Abstract
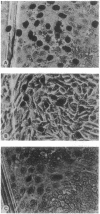
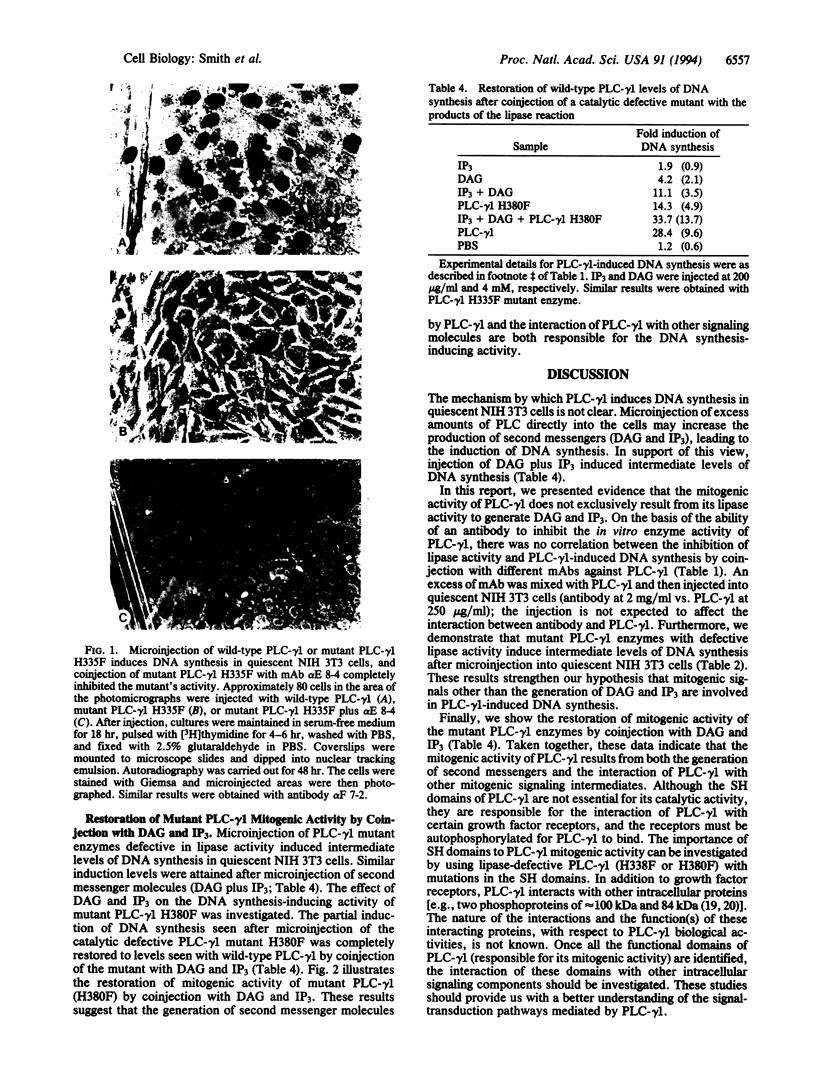
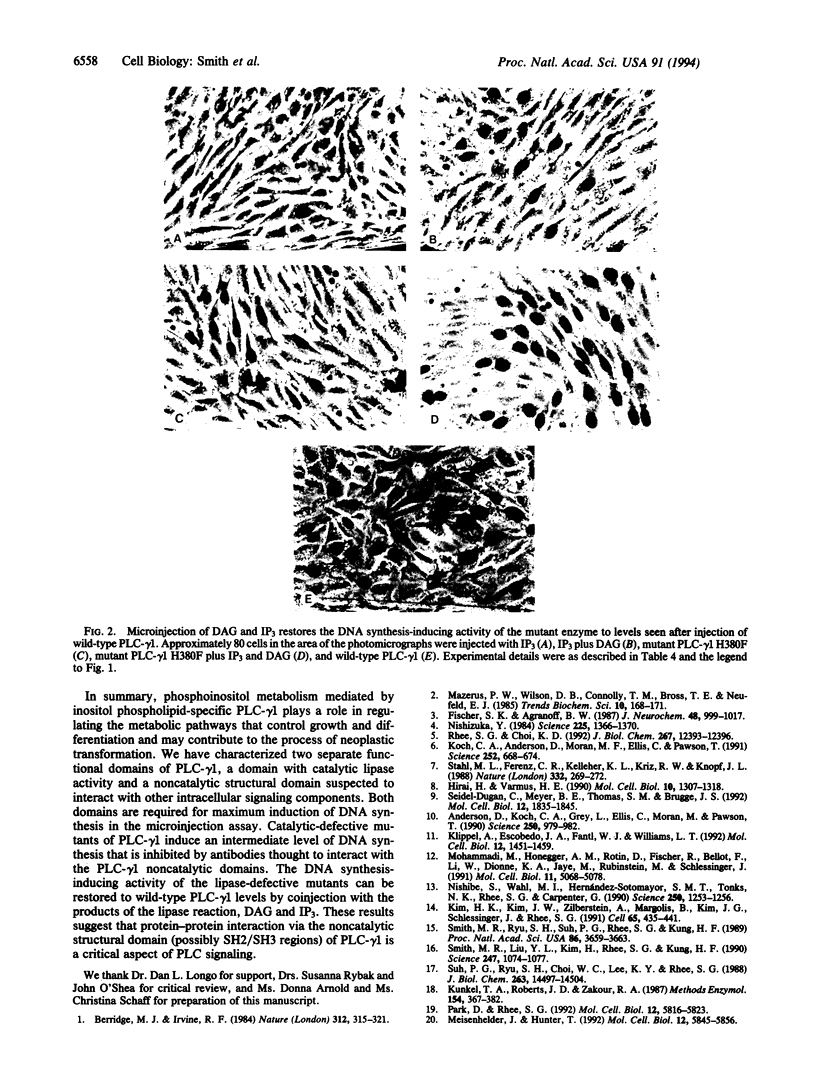
Inositol phospholipid-specific phospholipase C (PLC) is involved in several signaling pathways leading to cellular growth and differentiation. Our previous studies reported the induction of DNA synthesis in quiescent NIH 3T3 cells after microinjection of PLC and the inhibition of serum- or Ras-stimulated DNA synthesis by a mixture of monoclonal antibodies to PLC-gamma 1. In the course of our investigation of anti-PLC-gamma 1 monoclonal antibodies, we found that each antibody exerts different inhibitory effects on the phosphatidylinositol-hydrolyzing activity of PLC-gamma 1 and that the inhibition of enzymatic activity does not correlate with the inhibition of DNA synthesis observed in the microinjection assay. PLC-gamma 1 with defective enzymatic activity was synthesized by substituting phenylalanine for histidine within the PLC-gamma 1 catalytic domain at amino acids 335 and 380, and mutant enzymes were expressed using a vaccinia expression system. The mutant enzymes were purified and microinjected into quiescent NIH 3T3 cells to evaluate their mitogenic activity. A moderate induction of DNA synthesis occurred after injection of mutant PLC-gamma 1. This mitogenic activity was inhibited by an antibody (alpha E 8-4) that does not significantly inhibit PLC-gamma 1 enzyme activity, which indicates that something else has to be inhibited. Furthermore, the partial induction of DNA synthesis observed with mutant PLC-gamma 1 was increased to levels seen with wild-type PLC-gamma 1 by coinjection of mutant PLC-gamma 1 with two second messengers, diacylglycerol and inositol trisphosphate. These results suggest that the mitogenic activity of PLC-gamma 1 does not exclusively result from the enzymatic activity of the lipase and that another activity inherent to the PLC-gamma 1 molecule can also induce DNA synthesis in quiescent cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Koch C. A., Grey L., Ellis C., Moran M. F., Pawson T. Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science. 1990 Nov 16;250(4983):979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Agranoff B. W. Receptor activation and inositol lipid hydrolysis in neural tissues. J Neurochem. 1987 Apr;48(4):999–1017. doi: 10.1111/j.1471-4159.1987.tb05618.x. [DOI] [PubMed] [Google Scholar]
- Hirai H., Varmus H. E. Site-directed mutagenesis of the SH2- and SH3-coding domains of c-src produces varied phenotypes, including oncogenic activation of p60c-src. Mol Cell Biol. 1990 Apr;10(4):1307–1318. doi: 10.1128/mcb.10.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. K., Kim J. W., Zilberstein A., Margolis B., Kim J. G., Schlessinger J., Rhee S. G. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991 May 3;65(3):435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- Klippel A., Escobedo J. A., Fantl W. J., Williams L. T. The C-terminal SH2 domain of p85 accounts for the high affinity and specificity of the binding of phosphatidylinositol 3-kinase to phosphorylated platelet-derived growth factor beta receptor. Mol Cell Biol. 1992 Apr;12(4):1451–1459. doi: 10.1128/mcb.12.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Hunter T. The SH2/SH3 domain-containing protein Nck is recognized by certain anti-phospholipase C-gamma 1 monoclonal antibodies, and its phosphorylation on tyrosine is stimulated by platelet-derived growth factor and epidermal growth factor treatment. Mol Cell Biol. 1992 Dec;12(12):5843–5856. doi: 10.1128/mcb.12.12.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M., Honegger A. M., Rotin D., Fischer R., Bellot F., Li W., Dionne C. A., Jaye M., Rubinstein M., Schlessinger J. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-gamma 1. Mol Cell Biol. 1991 Oct;11(10):5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Park D., Rhee S. G. Phosphorylation of Nck in response to a variety of receptors, phorbol myristate acetate, and cyclic AMP. Mol Cell Biol. 1992 Dec;12(12):5816–5823. doi: 10.1128/mcb.12.12.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992 Jun 25;267(18):12393–12396. [PubMed] [Google Scholar]
- Seidel-Dugan C., Meyer B. E., Thomas S. M., Brugge J. S. Effects of SH2 and SH3 deletions on the functional activities of wild-type and transforming variants of c-Src. Mol Cell Biol. 1992 Apr;12(4):1835–1845. doi: 10.1128/mcb.12.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Liu Y. L., Kim H., Rhee S. G., Kung H. F. Inhibition of serum- and ras-stimulated DNA synthesis by antibodies to phospholipase C. Science. 1990 Mar 2;247(4946):1074–1077. doi: 10.1126/science.2408147. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Ryu S. H., Suh P. G., Rhee S. G., Kung H. F. S-phase induction and transformation of quiescent NIH 3T3 cells by microinjection of phospholipase C. Proc Natl Acad Sci U S A. 1989 May;86(10):3659–3663. doi: 10.1073/pnas.86.10.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Choi W. C., Lee K. Y., Rhee S. G. Monoclonal antibodies to three phospholipase C isozymes from bovine brain. J Biol Chem. 1988 Oct 5;263(28):14497–14504. [PubMed] [Google Scholar]