Abstract
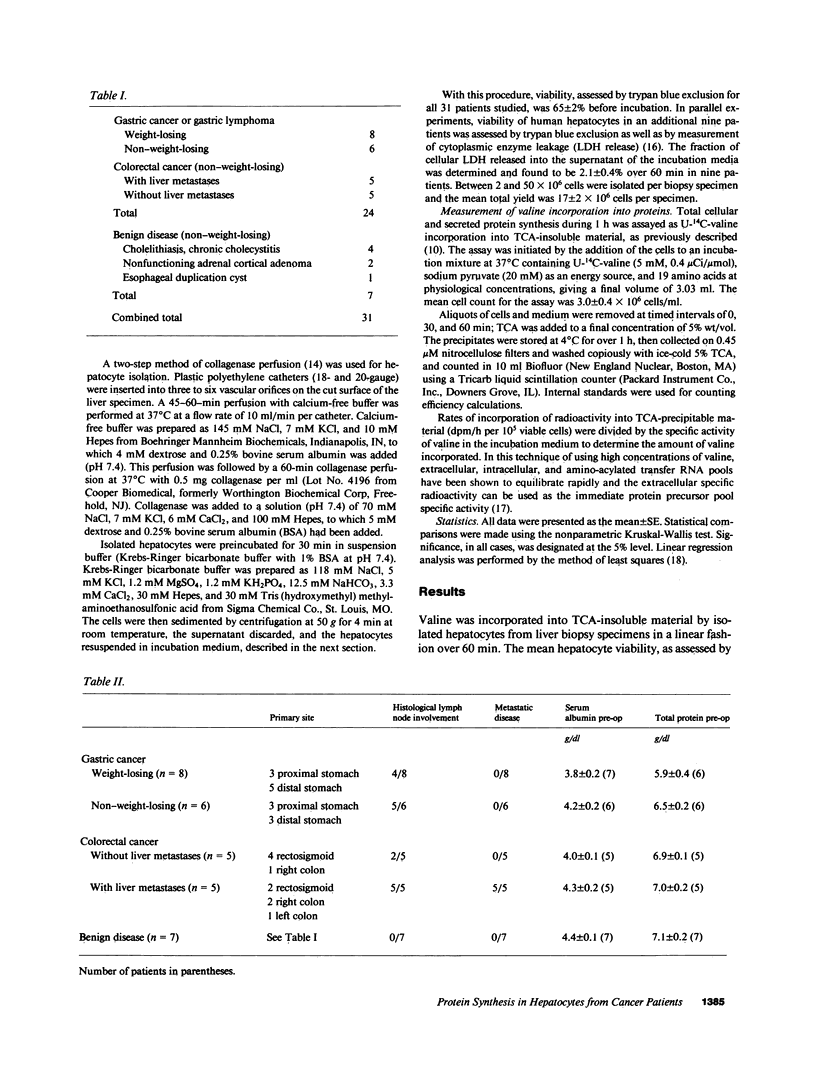
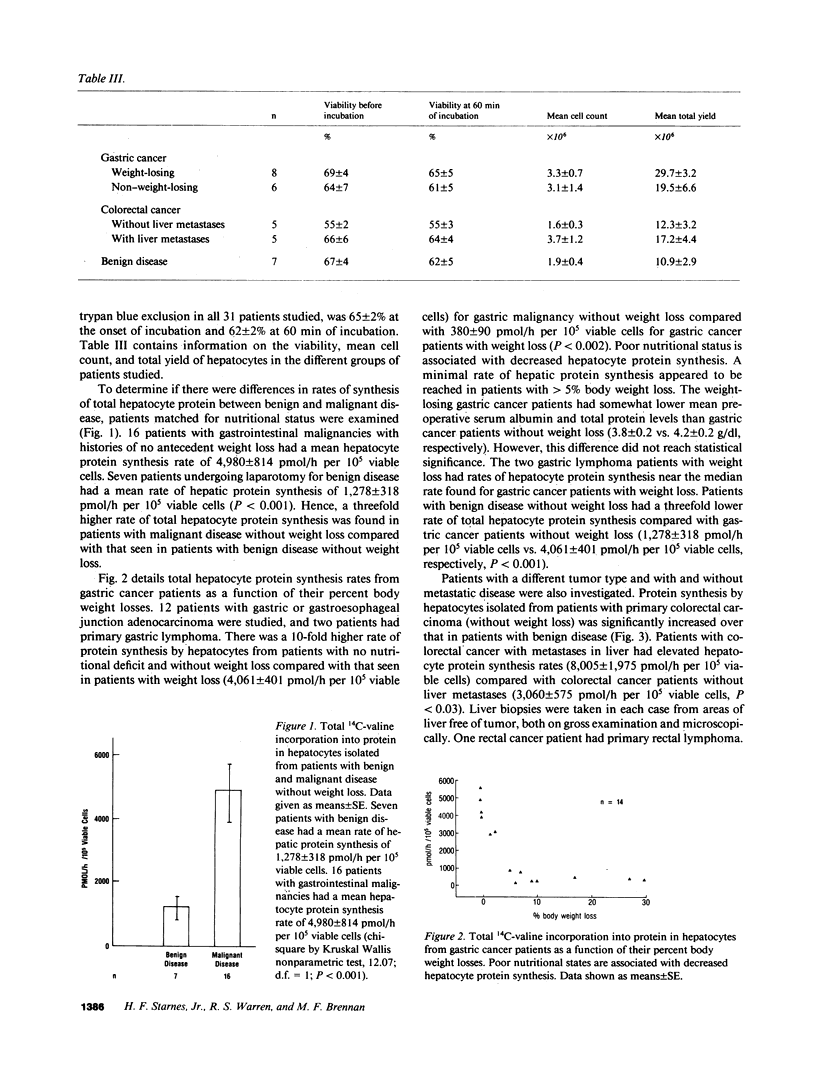
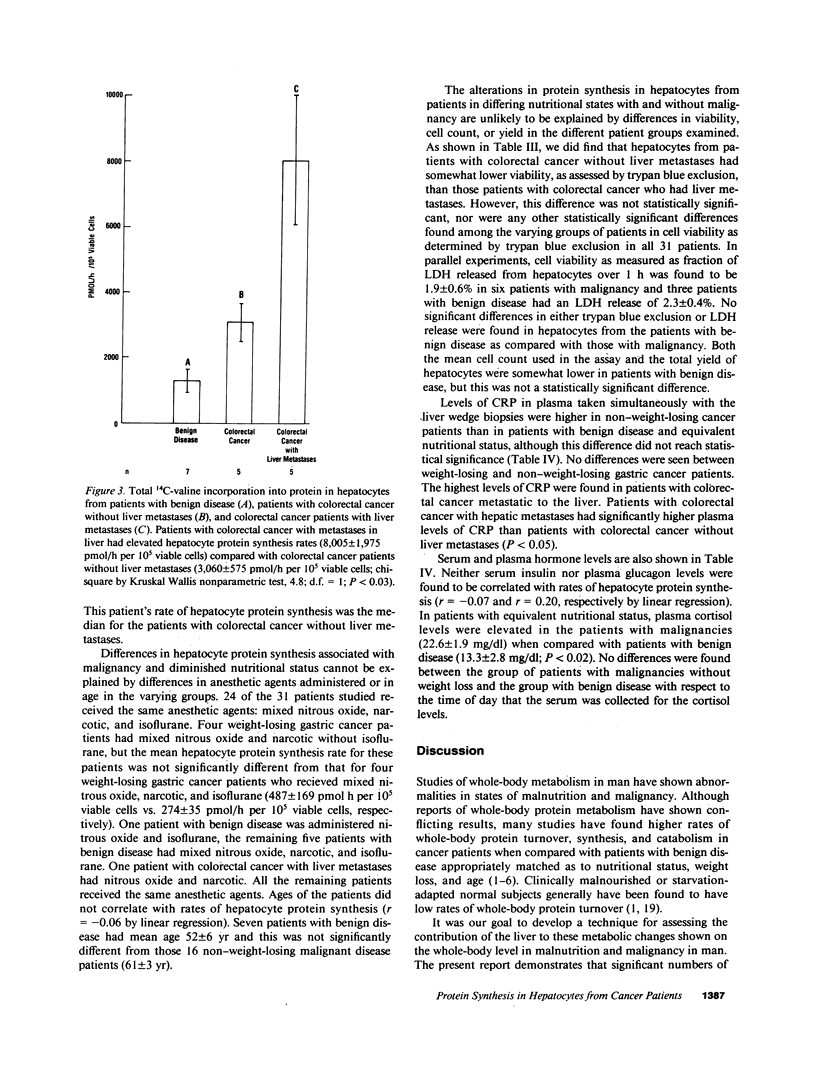
To investigate the effect of remote and proximate cancer on hepatic protein metabolism, we determined rates of total protein synthesis by hepatocytes (HPS) isolated from 31 patients undergoing liver wedge biopsy: 7 patients with benign disease, 14 with gastric cancer, and 10 with colorectal cancer (5 of whom had liver metastases). Patients with malignant disease without weight loss had a threefold higher rate of total HPS (4,980 +/- 814 pmol/h per 10(5) viable cells) than patients with benign disease without weight loss (1,278 +/- 318 pmol/h per 10(5) viable cells, P less than 0.001). Among the patients with gastric cancer, eight with preoperative weight loss had lower rates of HPS (380 +/- 90 pmol/h per 10(5) viable cells) than those without weight loss (4,061 +/- 401 pmol/h per 10(5) viable cells, P less than 0.002). The highest rates of HPS were seen in patients with colorectal cancer with liver metastases (8,005 +/- 1,975 pmol/h per 10(5) viable cells) vs. colorectal cancer patients without liver metastases (3,060 +/- 575 pmol/h per 10(5) viable cells, P less than 0.03). These data indicate that modulation of hepatic protein synthesis occurs in malignancy in man. However, the stimulatory influence of the tumor-bearing state may be overridden by the inhibitory effects of cachexia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayuso-Parrilla M. S., Martín-Requero A., Pérez-Días J., Parrilla R. Role of glucagon on the control of hepatic protein synthesis and degradation in the rat in vivo. J Biol Chem. 1976 Dec 25;251(24):7785–7790. [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Bourel M., Le Guilly Y. M., Lenoir P., Ferrand B., Febvre H. Culture de foie humain adulte. C R Seances Soc Biol Fil. 1968 Nov 23;162(4):979–983. [PubMed] [Google Scholar]
- Burt M. E., Stein T. P., Brennan M. F. A controlled, randomized trial evaluating the effects of enteral and parenteral nutrition on protein metabolism in cancer-bearing man. J Surg Res. 1983 Apr;34(4):303–314. doi: 10.1016/0022-4804(83)90076-8. [DOI] [PubMed] [Google Scholar]
- Carmichael M. J., Clague M. B., Keir M. J., Johnston I. D. Whole body protein turnover, synthesis and breakdown in patients with colorectal carcinoma. Br J Surg. 1980 Oct;67(10):736–739. doi: 10.1002/bjs.1800671015. [DOI] [PubMed] [Google Scholar]
- Cooper E. H., Stone J. Acute phase reactant proteins in cancer. Adv Cancer Res. 1979;30:1–44. doi: 10.1016/s0065-230x(08)60893-3. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Emery P. W., Lovell L., Rennie M. J. Protein synthesis measured in vivo in muscle and liver of cachectic tumor-bearing mice. Cancer Res. 1984 Jul;44(7):2779–2784. [PubMed] [Google Scholar]
- Fornander J., Bergmark J., Jagenburg R., Hasselgren P. O. Evaluation of an in vitro method for the study of hepatic protein synthesis in liver ischemia. Eur Surg Res. 1985;17(2):91–100. doi: 10.1159/000128453. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Glass R. E., Fern E. B., Garlick P. J. Whole-body protein turnover before and after resection of colorectal tumours. Clin Sci (Lond) 1983 Jan;64(1):101–108. doi: 10.1042/cs0640101. [DOI] [PubMed] [Google Scholar]
- Goodlad G. A., Mitchell A. J., McPhail L., Clark C. M. Serum insulin and somatomedin levels in the tumour-bearing rat. Eur J Cancer. 1975 Oct;11(10):733–737. doi: 10.1016/0014-2964(75)90048-1. [DOI] [PubMed] [Google Scholar]
- Heber D., Chlebowski R. T., Ishibashi D. E., Herrold J. N., Block J. B. Abnormalities in glucose and protein metabolism in noncachectic lung cancer patients. Cancer Res. 1982 Nov;42(11):4815–4819. [PubMed] [Google Scholar]
- Jeevanandam M., Horowitz G. D., Lowry S. F., Brennan M. F. Cancer cachexia and protein metabolism. Lancet. 1984 Jun 30;1(8392):1423–1426. doi: 10.1016/s0140-6736(84)91929-9. [DOI] [PubMed] [Google Scholar]
- Kawamura I., Moldawer L. L., Keenan R. A., Batist G., Bothe A., Jr, Bistrian B. R., Blackburn G. L. Altered amino acid kinetics in rats with progressive tumor growth. Cancer Res. 1982 Mar;42(3):824–829. [PubMed] [Google Scholar]
- Lundholm K., Edström S., Ekman L., Karlberg I., Bylund A. C., Scherstén T. A comparative study of the influence of malignant tumor on host metabolism in mice and man: evaluation of an experimental model. Cancer. 1978 Aug;42(2):453–461. doi: 10.1002/1097-0142(197808)42:2<453::aid-cncr2820420212>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Garlick P. J. Protein synthesis in liver and small intestine in protein deprivation and diabetes. Am J Physiol. 1981 Sep;241(3):E238–E245. doi: 10.1152/ajpendo.1981.241.3.E238. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Tomkins A. M., Garlick P. J. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979 Feb 15;178(2):373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. A., Stein T. P., Brennan M. F. Whole body protein synthesis and turnover in normal man and malnourished patients with and without known cancer. Ann Surg. 1981 Aug;194(2):123–128. doi: 10.1097/00000658-198108000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odedra B. R., Bates P. C., Millward D. J. Time course of the effect of catabolic doses of corticosterone on protein turnover in rat skeletal muscle and liver. Biochem J. 1983 Aug 15;214(2):617–627. doi: 10.1042/bj2140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Randall D. P., Garlick P. J. Protein synthesis in liver and skeletal muscle of mice bearing an ascites tumor. Cancer Res. 1984 Mar;44(3):1054–1057. [PubMed] [Google Scholar]
- Rennie M. J., Edwards R. H., Halliday D., Matthews D. E., Wolman S. L., Millward D. J. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982 Dec;63(6):519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Schaur R. J., Fellier H., Gleispach H., Fink E., Kronberger L. Tumor host relations. I. Increased plasma cortisol in tumor-bearing humans compared with patients with benign surgical diseases. J Cancer Res Clin Oncol. 1979 Apr 12;93(3):281–285. doi: 10.1007/BF00964584. [DOI] [PubMed] [Google Scholar]
- Schworer C. M., Mortimore G. E. Glucagon-induced autophagy and proteolysis in rat liver: mediation by selective deprivation of intracellular amino acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3169–3173. doi: 10.1073/pnas.76.7.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi P., Sterling K. E., Lam K. S., Finley P. R., Ryan K. J., Ray C. G., Petersen E., Slymen D. J., Salmon S. E. Raised serum levels of tumour necrosis factor in parasitic infections. Lancet. 1986 Dec 13;2(8520):1364–1365. doi: 10.1016/s0140-6736(86)92007-6. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E., Grinde B., Gordon P. B., Schwarze P. E., Gjessing R., Poli A. Amino acid control of protein synthesis and degradation in isolated rat hepatocytes. Ann N Y Acad Sci. 1980;349:1–17. doi: 10.1111/j.1749-6632.1980.tb29510.x. [DOI] [PubMed] [Google Scholar]
- Sganga G., Siegel J. H., Brown G., Coleman B., Wiles C. E., 3rd, Belzberg H., Wedel S., Placko R. Reprioritization of hepatic plasma protein release in trauma and sepsis. Arch Surg. 1985 Feb;120(2):187–199. doi: 10.1001/archsurg.1985.01390260051008. [DOI] [PubMed] [Google Scholar]
- Strom S. C., Jirtle R. L., Jones R. S., Novicki D. L., Rosenberg M. R., Novotny A., Irons G., McLain J. R., Michalopoulos G. Isolation, culture, and transplantation of human hepatocytes. J Natl Cancer Inst. 1982 May;68(5):771–778. [PubMed] [Google Scholar]
- Warren R. S., Jeevanandam M., Brennan M. F. Comparison of hepatic protein synthesis in vivo versus in vitro in the tumor-bearing rat. J Surg Res. 1987 Jan;42(1):43–50. doi: 10.1016/0022-4804(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Warren R. S., Jeevanandam M., Brennan M. F. Protein synthesis in the tumor-influenced hepatocyte. Surgery. 1985 Aug;98(2):275–282. [PubMed] [Google Scholar]


