremia. J Clin Invest. 1983 Jun;71(6):1661–1675. doi: 10.1172/JCI110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezi J., Michoud P., Aceves J., Giebisch G. Micropuncture study of electrolyte transport across papillary collecting duct of the rat. Am J Physiol. 1973 Mar;224(3):623–634. doi: 10.1152/ajplegacy.1973.224.3.623. [DOI] [PubMed] [Google Scholar]
- Dobyan D. C., Lacy F. B., Jamison R. L. Suppression of potassium-recycling in the renal medulla by short-term potassium deprivation. Kidney Int. 1979 Dec;16(6):704–709. doi: 10.1038/ki.1979.186. [DOI] [PubMed] [Google Scholar]
- Garvin J. L., Burg M. B., Knepper M. A. NH3 and NH4+ transport by rabbit renal proximal straight tubules. Am J Physiol. 1987 Feb;252(2 Pt 2):F232–F239. doi: 10.1152/ajprenal.1987.252.2.F232. [DOI] [PubMed] [Google Scholar]
- Garvin J. L., Knepper M. A. Bicarbonate and ammonia transport in isolated perfused rat proximal straight tubules. Am J Physiol. 1987 Aug;253(2 Pt 2):F277–F281. doi: 10.1152/ajprenal.1987.253.2.F277. [DOI] [PubMed] [Google Scholar]
- Gelbart D. R., Battilana C. A., Bhattacharya J., Lacy F. B., Jamison R. L. Transepithelial gradient and fractional delivery of chloride in thin loop of Henle. Am J Physiol. 1978 Sep;235(3):F192–F198. doi: 10.1152/ajprenal.1978.235.3.F192. [DOI] [PubMed] [Google Scholar]
- Good D. W., Caflisch C. R., DuBose T. D., Jr Transepithelial ammonia concentration gradients in inner medulla of the rat. Am J Physiol. 1987 Mar;252(3 Pt 2):F491–F500. doi: 10.1152/ajprenal.1987.252.3.F491. [DOI] [PubMed] [Google Scholar]
- Good D. W., Knepper M. A. Ammonia transport in the mammalian kidney. Am J Physiol. 1985 Apr;248(4 Pt 2):F459–F471. doi: 10.1152/ajprenal.1985.248.4.F459. [DOI] [PubMed] [Google Scholar]
- Good D. W., Knepper M. A., Burg M. B. Ammonia absorption by the thick ascending limb of Henle's loop. Contrib Nephrol. 1985;47:110–115. doi: 10.1159/000411216. [DOI] [PubMed] [Google Scholar]
- Good D. W., Knepper M. A., Burg M. B. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol. 1984 Jul;247(1 Pt 2):F35–F44. doi: 10.1152/ajprenal.1984.247.1.F35. [DOI] [PubMed] [Google Scholar]
- Good D. W. Sodium-dependent bicarbonate absorption by cortical thick ascending limb of rat kidney. Am J Physiol. 1985 Jun;248(6 Pt 2):F821–F829. doi: 10.1152/ajprenal.1985.248.6.F821. [DOI] [PubMed] [Google Scholar]
- Good D. W., Vurek G. G. Picomole quantitation of ammonia by flow-through fluorometry. Anal Biochem. 1983 Apr 1;130(1):199–202. doi: 10.1016/0003-2697(83)90670-x. [DOI] [PubMed] [Google Scholar]
- Hamm L. L., Trigg D., Martin D., Gillespie C., Buerkert J. Transport of ammonia in the rabbit cortical collecting tubule. J Clin Invest. 1985 Feb;75(2):478–485. doi: 10.1172/JCI111723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashihara E., Stokes J. B., Kokko J. P., Campbell W. B., DuBose T. D., Jr Cortical and papillary micropuncture examination of chloride transport in segments of the rat kidney during inhibition of prostaglandin production. Possible role for prostaglandins in the chloruresis of acute volume expansion. J Clin Invest. 1979 Nov;64(5):1277–1287. doi: 10.1172/JCI109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger P., Karlmark B., Giebisch G. Ammonium transport in rat cortical tubule: relationship to potassium metabolism. Am J Physiol. 1983 Nov;245(5 Pt 1):F593–F600. doi: 10.1152/ajprenal.1983.245.5.F593. [DOI] [PubMed] [Google Scholar]
- Jamison R. L. Micropuncture study of superficial and juxtamedullary nephrons in the rat. Am J Physiol. 1970 Jan;218(1):46–55. doi: 10.1152/ajplegacy.1970.218.1.46. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Good D. W., Burg M. B. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol. 1985 Dec;249(6 Pt 2):F870–F877. doi: 10.1152/ajprenal.1985.249.6.F870. [DOI] [PubMed] [Google Scholar]
- Kurtz I., Star R., Balaban R. S., Garvin J. L., Knepper M. A. Spontaneous luminal disequilibrium pH in S3 proximal tubules. Role in ammonia and bicarbonate transport. J Clin Invest. 1986 Oct;78(4):989–996. doi: 10.1172/JCI112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoguchi H., Takehara Y., Endou H. Intra- and inter-nephron heterogeneity of ammoniagenesis in rats: effects of chronic metabolic acidosis and potassium depletion. Pflugers Arch. 1986 Sep;407(3):245–251. doi: 10.1007/BF00585298. [DOI] [PubMed] [Google Scholar]
- ROBINSON R. R., OWEN E. E. INTRARENAL DISTRIBUTION OF AMMONIA DURING DIURESIS AND ANTIDIURESIS. Am J Physiol. 1965 Jun;208:1129–1134. doi: 10.1152/ajplegacy.1965.208.6.1129. [DOI] [PubMed] [Google Scholar]
- Roy D. R., Blouch K. L., Jamison R. L. Effects of acute acid-base disturbances on K+ delivery to the juxtamedullary end-descending limb. Am J Physiol. 1982 Aug;243(2):F188–F196. doi: 10.1152/ajprenal.1982.243.2.F188. [DOI] [PubMed] [Google Scholar]
- SULLIVAN L. P. AMMONIUM EXCRETION DURING STOPPED FLOW: A HYPOTHETICAL AMMONIUM COUNTERCURRENT SYSTEM. Am J Physiol. 1965 Aug;209:273–282. doi: 10.1152/ajplegacy.1965.209.2.273. [DOI] [PubMed] [Google Scholar]
- Sajo I. M., Goldstein M. B., Sonnenberg H., Stinebaugh B. J., Wilson D. R., Halperin M. L. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1981 Sep;20(3):353–358. doi: 10.1038/ki.1981.146. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Imai M. Effects of vasopressin on water and NaCl transport across the in vitro perfused medullary thick ascending limb of Henle's loop of mouse, rat, and rabbit kidneys. Pflugers Arch. 1980 Feb;383(3):215–221. doi: 10.1007/BF00587521. [DOI] [PubMed] [Google Scholar]
- Sastrasinh S., Tannen R. L. Effect of potassium on renal NH3 production. Am J Physiol. 1983 Apr;244(4):F383–F391. doi: 10.1152/ajprenal.1983.244.4.F383. [DOI] [PubMed] [Google Scholar]
- Simon E., Martin D., Buerkert J. Contribution of individual superficial nephron segments to ammonium handling in chronic metabolic acidosis in the rat. Evidence for ammonia disequilibrium in the renal cortex. J Clin Invest. 1985 Aug;76(2):855–864. doi: 10.1172/JCI112043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeper R. S., Belanger P., Lemieux G., Preuss H. G. Effects of in vitro potassium on ammoniagenesis in rat and canine kidney tissue. Kidney Int. 1982 Feb;21(2):345–353. doi: 10.1038/ki.1982.28. [DOI] [PubMed] [Google Scholar]
- Stern L., Backman K. A., Hayslett J. P. Effect of cortical-medullary gradient for ammonia on urinary excretion of ammonia. Kidney Int. 1985 Apr;27(4):652–661. doi: 10.1038/ki.1985.60. [DOI] [PubMed] [Google Scholar]
- Tannen R. L. Relationship of renal ammonia production and potassium homeostasis. Kidney Int. 1977 Jun;11(6):453–465. doi: 10.1038/ki.1977.63. [DOI] [PubMed] [Google Scholar]
- Whinnery M. A., Kunau R. T., Jr Effect of potassium deficiency on papillary plasma flow in the rat. Am J Physiol. 1979 Sep;237(3):F226–F231. doi: 10.1152/ajprenal.1979.237.3.F226. [DOI] [PubMed] [Google Scholar]
- Work J., Galla J. H., Booker B. B., Schafer J. A., Luke R. G. Effect of ADH on chloride reabsorption in the loop of Henle of the Brattleboro rat. Am J Physiol. 1985 Nov;249(5 Pt 2):F698–F703. doi: 10.1152/ajprenal.1985.249.5.F698. [DOI] [PubMed] [Google Scholar]


