Abstract
Atherosclerosis is a chronic immune-inflammatory disorder that integrates multiple cell types and a diverse set of inflammatory mediators. miRNAs are emerging as important post-transcriptional regulators of gene expression in most, if not all, vertebrate cells and constitute central players in many physiological and pathological processes. Rapidly accumulating experimental studies reveal their key role in cellular and molecular processes related to the development of atherosclerosis. Here, we review the current evidence for the involvement of miRNAs in early atherosclerotic lesion formation to plaque rupture and erosion. We conclude with a perspective on the clinical relevance, therapeutic opportunities, and future challenges of miRNA biology in the pathogenesis of this complex disease.
Keywords: atherosclerosis, miRNAs, inflammation, shear stress, vulnerable plaque
miRNAs: novel players in atherosclerotic processes
miRNAs are highly conserved, single-stranded noncoding RNA molecules, ~22 nucleotide-long, that exert post-transcriptional effects on gene expression by promoting degradation of mRNA target and/or inhibiting mRNA translation [1]. Their biology constitutes a complex and highly orchestrated mode of gene regulation, potentially participating in nearly all biological processes in mammals and having essential roles in health and disease states.
Atherosclerosis (see Glossary) is a chronic immune-inflammatory vascular disease and the leading cause of death worldwide. Atherosclerotic lesions progress from the early fatty streaks to the complex vulnerable plaques that are responsible for the acute consequences of the disease. The pathogenesis of atherosclerosis is a result of interactions between multiple cell types in the vessel wall and a diverse set of inflammatory factors that occur at predilection sites with disturbed laminar blood flow. Given the diversity of underlying mechanisms, it is not surprising that miRNAs have emerged as an additional crucial regulatory network intersecting with the cellular and molecular mechanisms that govern the development of atherosclerosis (Figure 1).
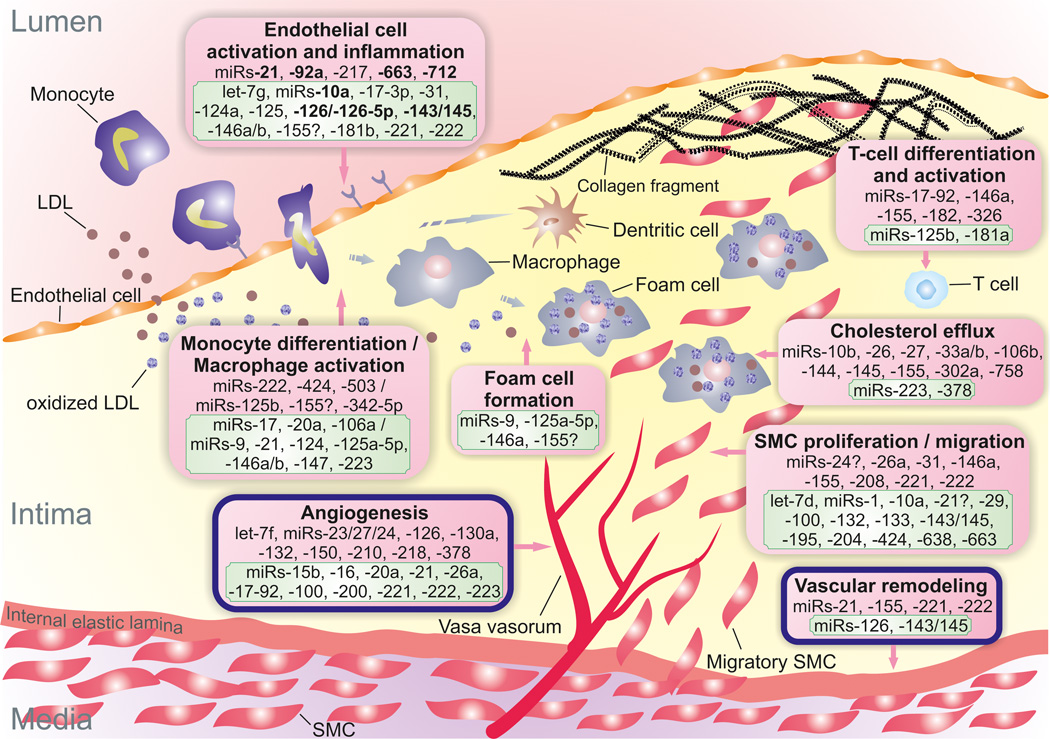
Figure 1. miRNAs implicated in atherosclerotic processes.
Positive/atheroprotective (in green frame) or negative/atherogenic (in red frame) effects of miRNAs on atherosclerotic process are shown. Question marks next to miRNAs indicate controversial or contradictory evidence. miRNAs in bold are those reported to be regulated by blood flow/shear stress. LDL diffuses from the blood into the intima and undergoes oxidative modification. Oxidized LDL triggers the expression of leukocyte adhesion molecules by endothelial cells. The initial steps of atherosclerosis include adhesion of blood monocytes to the activated endothelium, their migration into the intima, their maturation into macrophages (or dendritic cells), and their uptake of lipid yielding foam cells. Although fewer in number than macrophages, other leukocyte subsets, such as T cells, also enter the intima and regulate cellular and humoral immune responses. Lesion progression involves the proliferation and migration of SMCs into the intima, as well as increased extracellular matrix protein synthesis, including collagen. Advanced lesions also exhibit intraplaque neovascularization and outward remodeling.
Abbreviations: LDL, low-density lipoprotein; SMC, smooth muscle cell.
The role of miRNAs in atherosclerotic plaque initiation and progression
Accumulating experimental evidence indicates that cellular and molecular processes related to the development of atherosclerosis are affected by a plethora of miRNAs.
Endothelial activation and inflammation
Induced by both biochemical and biomechanical stimuli, the early phase of atherosclerotic disease is characterized by the activation of endothelial cells (ECs) that express a set of adhesion molecules, such as vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1, and E-selectin. Several miRNAs have been implicated in the regulation of the inflammatory response in ECs (Figure 2). For example, miR-31 and miR-17-3p directly inhibit tumor necrosis factor (TNF)-α induced E-selectin and ICAM-1 expression respectively, thus acting in a negative feedback loop to control EC activation [2]. miR-155 and miR-221/222 inhibit the angiotensin II-induced inflammatory response in ECs in vitro via targeting of transcription factor Ets-1 and its downstream genes, including VCAM-1 and monocyte chemoattractant protein (MCP)-1 [3]. However, the role of miR-155 in EC function remains controversial as it has also been found to directly target endothelial nitric oxide synthase (eNOS) mRNA [4].
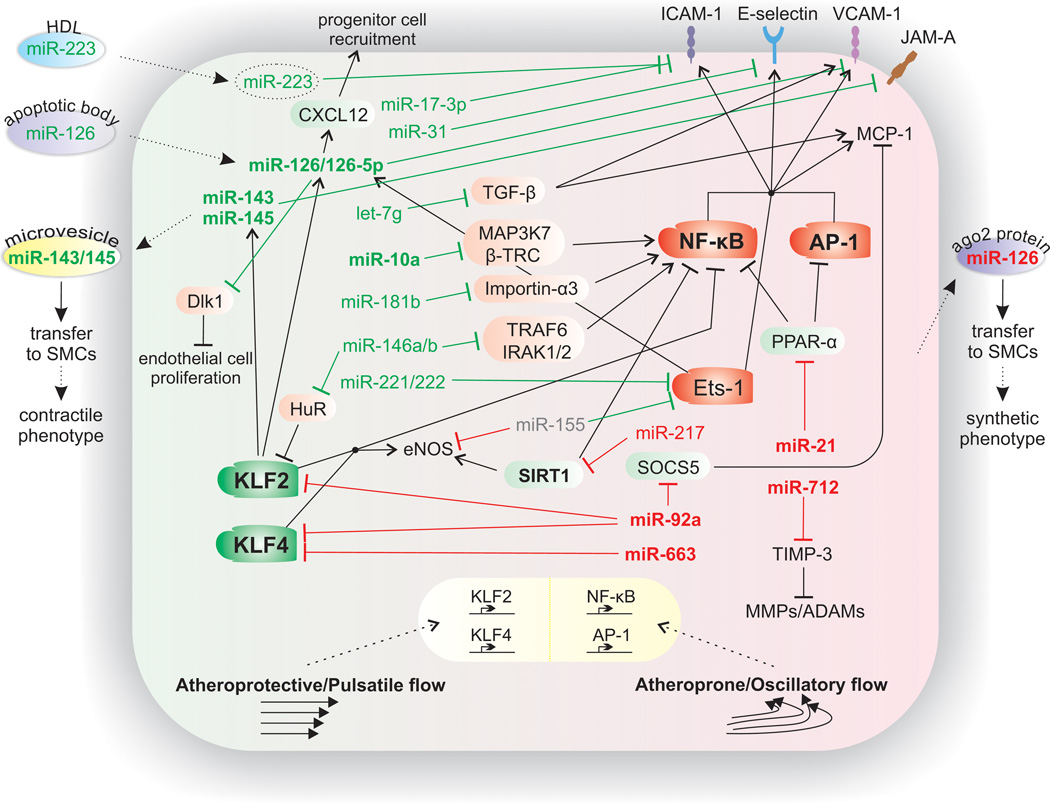
Figure 2. Endothelial miRNAs implicated in atherosclerosis.
Established miRNAs that regulate, either in an atheroprotective (shown in green) or an atherogenic (shown in red) manner, inflammatory pathways in the endothelium and the impact of shear stress on these pathways are depicted. Arrows between miRNAs and mechanistic processes indicate stimulation of the processes by the corresponding miRNA. Blunted arrows indicate inhibition. miRNAs (and other factors) in bold are those shown to be regulated by blood flow/shear stress. Transcription factors KLF2 and KLF4 mediate the anti-inflammatory effects of high shear stress (atheroprotective/pulsatile flow) by inducing eNOS. In contrast, low shear stress (atheroprone/oscillatory flow) induces the activation of proinflammatory NF-κB and AP-1, thus promoting endothelial cell dysfunction and activation. The increased expression of the downstream target genes of these transcription factors, such as VCAM-1, ICAM-1, E-selectin, and MCP-1, promotes the attachment and infiltration of lipid and inflammatory cells into the subendothelial space.
Abbreviations: ADAMs, a disintegrin and metalloproteinases; AP-1, activator protein-1; β-TRC, β-transducin repeat-containing gene; Dlk1, delta-like 1 homolog; eNOS, endothelial nitric oxide synthase; ICAM-1, intercellular adhesion molecule-1; IRAK1/2, interleukin-1receptor-associated kinase 1/2; KLF, Krüppel-like factor 2; JAM-A, junctional adhesion molecule-A; MAP3K7, mitogen-activated protein kinase kinase kinase 7; MCP-1, monocyte chemotactic protein-1; MMPs, matrix metalloproteinases; NF-κB, nuclear factor-κB; PPAR-α, peroxisome proliferator-activated receptor-α; SMC, smooth muscle cell; SIRT1, silent information regulator 1; SOCS5, suppressor of cytokine signaling 5; TIMP-3, tissue inhibitor of metalloproteinase-3; TGF-β, transforming growth factor-β; and TRAF6, tumor necrosis factor receptor-associated factor 6; VCAM-1, vascular cell adhesion molecule-1.
Recent studies in mice and human subjects highlight an important role for miR-181b as a suppressor of endothelial inflammatory responses in both acute (e.g. sepsis) and chronic (e.g. atherosclerosis) vascular disease states [5, 6]. miR-181b inhibits an enriched set of nuclear factor (NF)-κB-responsive genes, such as VCAM-1 and E-selectin, by targeting importin-α3, a protein required for nuclear translocation of NF-κB [5]. Systemic delivery of miR-181b reduces NF-κB activity and atherosclerotic lesion formation in the aortic arch of mice, accompanied by decreased numbers of lesional macrophages and CD4+ T cells [6]. Additional cytokine-responsive miRNAs include miR-146a and miR-146b, which inhibit endothelial activation by promoting eNOS expression through targeting the RNA-binding protein HuR and by repressing the induction of adhesion molecules through targeting TRAF6 and IRAK1/2 [7]. Finally, let-7g exerts antiinflammatory effects on ECs through downmodulation of transforming growth factor (TGF)-β and silent information regulator 1 (SIRT1) signaling [8]. Consistent with these observations, systemic lentiviral delivery of let-7g inhibitors increased plasminogen activator inhibitor (PAI)-1 expression and induced overgrowth of the carotid intima-media layer in mice [8].
It has long been appreciated that shear stress generated by complex hemodynamic blood flow impacts blood vessel function and contributes to the pathogenesis of atherosclerosis. Accumulating evidence suggests that several miRNA-based regulatory networks converge on shear stress-induced inflammatory pathways and regulate the endothelial inflammatory phenotype. For example, miR-126 is highly expressed in ECs and has been demonstrated in vitro to suppress VCAM-1 expression [9]. This miRNA was shown in a later study to act downstream of Krüppel-like factor 2 (KLF2) to drive flow-stimulated angiogenesis [10]. Interestingly, miR-126-carrying endothelial apoptotic bodies have been identified as atheroprotective in mice by targeting RGS16, a negative regulator of CXCR4, and promoting CXCL12-dependent recruitment of progenitor cells to the endothelial lining [11]. The passenger strand miR-126-5p, which is processed from the miR-126 duplex, is also functional and takes part in the control of leukocyte trafficking by modulating the expression of ALCAM and SetD5 [12]. Disturbed flow also suppresses miR-126-5p and promotes lesion formation through upregulation of delta-like 1 homolog (Dlk1), a negative regulator of EC proliferation [13]. Indeed, systemically treated ApoE−/− mice with miR-126-5p mimics rescued EC proliferation at predilection sites and limited lesion progression [13]. Intriguingly, miR-126 was also shown to be induced by Ets-1 and Ets-2 suggesting a potential negative feedback mechanism by which effects of proinflammatory factors, such as TNF-α or angiotensin II, may be limited by the antiinflammatory effect of miR-126 [14].
Another shear stress-regulated miRNA, miR-10a, was reported to have significantly lower expression in atherosusceptible regions of large swine vessels [15]. This miRNA was identified in vitro as a post-transcriptional inhibitor of NF-κB activation through suppression of mitogen-activated protein kinase kinase kinase 7 (MAP3K7) and the β-transducin repeat-containing gene (β-TRC), two factors involved in IκΒα degradation [15]. The role of miR-10a in atherosclerotic lesion formation remains to be elucidated. In a recent study, atheroprotective laminar flow conditions induced miR-145 to repress junctional adhesion molecule-A expression, a molecule that increases endothelial intercellular permeability and leukocyte extravasation [16].
In contrast, miR-92a is upregulated by oscillatory shear stress in vitro [17] and is expressed at a higher level in regions of atherosusceptibility in vivo [18]. In silico predictions and experimental validation demonstrated that two targets of miR-92a are the laminar flow-responsive transcription factors KLF2 [17, 18] and KLF4 [18]. More specifically, miR-92a decreases KLF2 mRNA and KLF2-regulated eNOS and thrombomodulin expression [17]. Moreover, a recent study showed that oxidized low density lipoprotein (oxLDL) increases miR-92a expression primarily in low shear stress areas, while therapeutic inhibition of miR-92a attenuates endothelial NF-κB activation and limits the development of atherosclerotic lesions in mice, in part, by derepression of KLF2 and KLF4 [19]. It has also been shown in vitro that oscillatory shear stress induces monocyte adhesion to ECs by upregulating miR-663. This inflammatory response was associated with altered expression of several transcription factors, including KLF4 [20]. Similarly, oscillatory shear stress induces a sustained miR-21 expression in human umbilical vein ECs (HUVECs), which promotes increased activity of activator protein-1 by targeting peroxisome proliferator-activated receptor-α (PPAR-α), thus enhancing expression of VCAM-1 and MCP-1 [21]. However, this report comes in contrast to data pointing to an atheroprotective function of miR-21 in HUVECs exposed to unidirectional shear stress, by decreasing apoptosis and increasing eNOS phosphorylation [22]. miR-712 expression, as well as its human homologue miR-205, is also upregulated in regions of disturbed flow in vitro and in vivo. This miRNA stimulates endothelial inflammation, permeability, and extracellular matrix fragmentation via direct repression of tissue inhibitor of metalloproteinase (TIMP)-3, while inhibiting miR-712 rescues TIMP-3 expression and reduces lesion formation in response to partial carotid artery ligation in ApoE−/− mice [23]. Finally, miR-217 induces EC senescence by direct inhibition of SIRT1 [24], an atheroprotective flow-induced anti-inflammatory deacetylase that activates eNOS and inactivates NF-κB.
Recent studies also highlight the role of emerging paradigms of miRNA-mediated cellular regulation in endothelial activation and inflammation. These include novel forms of inter- and extracellular communication, such as via microvesicles, exosomes, and lipoproteins (Box 1).
Box 1. miRNA extracellular release and transport in atherosclerosis.
miRNAs can be secreted from the cell, packaged into extracellular vesicles (e.g. apoptotic bodies, microparticles, and exosomes), and transferred to recipient cells, where they alter gene expression. miRNAs are also found in circulation in complex with HDL or RNA-binding proteins, such as Ago2. Interestingly, in a recent study by Tabet et al. [107], it was demonstrated that the anti-inflammatory properties of HDL are conferred, in part, by the transfer of miR-223, which is highly expressed in myeloid cells, to recipient ECs, where it decreases ICAM-1 expression. However, HDL-associated miR-223 contributes to only 8% of the total circulating pool of miRNAs, and other groups have not observed significant uptake of HDL-associated miRNAs to ECs, SMCs, or peripheral leukocytes in vitro [108]. Future studies will be required to provide clarity to the functional consequences of HDL-associated miR-223 in the progression of atherosclerotic lesions and the cell types that are directly affected in vivo.
Communication and signaling between ECs and SMCs are also critical for cardiovascular homeostasis and the pathogenesis of vascular disease states, including atherosclerosis. In this context, laminar shear stress in a physiological range was shown to upregulate expression of miR-143/145 cluster in ECs through a KLF2-dependent mechanism [109]. The release and transport of these miRNAs in extracellular microvesicles to SMCs represses miR-143/145 target genes (e.g. KLF4, ELK1, CAMK2d, and SSH2) involved in dedifferentiation of these cells. Moreover, delivery of these microvesicles to ApoE−/− mice reduced aortic lesion areas, an effect that was abrogated by ex vivo inhibition of miR-143/145 [109]. Conversely, non-laminar or turbulent flow conditions induce secretion of miR-126 by ECs both in vitro and in vivo. This miRNA, bound to Ago2, is then transported to adjacent SMCs where it represses the expression of specific target genes (e.g. FOXO3, BCL2, and IRS1) that normally keep these cells in the atheroprotective contractile phenotype [110]. Therefore, miR-126, despite its beneficial effects on endothelial homeostasis [9], may play an atheroprone role in SMCs.
Immune activation
Immunity is a major contributor to atherosclerosis. It is mediated by components of the innate immune system, such as macrophages and dendritic cells, as well as adaptive immune system, such as T lymphocytes.
Macrophages play a central role in the pathophysiology of atherosclerosis through both maintaining vessel wall lipid homeostasis and orchestrating inflammatory responses. miRNAs are involved in macrophage biology by regulating their differentiation from precursor cells and modifying their inflammatory capacity, thus exerting profound effects during plaque evolution. For example, the multifunctional miRNA, miR-155, was induced in macrophages in atherosclerotic regions of ApoE−/− mice and suppressed B-cell leukemia/lymphoma 6 (BCL6), a transcription factor that counter-regulates NF-κB activation [25]. Consistent with these findings, ApoE−/− mice with bone marrow miR-155 deficiency exhibited reduced macrophage inflammatory responses, enhanced macrophage cholesterol efflux, and reduced lesion size [26]. In contrast, in LDL receptor knockout (LDLR−/−) mice, bone marrow miR-155 deficiency enhanced atherosclerosis by generating a more pro-inflammatory macrophage phenotype [27]. Collectively, these data highlight the importance of miR-155 in regulating the inflammatory signaling pathways of the macrophage lineage, an effect that may reflect atherosclerotic stage-dependent actions of miR-155 on its target genes in response to hyperlipidemic conditions achieved in ApoE−/− and LDLR−/− mice. Future studies will be needed to clarify the role of macrophage-specific miRNA expression in atherogenesis.
Toll-like receptor (TLR) signaling pathways are highly active in human atherosclerotic plaques and have been implicated in the promotion of atherosclerosis. Several miRNAs have been shown to operate a negative feedback regulation to thwart inflammation in response to TLR activation warranting an investigation of their functions as anti-atherogenic mediators of vascular disease. For example, miR-146a and miR-146b are induced in macrophages in a NF-κB-dependent manner and are involved in inflammation resolution by limiting TLR and cytokine signaling [28]. Likewise, miR-147 attenuates TLR-associated signaling events in macrophages in a negative feedback manner [29], while miR-21 represses different components of NF-κB signaling during TLR-mediated activation of human peripheral blood mononuclear cells [30]. Intriguingly, similar to the controversy regarding its action in ECs [21, 22], miR-21 may promote TLR-mediated NF-κB activation [31]. While the in vivo role of miR-21 in atherosclerotic lesion formation has not been defined, it can be hypothesized that at the early phases miR-21 induction may be pro-inflammatory, while at later stages it may facilitate resolution of inflammation, thereby maintaining homeostasis of immune responses.
Other miRNAs have been implicated in regulating the macrophage response to inflammatory stimuli. For instance, overexpression of miR-125b induces an activated phenotype in macrophages and elevates their responsiveness to interferon (IFN)-γ, an effect mediated by miR-125b suppression of interferon regulatory factor 4 (IRF4) [32]. Interestingly, during early atherosclerosis the most prominently induced miRNA is miR-342-5p, which is expressed in lesional macrophages [33]. miR-342-5p enhances proinflammatory macrophage mediators, such as inducible nitric oxide synthase (iNOS) and IL-6, by suppressing the Akt1-mediated inhibition of miR-155 expression. Accordingly, miR-342-5p inhibition reduces atherosclerotic lesion formation in ApoE−/− mice [33].
Lipoprotein uptake by macrophages is thought to be one of the earliest pathogenic events in the nascent plaque and results in the formation of foam cells. miR-125a-5p and miR-146a were found to decrease lipid uptake and cytokine release in oxLDL-stimulated macrophages, in part by targeting the genes oxysterol binding protein-like 9 and TLR4, respectively, suggesting that they may play a protective role against the development of atherosclerosis [34, 35]. Similarly, overexpression of miR-155 decreased lipid uptake in monocytic cell lines and primary monocyte-derived dendritic cells in vitro [36]. However, in primary macrophages from atherosclerotic ApoE−/− mice, miR-155 was found to enhance oxLDL-induced foam cell formation by targeting HMG box-transcription protein1 [37]. Furthermore, injection of anti-miR-155 in ApoE−/− mice effectively decreased lipid-laden macrophage accumulation in lesions and the formation of aortic atherosclerotic plaques [37]. Finally, miR-155 expression was significantly higher in CD14+ monocytes from patients with coronary artery disease than healthy controls, implicating this miRNA as a potentially relevant myeloid target under atherosclerotic conditions.
Several studies also support an essential role for miRNAs in regulating macrophage polarization, which is a critical component of the inflammatory response. For example, miR-124 has a key role in inhibiting macrophage activation and skewing their polarization from a pro-inflammatory M1 toward an anti-inflammatory M2 phenotype, in part, by targeting the transcription factor C/EBP-α [38]. Moreover, miR-223-deficient macrophages exhibited increases in M1 and decreases in M2 polarization biomarkers, suggesting a suppressive effect of this miRNA on macrophage pro-inflammatory activation, in part, by targeting the protein Pknox1 [39]. Likewise, miR-125a-5p diminished M1 phenotype expression induced by lipopolysaccharide (LPS), but promoted M2 marker expression induced by IL-4 [40]. Conversely, miR-155 has been shown to induce M1 polarization by targeting C/EBP-β [41]. The kinetics of miRNA expression associated with M1/M2 polarization in the atherosclerotic plaque will require future investigation.
Other immune cells, such as dendritic cells and T cells, predominantly type 1 T helper cells, are also present throughout the natural history of plaque development and are considered to play an important role in atherogenesis. Several studies highlight that miRNAs play important roles in their function. Representatively, miR-181a attenuates the oxLDL-induced immune inflammatory response by targeting transcription factor c-Fos and reducing dendritic maturation cell surface molecules, including CD40 and CD83 [42]. In addition, T-cell receptor (TCR) activation increases expression of miR-146a, which protects T cells from cell death and controls the resolution of T-cell responses by targeting, respectively, the pro-apoptotic factor Fas-associated death domain and the NF-κB mediators TRAF6/IRAK1 [43]. Consistent with the latter findings, T cells lacking miR-146a are hyperactivated to antigen stimulation and are prone to induce T-cell-mediated inflammatory disease [43]. Future studies will be required to explore the role of dendritic- and T-cell-specific miRNA-mediated effects in the context of atherogenesis.
Cholesterol homeostasis
Excessive LDL cholesterol in the circulation leads to increased subendothelial accumulation and its local oxidative and enzymatic modification elicits an immune response from resident and recruited immune cells resulting in a chronically inflamed environment that promotes plaque growth. Several hepatic-enriched miRNAs have demonstrated important functional properties in lipoprotein homeostasis. miR-122, expressed primarily in liver, plays a critical role in regulating lipid metabolism by controlling AMPK activation, cholesterol synthesis, and lipoprotein secretion [44]. Hepatic miR-27b also regulates the expression of multiple key metabolic genes which have been implicated in the pathobiology of lipid-related disorders [45]. Finally, overexpression of miR-30c reduced hyperlipidemia and attenuated atherosclerosis in ApoE−/− mice by targeting microsomal triglyceride transfer protein (MTTP), a key protein for effective hepatic assembly of very low-density lipoprotein (VLDL) and ApoB-containing lipoproteins [46].
Cholesterol efflux capacity is essential for maintaining cholesterol homeostasis and constitutes a robust predictor of atherosclerosis in humans. miR-33a and miR-33b have been shown to act as post-transcriptional inhibitors of adenosine triphosphate-binding cassette A1 (ABCA1) and ABCG1 expression in macrophages, resulting in reduced cholesterol efflux to high-density lipoprotein (HDL) [47–50]. Treatment with anti-miR-33 oligonucleotides increased ABCA1 and ABCG1 expression, thus enhancing cholesterol efflux and regression of atherosclerotic plaques in LDLR−/− mice [51]. Subsequent studies in nonhuman primates showed that antisense oligonucleotides targeting miR-33a/b are effective in increasing HDL cholesterol and lowering VLDL-associated triglycerides by inducing ABCA1 expression [52, 53]. Mechanistically, these miRNAs were demonstrated to regulate key genes involved in fatty acid metabolism and insulin signaling [54]. Interestingly, miR-33 inhibition was recently shown to overcome the deleterious effects of hyperglycemia on plaque regression in atherosclerotic mice after their plasma lipids were aggressively lowered [55]. Moreover, long-term anti-miR-33 therapy significantly reduced the progression of atherosclerosis independently of plasma HDL-cholesterol levels [56]. However, prolonged anti-miR-33 treatment failed to maintain elevated plasma HDL and did not prevent the progression of atherosclerosis in LDLR−/− mice [57]. In addition, miR-33 inhibition increased circulating VLDL-triglyceride secretion, an effect mediated by miR-33 targeting hepatic expression of N-ethylmaleimide-sensitive factor, a key protein in VLDL vesicular trafficking [58]. Finally, genetic loss of miR-33 or long-term anti-miR-33 treatment exacerbated high fat diet-induced obesity and liver steatosis in mice [59, 60]. Collectively, future studies will be required to sort out the experimental conditions in miR-33 inhibitor studies that may, in part, account for differences observed across studies on lipoprotein metabolism and atherosclerosis.
Although miR-33 has been the most extensively studied in vivo, several other miRNAs have also been shown to modulate cholesterol efflux in macrophages and other cell types, indicating that HDL regulation by miRNAs is likely to be complex. In a recent study, miR-302a was demonstrated to suppress ABCA1, while anti-miR-302a treatment attenuated atherosclerosis progression in mice [61]. miR-10b also directly repressed ABCA1 and ABCG1 and negatively regulated cholesterol efflux from lipid-loaded macrophages [62]. Similarly, miR-27 [63], miR-144 [64, 65], miR-145 [66, 67], miR-223 [68], and miR-758 [69] post-transcriptionally regulate cellular cholesterol efflux to apolipoprotein A-I by suppression of ABCA1 expression. Moreover, miR-26 suppresses other genes involved in cholesterol mobilization in addition to ABCA1, such as ADP-ribosylation factor-like 7, an intracellular transport protein that moves cholesterol to the membrane for removal by ABCA1 in response to activation of the liver X receptor (LXR) nuclear hormone signaling pathway [70]. Recently, coenzyme Q10 was demonstrated to inhibit activator protein-1 and reduce miR-378 expression, which increased the expression of ABCG1 in mouse and human macrophages and, thus, facilitated macrophage cholesterol efflux in vitro and in vivo [71].
The selective uptake of HDL cholesterol into the liver, the pivotal final step of reverse cholesterol transport, is mediated by scavenger receptor B-I, which possesses atheroprotective activity. In this context, miR-96, miR-185, and miR-223 have been demonstrated to repress hepatic scavenger receptor B-I providing an additional important regulatory mechanism that modulates HDL cholesterol transport [68, 72].
Vascular smooth muscle cells
In healthy arteries, medial smooth muscle cells (SMCs) are specialized to maintain a differentiated contractile phenotype. In response to vascular injury or inflammatory signaling, SMCs dedifferentiate and adopt a synthetic phenotype. Critical regulators in the maintenance of the mature SMC phenotype include the transcription factor serum-response factor (SRF), SRF-associated coactivators, such as myocardin, and TGF-β signaling effectors, whereas soluble factors, such as platelet-derived growth factor (PDGF), promote the dedifferentiation of SMCs. Accumulating studies indicate that multiple miRNAs play a central role in the mechanisms determining SMC phenotype. The miRNA cluster containing miR-143 and miR-145, the most abundant SMC miRNAs, is a key player in SMC differentiation through the enhancement of myocardin expression [73]. Deficiency of these miRNAs results in inappropriate SMC plasticity and promotes a phenotypic switch from a contractile to a proliferative, migratory state via regulation of the angiotensin-converting enzyme gene [74]. Myocardin was also found to inhibit SMC migration via induction of miR-24 and miR-29a, and subsequent inhibition of PDGF-β [75]. Moreover, oxLDL-treated human SMCs transfected with miR-195 precursor exhibited reduced proliferation, migration, and pro-inflammatory cytokine secretion, an effect mediated in part by miR-195 repression of the cell cycle regulator cdc42 [76]. miR-638, another miRNA robustly expressed in human SMCs, inhibits human SMC proliferation and migration by targeting the orphan nuclear receptor NOR1, which is a critical regulator implicated in atherosclerosis [77]. Similarly, overexpression of miR-663 increased expression of SMC differentiation marker genes and potently inhibited PDGF-induced SMC proliferation and migration [78]. Other miRNAs that promote the acquisition of a contractile phenotype of SMCs include miR-133 [79] and miR-424 [80]. In contrast, miR-221, expressed under PDGF stimulation, decreases the differentiation and increases the proliferative capacity of SMCs via downregulation of c-kit and p27Kip1, respectively [81]. Knockdown of miR-221 and miR-222 resulted in decreased SMC proliferation both in vitro and in vivo [82]. Other miRNAs promoting a switch to the synthetic phenotype include miR-26a [83] and miR-146a [84].
Like monocyte-derived macrophages, intimal SMCs express scavenger receptors that facilitate lipid uptake and foam cell formation. The oxLDL receptor LOX-1, which plays an important role in the atherogenic process by mediating the internalization of oxLDL into cells and inducing a pro-inflammatory phenotype, was suppressed by let-7g in human aortic SMCs [85]. Indeed, circulating levels of let-7g are reduced in hypercholesterolemic human subjects implicating a potential relationship between let-7g and hyperlipidemia [85].
The role of miRNAs in atherosclerotic plaque rupture
Rupture-prone vulnerable plaques, referred to as thin-cap fibroatheromas, are typically associated with the presence of highly inflammatory cell content and a large necrotic core covered by a thin fibrous cap. Accumulating studies implicate various roles for miRNAs in processes related to the risk of atherosclerotic plaque rupture (Figure 3).
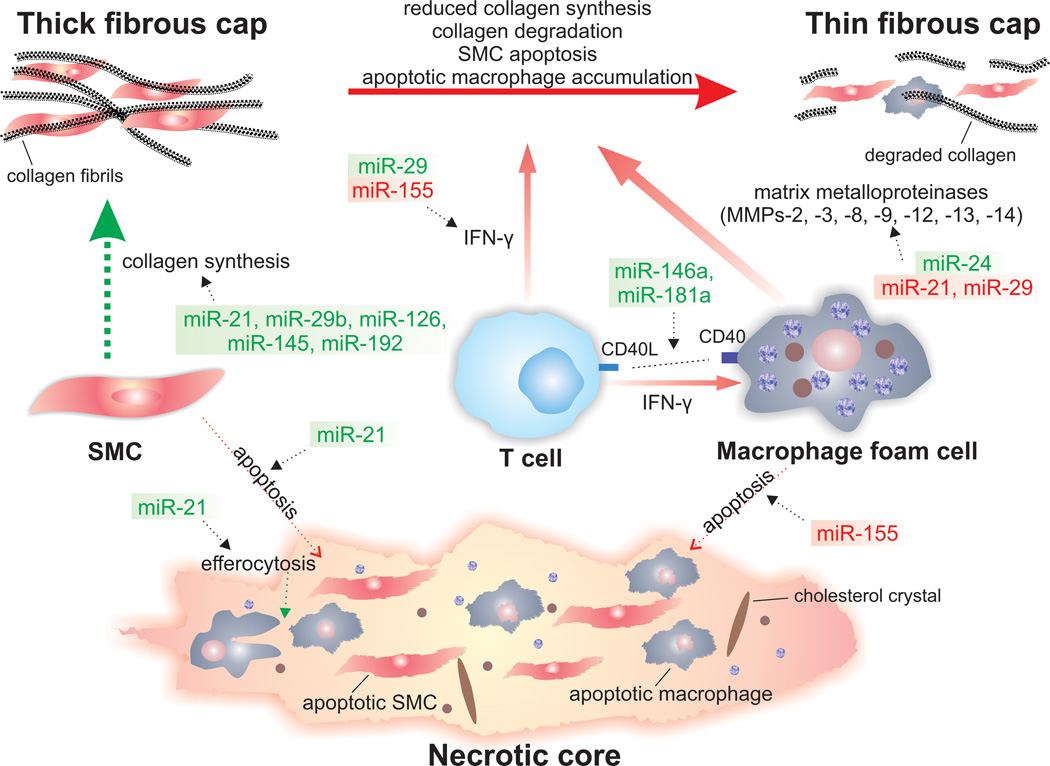
Figure 3. miRNAs implicated in atherosclerotic plaque destabilization.
The potential involvements of miRNAs in molecular and cellular mechanisms associated with atherosclerotic plaque instability are illustrated. Green miRNAs indicate those with stabilizing properties, while red miRNAs indicates those with destabilizing properties. The advanced atherosclerotic plaque consists of a fibrous cap rich in SMCs and collagen. The gradual loss of SMCs by apoptosis and the increased activity of matrix-degrading enzymes result in a fragile and rupture-prone fibrous cap. T cells produce proinflammatory mediators, such as IFN-γ and CD40L, which contribute to the amplification of local inflammation and plaque instability. Moreover, IFN-γ inhibits the collagen synthesis by SMCs. CD40L interactions with CD40 lead further to the release and activation of MMPs from activated macrophages. Apoptotic macrophages and SMCs, as well as extracellular lipid derived from dead cells, can accumulate in advanced plaques leading to the formation of a soft, destabilizing necrotic core within the intima.
Abbreviations: CD40L, CD40 ligand; IFN-γ, interferon-γ; MMPs, matrix metalloproteinases; SMC, smooth muscle cell.
Fibrous cap thinning
Vulnerable plaques show evidence of SMC death and decreased number of SMCs in the fibrous cap, suggesting that therapeutic stabilization of existing plaques could involve SMCs as a primary target. Respectively, upregulated miR-21 expression inhibits reactive oxygen species-induced SMC apoptosis and death [86]. Moreover, miR-126-treated arteries in mice exhibited increased number of intimal SMCs, higher collagen content, and reduced apoptotic cells, consistent with a more stable plaque phenotype [11]. Promoting a contractile SMC phenotype may lead to increased fibrous cap integrity. In this context, overexpression of miR-145 in SMCs reduced plaque volume in ApoE−/− mice and increased features of plaque stability, such as increased collagen content and fibrous cap area, in a fashion consistent with the promotion of a quiescent SMC phenotype [87]. Therefore, upregulation of miR-143/145 may not only decrease SMC proliferation in the initial stages of atherosclerotic plaque evolution, but also stabilize fibrous cap in more advanced plaques. Moreover, IFN-γ compromises fibrous cap integrity by inhibiting the ability of SMCs to express the genes encoding procollagens. In this respect, miR-29 directly suppresses IFN-γ production by targeting IFN-γ mRNA [88].
Macrophage-derived matrix metalloproteinases (MMPs) play a crucial role of in fibrous cap thinning and plaque destabilization. In a recent study, miR-24 downregulation was demonstrated to enhance macrophage apoptosis and MMP-14 proteolytic activity, promoting atherosclerotic plaque progression and characteristics associated with plaque instability [89]. Finally, miR-29 represses the expression of target genes that encode extracellular matrix proteins and may, thus, sensitize the aorta for aneurysm formation, while treatment with a miR-29 inhibitor augmented matrix synthesis [90].
Necrotic core
Exacerbated macrophage and SMC apoptosis is a main contributor of necrotic core formation and expansion. Endoplasmic reticulum stress-induced pathways, primarily the unfolded protein response, contribute to macrophage death and subsequent plaque necrosis in advanced atheromata. In this regard, miR-155 has been implicated in the induction of macrophage apoptosis in response to specific stimuli [91], while several miRNAs have emerged as regulators of the unfolded protein response [92]. During advanced atherosclerosis, defective apoptotic cell clearance by activated macrophages through the process of efferocytosis leads to apoptotic cell accumulation, delayed resolution of inflammation, and expansion of the necrotic core. Elevated macrophage miR-21 was recently demonstrated to promote efferocytosis and suppress innate immune response [93]. Moreover, cholesterol crystals in the necrotic core, apart from having a direct effect on breaching the fibrous cap, may trigger an inflammatory cascade via activation of the NLRP3 inflammasome that further promotes the instability of atherosclerotic plaque. miR-223 has been shown to be a negative regulator of NLRP3 inflammasome and IL-1β production, thereby preventing the associated inflammatory response [94].
Other advanced lesion characteristics
Within more advanced plaques, EC-driven angiogenesis eventually leads to neovessels invading the intima, a process that is closely related to plaque growth, destabilization, and rupture. miRNAs can function either as pro-angiogenic or antiangiogenic factors [95] and may, therefore, alter lesion characteristics. However, their role in the context of atherosclerosis has not been well characterized. Moreover, vascular regions with lesions related to plaque rupture exhibit an expansive remodeling process. Several studies have demonstrated the functional role of miRNAs in vascular remodeling [96], reflecting the miRNA modulation of the function of ECs, SMCs, and leukocytes, which are the main cellular players orchestrating remodeling. Another common feature of plaque progression is the focal calcification in atherosclerotic lesions. In this context, several studies implicate miRNAs in various aspects of SMC mineralization as novel links in the mechanisms of vascular calcification. For instance, miR-125b is involved in vascular calcification in vitro and in vivo, at least partially, by targeting the osteoblast transcription factor SP7 [97].
Plaque erosion
Superficial erosion of the plaques accounts for approximately 25% of cases of acute coronary syndromes. Although no distinct morphological features have been identified for the erosion-prone plaques, such plaques characteristically exhibit de-endothelialization and accumulation of proteoglycan, including versican. Myocardin has been shown to simultaneously suppress versican gene expression and promote SMC differentiation by inducing miR-143 transcription [98]. In the same context, miR-712 activates a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS4) via decreased TIMP-3 expression leading to versican fragmentation [23]. Moreover, endothelial apoptosis, likely contributing to EC loss, may expose surface extracellular matrix, which could potentially stimulate thrombus formation through platelet adherence and aggregation. Interestingly, platelets can remotely modulate the apoptosis of ECs through release of miR-223-containing microvesicles that are taken up by ECs and induce the formation of advanced glycation end products [99].
miRNA-directed diagnostics and therapeutics in atherosclerosis
Innovative and reliable biomarkers for atherosclerosis and plaque stability are desperately needed. miRNAs are currently explored as biomarkers in a wide range of cardiovascular conditions including atherosclerotic disease. The origin of extracellular circulating miRNAs is diverse and may be associated with active secretion via microvesicles or cell death. Circulating miRNAs have many properties that make them promising new biomarker candidates: they are remarkably stable and detectable in easily accessible extracellular fluids, such as blood and urine, by specific microarray or other quantitative reverse-transcription polymerase chain reaction techniques. Moreover, changes in their expression are often tissue or disease specific.
Patients with coronary artery disease display decreased plasma levels of miR-17, miR-92a, miR-126, and miR-181b, which are expressed in ECs, miR-145, which is expressed in SMCs, and miR-155, which is expressed in both monocytes/macrophages and activated T cells [6, 100]. In the context of plaque rupture, a distinct miRNA expression signature has been identified in the plasma of patients with acute coronary syndrome [101]. It is speculated that combining multiple miRNAs into a miRNA profile may provide greater accuracy than can be expected from the assessment of a single miRNA. Prospective large-scale studies are needed to determine the true potential of circulating miRNAs as biomarkers for atherosclerotic-related disease states.
Pharmacological targeting of dysregulated miRNAs is a promising concept that has enormous therapeutic potential. The multiplicity of miRNA targets enables miRNAs to bypass mechanisms that render cells or tissues insensitive to certain drugs. For example, cells can develop insensitivity to single drugs through rare mutations in drug targets or desensitization of cell-surface receptors. Such mechanisms are unlikely to diminish sensitivity to miRNA-based therapies, which target several steps in a disease pathway by modulating multiple downstream signaling mediators in parallel. An attractive therapeutic approach may involve the delivery of a cassette of miRNA mimics or inhibitors to facilitate “fine-tuning” of specific stages of atherosclerotic progression. Therapies based on miRNA inhibitors, such as locked nucleic acids, antagomirs, and miRNA sponges, or miRNA mimics are now being developed to repress pathological miRNAs or overexpress protective miRNAs, respectively.
Although most miRNA therapeutics are still in preclinical development, two have reached clinical trials. The first miRNA-targeted therapy, a locked nucleotide acid-based antisense miR-122 inhibitor (miravirsen), was shown to downregulate hepatitis C virus RNA levels in patients with chronic hepatitis C infection [102], while one liposome-based miR-34 mimic (MRX34) has entered a clinical Phase I trial in patients with advanced liver cancer [103]. miRNA replacement therapies and anti-miRNA oligonucleotides may be taken up more efficiently in the liver and kidney, but many additional peripheral tissues, such as vessels and heart, have also been successfully targeted using currently available delivery approaches. However, much additional work is needed to establish whether therapeutic manipulation of miRNA function may indeed represent an efficacious and safe atherosclerosis treatment in humans.
Although it is apparent that several miRNAs have an important regulatory impact on atherosclerotic processes in vivo, it is unclear how widespread is the contribution of individual miRNAs. Furthermore, in most of the animal studies to date, the phenotypic effects of miRNA inhibition have only been studied in the target tissue of interest, which might overlook off-target or even opposing effects of miRNAs in different tissues, stages, or in response to diverse pathophysiological stimuli. Given that only a minority of miRNAs is tissue-specific, delivering of miRNA mimics or inhibitors in a targeted rather than a systemic manner may represent a novel opportunity to prevent the development of atherosclerosis. With the growing number of tools and animals to study the role of miRNAs in complex biologic systems, such as animal models that reproduce plaque instability observed in humans [104], in the future we will be able to have a broader, more detailed picture on the role of these regulatory molecules in specific stages of atherosclerotic lesion formation.
Concluding remarks
Accumulating evidence indicates that numerous miRNAs, which participate in positive as well as negative regulatory loops and may function as hierarchical networks rather than individual regulators, play a pivotal role in atherosclerotic disease processes in cell cultures and animal model experiments (Table 1). The list of miRNAs targeting the various aspects of atherogenesis is rapidly increasing and will likely expand to include miRNAs targeting additional genes. An avenue that remains to be elucidated is the contribution and roles of other emerging noncoding RNAs, including long noncoding RNAs, in the development of atherosclerosis and their interaction with miRNAs [105]. Although many questions remain (Box 2), recent biological insights and experimental progress in understanding the impact of miRNAs on the mechanisms of atherosclerosis and its complications may pave the way for the use of miRNA-based strategies in the management of this disease.
Table 1.
Regulation of the initiation, progression, and thrombotic complications of atherosclerosis by miRNAs in mice
| miRNA | Target genes | Functions | Refs |
|---|---|---|---|
| miR-30c | MTTP | Reduces lipid biosynthesis, lipoprotein secretion, prevents atherosclerosis | [46] |
| miR-33 | ABCA1, ABCG1 | Genetic deficiency of miR-33 increases plasma HDL level and cholesterol efflux, reduces plaque size and lipid content, prevents atherosclerosis | [49] |
| ABCA1, ABCG1 | Inhibition of miR-33 improves HDL functionality, increases cholesterol efflux, reduces plaque size, prevents atherosclerosis | [56] | |
| ABCA1, CPT1α | Inhibition of miR-33 increases circulating triglycerides, reduces hepatic free fatty acids, does not change plasma HDL level and plaque size | [57] | |
| miR-92a | KLF2/4, SOCS5 | Increases endothelial activation, decreases plaque stability, promotes atherosclerosis | [19] |
| miR-126-5p | Dlk1 | Promotes EC proliferation, prevents atherosclerosis | [13] |
| miR-126-3p | RGS16 | Induces CXCL-12 in apoptotic bodies, prevents atherosclerosis | [11] |
| miR-145 | KLF4 | SMC-lentiviral miR-145 delivery increases plaque collagen content, fibrous cap area, and plaque stability, reduces plaque size and necrotic core area, prevents atherosclerosis | [87] |
| miR-155 | Bcl6 | Enhances inflammation, promotes atherosclerosis | [25] |
| SOCS1 | Enhances inflammation, impairs cholesterol efflux, promotes atherosclerosis | [26] | |
| ? | Hematopoietic miR-155 deficiency promotes atherosclerosis, decreases plaque stability | [27] | |
| MAP3K10 | Reduces inflammation, prevents atherosclerosis | [106] | |
| miR-181b | Importin-α3 | Inhibits NF-κB signaling and vascular inflammation in ECs of arterial wall, reduces atherosclerosis | [6] |
| miR-302a | ABCA1 | Inhibition of miR-302a increases plasma HDL level and cholesterol efflux, reduces inflammation and plaque size, prevents atherosclerosis | [61] |
| miR-342-5p | AKT1 | Induces pro-inflammatory mediators (Nos2, IL-6), promotes atherosclerosis | [33] |
| miR-712 | TIMP3 | Activates MMPs and ADAMs, stimulates endothelial inflammation and permeability, promotes atherosclerosis | [23] |
Box 2. Outstanding questions.
What are the optimal pharmacokinetics and pharmacodynamics of miRNA inhibitors or mimics in vivo?
How does therapeutic manipulation in vivo affect miRNA compensation or redundancy?
To what extent is effective miRNA-specific gene targeting dependent upon the route of delivery?
Can we create “designer” miRNA therapeutics for optimal penetration of the vascular wall (e.g. miRNA-coated stents)?
Which miRNAs actively contribute to regression or resolution of atherosclerosis?
Do miRNAs function equally during early versus advanced lesion formation?
Highlights.
miRNAs regulate diverse cell types involved in atherosclerotic disease progression.
miRNAs are implicated in advanced atherosclerotic plaque rupture.
Circulating miRNAs are promising new candidates as biomarkers for atherosclerosis.
Pharmacological targeting of dysregulated miRNAs is a promising therapeutic concept.
Acknowledgments
This work was supported by George D. Behrakis Cardiovascular Research Program and by funding from the NIH (HL115141 and HL117994 to M.W.F. and GM49039 to E.R.E.).
Glossary
- Antagomir
cholesterol-conjugated antisense oligonucleotide used to antagonize endogenous miRNAs. Antagomirs are fully complementary to mature miRNAs
- Anti-miRNA (anti-miR)
a chemically modified, single-stranded antisense oligonucleotide designed to inhibit target-specific miRNAs
- Cholesterol efflux
the process whereby peripheral cells, notably macrophages, get rid of excess cholesterol. The final step of cholesterol efflux from the plasma membrane is mediated by adenosine triphosphate-binding cassette A1 (ABCA1) cholesterol transporter to lipid-poor apolipoprotein A-I, which generates nascent HDL, and by ABCG1 transporter to mature HDL particles
- Interferon-γ
a signature cytokine of type 1 T helper cells that causes a range of effects, such as induction of other pro-inflammatory cytokines, enhanced activation of macrophages and endothelial cells, higher expression of major histocompatibility complex class II, less collagen fiber formation, increased protease and chemokine secretion, and upregulation of adhesion molecule expression
- Locked nucleic acid
a high-affinity RNA analogue, in which the ribose moiety is locked in an extra bridge connecting the 2′-O and 4′-C
- miRNA mimic
a small, chemically modified double-stranded RNA that mimics the function of an endogenous miRNA
- miRNA replacement therapy
a therapeutic approach to treat human disease, in which the function of a lost or down-regulated miRNA is restored by introduction of a synthetic miRNA to the diseased tissue. The synthetic miRNA mimic counteracts disease-dependent processes and induces a therapeutic response
- miRNA sponge
a transcript expressed from strong promoters, containing multiple, tandem binding sites to a miRNA of interest, thereby acting as competitive inhibitors of miRNA function
- Shear stress
the frictional drag of blood flowing tangentially across the endothelial surface of the arterial wall. Atherosclerosis develops preferentially at sites of branching, curvatures, and bifurcations in large arteries, where flow conditions are disturbed with prevalence of pro-atherogenic low or oscillatory shear stress. Regions of moderate to high shear stress, where the flow remains unidirectional and axially aligned, are relatively protected from atherosclerosis formation
- Toll-like receptors (TLRs)
a family of pattern-recognition receptors in mammals that can discriminate between chemically diverse classes of microbial products, including bacterial cell-wall components. Binding of the TLRs to their respective ligands initiates the production of a variety of cytokines, which, in turn, shape and enhance the inflammatory and adaptive immune responses
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suarez Y, et al. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010;184:21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Sun HX, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–1414. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 5.Sun X, et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. J Clin Invest. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X, et al. Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2014;114:32–40. doi: 10.1161/CIRCRESAHA.113.302089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng HS, et al. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5:949–966. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao YC, et al. Let-7g improves multiple endothelial functions through targeting transforming growth factor-beta and SIRT-1 signaling. J Am Coll Cardiol. 2014;63:1685–1694. doi: 10.1016/j.jacc.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 9.Harris TA, et al. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicoli S, et al. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zernecke A, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 12.Poissonnier L, et al. miR126-5p repression of ALCAM and SetD5 in endothelial cells regulates leucocyte adhesion and transmigration. Cardiovasc Res. 2014;102:436–447. doi: 10.1093/cvr/cvu040. [DOI] [PubMed] [Google Scholar]
- 13.Schober A, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris TA, et al. Ets-1 and Ets-2 regulate the expression of microRNA-126 in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1990–1997. doi: 10.1161/ATVBAHA.110.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Y, et al. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt MM, et al. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014;129:66–76. doi: 10.1161/CIRCULATIONAHA.113.004149. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, et al. Flow-dependent regulation of Kruppel-like factor 2 is mediated by microRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loyer X, et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014;114:434–443. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- 20.Ni CW, et al. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762–H1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber M, et al. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son DJ, et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menghini R, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 25.Nazari-Jahantigh M, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du F, et al. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34:759–767. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donners MM, et al. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS One. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taganov KD, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, et al. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106:15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheedy FJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 31.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhuri AA, et al. MicroRNA-125b potentiates macrophage activation. J Immunol. 2011;187:5062–5068. doi: 10.4049/jimmunol.1102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Y, et al. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127:1609–1619. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 34.Chen T, et al. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res. 2009;83:131–139. doi: 10.1093/cvr/cvp121. [DOI] [PubMed] [Google Scholar]
- 35.Yang K, et al. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011;585:854–860. doi: 10.1016/j.febslet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Chen T, et al. MicroRNA-155 regulates lipid uptake, adhesion/chemokine marker secretion and SCG2 expression in oxLDL-stimulated dendritic cells/macrophages. Int J Cardiol. 2011;147:446–447. doi: 10.1016/j.ijcard.2010.10.133. [DOI] [PubMed] [Google Scholar]
- 37.Tian FJ, et al. Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc Res. 2014;103:100–110. doi: 10.1093/cvr/cvu070. [DOI] [PubMed] [Google Scholar]
- 38.Ponomarev ED, et al. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang G, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee S, et al. miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem. 2013;288:35428–35436. doi: 10.1074/jbc.M112.426866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arranz A, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109:9517–9522. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C, et al. microRNA-181a represses ox-LDL-stimulated inflammatory response in dendritic cell by targeting c-Fos. J Lipid Res. 2012;53:2355–2363. doi: 10.1194/jlr.M028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, et al. miR-146a controls the resolution of T cell responses in mice. J Exp Med. 2012;209:1655–1670. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esau C, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Vickers KC, et al. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology. 2013;57:533–542. doi: 10.1002/hep.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soh J, et al. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat Med. 2013;19:892–900. doi: 10.1038/nm.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rayner KJ, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Najafi-Shoushtari SH, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horie T, et al. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE−/− mice. J Am Heart Assoc. 2012;1:e003376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X, Feinberg MW. MicroRNA-Management of Lipoprotein Homeostasis. Circ Res. 2014;115:2–6. doi: 10.1161/CIRCRESAHA.114.304228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rayner KJ, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rayner KJ, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rottiers V, et al. Pharmacological inhibition of a microRNA family in nonhuman primates by a seed-targeting 8-mer antimiR. Sci Transl Med. 2013;5:212ra162. doi: 10.1126/scitranslmed.3006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davalos A, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Distel E, et al. miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice. Circ Res. 2014;115:759–769. doi: 10.1161/CIRCRESAHA.115.304164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rotllan N, et al. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr−/− mice--brief report. Arterioscler Thromb Vasc Biol. 2013;33:1973–1977. doi: 10.1161/ATVBAHA.113.301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marquart TJ, et al. Anti-miR-33 therapy does not alter the progression of atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33:455–458. doi: 10.1161/ATVBAHA.112.300639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen RM, et al. Control of very low-density lipoprotein secretion by N-ethylmaleimide-sensitive factor and miR-33. Circ Res. 2014;115:10–22. doi: 10.1161/CIRCRESAHA.115.303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horie T, et al. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat Commun. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goedeke L, et al. Long-term therapeutic silencing of miR-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO Mol Med. 2014;6:1133–1141. doi: 10.15252/emmm.201404046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meiler S, et al. MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.304878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111:967–981. doi: 10.1161/CIRCRESAHA.112.266502. [DOI] [PubMed] [Google Scholar]
- 63.Zhang M, et al. MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis. 2014;234:54–64. doi: 10.1016/j.atherosclerosis.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Ramirez CM, et al. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ Res. 2013;112:1592–1601. doi: 10.1161/CIRCRESAHA.112.300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Aguiar Vallim TQ, et al. MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor farnesoid X receptor. Circ Res. 2013;112:1602–1612. doi: 10.1161/CIRCRESAHA.112.300648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang MH, et al. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145. Arterioscler Thromb Vasc Biol. 2013;33:2724–2732. doi: 10.1161/ATVBAHA.113.302004. [DOI] [PubMed] [Google Scholar]
- 67.Sala F, et al. MiR-143/145 deficiency attenuates the progression of atherosclerosis in Ldlr−/−mice. Thromb Haemost. 2014;112:796–802. doi: 10.1160/TH13-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vickers KC, et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc Natl Acad Sci U S A. 2014;111:14518–14523. doi: 10.1073/pnas.1215767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramirez CM, et al. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler Thromb Vasc Biol. 2011;31:2707–2714. doi: 10.1161/ATVBAHA.111.232066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun D, et al. MiR-26 controls LXR-dependent cholesterol efflux by targeting ABCA1 and ARL7. FEBS Lett. 2012;586:1472–1479. doi: 10.1016/j.febslet.2012.03.068. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, et al. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler Thromb Vasc Biol. 2014;34:1860–1870. doi: 10.1161/ATVBAHA.113.302879. [DOI] [PubMed] [Google Scholar]
- 72.Wang L, et al. MicroRNAs 185, 96, and 223 repress selective high-density lipoprotein cholesterol uptake through posttranscriptional inhibition. Mol Cell Biol. 2013;33:1956–1964. doi: 10.1128/MCB.01580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cordes KR, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boettger T, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talasila A, et al. Myocardin regulates vascular response to injury through miR-24/-29a and platelet-derived growth factor receptor-beta. Arterioscler Thromb Vasc Biol. 2013;33:2355–2365. doi: 10.1161/ATVBAHA.112.301000. [DOI] [PubMed] [Google Scholar]
- 76.Wang YS, et al. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc Res. 2012;95:517–526. doi: 10.1093/cvr/cvs223. [DOI] [PubMed] [Google Scholar]
- 77.Li P, et al. MicroRNA-638 is highly expressed in human vascular smooth muscle cells and inhibits PDGF-BB-induced cell proliferation and migration through targeting orphan nuclear receptor NOR1. Cardiovasc Res. 2013;99:185–193. doi: 10.1093/cvr/cvt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li P, et al. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ Res. 2013;113:1117–1127. doi: 10.1161/CIRCRESAHA.113.301306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torella D, et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 80.Merlet E, et al. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res. 2013;98:458–468. doi: 10.1093/cvr/cvt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis BN, et al. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leeper NJ, et al. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011;226:1035–1043. doi: 10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun SG, et al. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen KC, et al. Negative feedback regulation between microRNA let-7g and the oxLDL receptor LOX-1. J Cell Sci. 2011;124:4115–4124. doi: 10.1242/jcs.092767. [DOI] [PubMed] [Google Scholar]
- 86.Lin Y, et al. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem. 2009;284:7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lovren F, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126:S81–S90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 88.Ma F, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 89.Di Gregoli K, et al. MicroRNA-24 regulates macrophage behavior and retards atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1990–2000. doi: 10.1161/ATVBAHA.114.304088. [DOI] [PubMed] [Google Scholar]
- 90.Boon RA, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 91.Ghorpade DS, et al. MicroRNA-155 is required for Mycobacterium bovis BCG-mediated apoptosis of macrophages. Mol Cell Biol. 2012;32:2239–2253. doi: 10.1128/MCB.06597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maurel M, Chevet E. Endoplasmic reticulum stress signaling: the microRNA connection. Am J Physiol Cell Physiol. 2013;304:C1117–C1126. doi: 10.1152/ajpcell.00061.2013. [DOI] [PubMed] [Google Scholar]
- 93.Das A, et al. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192:1120–1129. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haneklaus M, et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 95.Chang SH, Hla T. Gene regulation by RNA binding proteins and microRNAs in angiogenesis. Trends Mol Med. 2011;17:650–658. doi: 10.1016/j.molmed.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei Y, et al. Pathogenic arterial remodeling: the good and bad of microRNAs. Am J Physiol Heart Circ Physiol. 2013;304:H1050–H1059. doi: 10.1152/ajpheart.00267.2012. [DOI] [PubMed] [Google Scholar]
- 97.Goettsch C, et al. miR-125b regulates calcification of vascular smooth muscle cells. Am J Pathol. 2011;179:1594–1600. doi: 10.1016/j.ajpath.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X, et al. Repression of versican expression by microRNA-143. J Biol Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan Y, et al. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol. 2014;192:437–446. doi: 10.4049/jimmunol.1301790. [DOI] [PubMed] [Google Scholar]
- 100.Fichtlscherer S, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 101.Ren J, et al. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS One. 2013;8:e80738. doi: 10.1371/journal.pone.0080738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Janssen HL, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 103.Ling H, et al. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen YC, et al. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ Res. 2013;113:252–265. doi: 10.1161/CIRCRESAHA.113.301562. [DOI] [PubMed] [Google Scholar]
- 105.Hu YW, et al. RP5-833A20.1/miR-382-5p/NFIA-dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol. 2015;35:87–101. doi: 10.1161/ATVBAHA.114.304296. [DOI] [PubMed] [Google Scholar]
- 106.Zhu J, et al. Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS One. 2012;7:e46551. doi: 10.1371/journal.pone.0046551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tabet F, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wagner J, et al. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:1392–1400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- 109.Hergenreider E, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 110.Zhou J, et al. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res. 2013;113:40–51. doi: 10.1161/CIRCRESAHA.113.280883. [DOI] [PMC free article] [PubMed] [Google Scholar]