Abstract
BACKGROUND
To evaluate long-term disease control, survival, and functional outcomes after surgical and nonsurgical initial treatment for T4 larynx cancer.
METHODS
Demographics, disease stage, and treatment characteristics were reviewed for 221 sequential patients treated for T4 laryngeal squamous cell cancer at a single institution between 1983-2011. Survival and disease-control outcomes were calculated.
RESULTS
The median follow-up time was 47 months (71 months for patients alive at the time of analysis). The overall 5- and 10-year overall survival (OS) rates were 52% and 29%, and the corresponding disease free survival rates were 57% and 48%. Overall 5- and 10-year locoregional control (LRC) was 78% and 67% and freedom from distant metastasis 76% and 74%. In both univariate and multivariate analyses, node-positive disease at presentation was associated with overall mortality, (P<0.0001). Patients treated by laryngectomy followed by post-laryngectomy radiotherapy (161 patients) achieved better initial LRC than patients treated by a laryngeal preservation (LP) approach (60 patients) throughout the follow-up period (log-rank P<0.007) yet median OS times were equal (64 months) for both groups (95% confidence interval [CI] 47-87 months, and 38-87 months, respectively, P=0.7) LP Patients had a tracheostomy rate of 45% and any-event aspiration rate of 23%. Rates of high-grade dysphagia at last follow-up were worse for LP patients (P<0.01).
CONCLUSIONS
Surgery and postoperative RT can produce substantial long-term cancer control and survival rates for patients with T4 larynx cancer. Caution should be taken in selecting patients for initial nonsurgical treatment because of significant rates of functional impairment despite survival equivalence.
Keywords: Larynx cancer, T4, locally advanced, radiotherapy, survival, laryngectomy, laryngeal preservation
INTRODUCTION
The larynx was one of the first head-and-neck cancer sites to be considered for preservation by nonsurgical means because of the functional morbidity associated with loss of the larynx and the greater potential for surgical salvage relative to tumors at other sites.1 Although the published clinical trial experience for patients with T3 cancers is relatively extensive,1, 2 fewer details are available for patients with T4 cancers, so reliable retrospective data is still relevant. Patients with T4 larynx cancer are often treated with definitive concomitant chemoradiotherapy (CRT) by analogy because of the known responsiveness of T3 cancers to these modalities.3. However, tumors that have transgressed and are no longer confined by the laryngeal cartilage generally are thought to have low surgical salvage after failure of definitive larynx-preserving non-surgical approaches4-6. Preservation of laryngeal function, which requires local control with an intact, sensate airway without tracheotomy and functional oral alimentation without feeding tube, and aspiration, is an important outcome measure that has not been well captured in previous reports of organ preservation7-9. Consequently, we sought to assess these long-term therapeutic and functional outcomes for patients with T4 larynx cancer in a large single-institution retrospective analysis.
MATERIALS AND METHODS
This study was approved by the institutional review board. Sequential cases of locally advanced squamous cell carcinoma of the larynx from June 1983 through August 2011 were identified from The University of Texas, M.D. Anderson Cancer Center registry. Records were assessed for all cases that met the criteria for T4 cancer (as classified according to the 7th [2010] edition of the American Joint Committee on Cancer [AJCC] staging manual). Patient demographics (age at diagnosis, sex, ethnicity), tumor pathologic grade and subsite of origin, Eastern Cooperative Oncology Group (ECOG) performance status, and clinical TNM staging were extracted. Staging was reclassified as necessary (e.g., the AJCC 7th edition considers partial cartilage involvement to be T3 disease whereas the 5th and previous editions considered it T4). Patients with distant metastatic (M1) disease at presentation were excluded. Staging findings from computed tomography imaging (CT) were recorded, as was information on pathologic staging features (margins, lymph nodes, perineural, vascular, lymphatic, or cartilage invasion) for patients who had surgery as primary treatment. Disease recurrence was manually coded as local (in the treated primary site), locoregional (in the treated primary site or treated lymph nodes), and distant metastases (squamous carcinomas outside the treated head and neck). Notably, distant metastases and 2nd primary squamous carcinomas could not be reliably separated, so were grouped for the current analysis. Nonsurgical treatment factors which were coded included chemotherapy regimen(s) and their sequence with radiotherapy (RT) or surgery (neoadjuvant/concurrent/adjuvant), and RT dose, fractionation, technique, beam energy, and delivery interval. Biologically equivalent dose (BED) was calculated using the simple BED equation10, 11 without correction for repopulation. Other information extracted from the records was the need for long-term airway support (tracheostomy during therapy, at 6 and 12 months after therapy, and at last contact) for patients treated with RT with an intact larynx, and the need for gastrostomy (feeding) tube placement during therapy, at 6 and 12 months after therapy, and at last contact for all patients.
Statistical Analysis
Proportions were compared with chi-square test. Response to therapy was assessed in terms of recurrence status, time to recurrence, and site of recurrence (local, locoregional or distant). Overall survival (OS), local control (LC), locoregional control (LRC), freedom from distant disease (FDD), recurrence free survival (RFS; local, distant, or metastatic disease coded as events, censoring for death), cancer event free survival (EFS; recurrence and death coded as events, all others censored), disease specific survival (DSS, coding death from disease as an event and censoring all others), from the date of diagnosis were calculated using the Kaplan-Meier product limit method. Log-rank tests were used to compare univariate survival curves.
Four composite functional/mortality endpoints were calculated for patients dispositioned to larynx preservation (LP): laryngoesophageal dysfunction (LED)-free survival (LEDFS, which codes any death, local relapse, salvage total laryngectomy, tracheotomy and/or feeding tube placement/persistence recorded after 2 years as an event, censoring all others), laryngectomy-free survival (LxFS, which codes any death or salvage total laryngectomy as events, censoring all others), actuarial freedom from laryngectomy (FFL, which codes salvage laryngectomy as an event, censoring all others), and actuarial freedom from laryngoesophageal dysfunction (FFLED, which codes local relapse, salvage total laryngectomy, tracheotomy and/or feeding tube placement/persistence recorded after 2 years as an event, censoring all others, including deaths). Finally, non-cancer cause specific survival (NCCSS, wherein all deaths recorded in patients without active cancer at last follow-up are coded as events, and all others censored) was included as crude estimator of non-cancer mortality events though, obviously, not excluding potential therapy-related death. Univariate and multivariate survival analyses were performed using a Weibull parametric hazards model to investigate the following binary variables as correlates of hazard ratio of all survival endpoints: surgical therapy cohort (larynx preservation vs. post-laryngectomy radiotherapy), ECOG performance status at treatment (0-1 vs. 2-3), age (<65 years at diagnosis vs. >65 years), nodal positivity (vs. node negative), chemotherapy cohort (chemotherapy vs. no chemotherapy), sex (female vs. male), and primary anatomic site (glottic vs. supra- and/or sub-glottic disease. Analysis of the role of chemotherapy regimen and radiotherapy delivery (i.e. 3DCRT vs. IMRT) was precluded in our survey owing to the multicollinearity of these treatments with surgical status, as most were dispositioned to LP approaches, as a function of longitudinal practice patterns. Consequently, we did not specifically include these as covariates in our presented survival models.
Weibull parametric analysis was selected as it is robust with regard to hazard proportionality12, 13. That is, if the proportional hazards assumption is true, the Weibull model will generate hazard ratios comparable to a Cox proportional hazard model, while if the assumption is false (e.g. the survival curves cross during follow-up) the Weibull represents an acceptable parametric alternative12. Data were analyzed with JMP 11.0 statistical software (SAS Institute, Cary, NC), with a specified α=0.05, without correction for multiple comparisons.
RESULTS
Patient and Treatment characteristics
Two hundred thirty patients were identified as presenting with previously untreated T4 larynx cancer. Nine were excluded for having unknown surgical or salvage procedure before presentation, leaving 221 patients for the current analysis. The median age at diagnosis was 57 years (range 31-90 years), and 180 patients (81%) were men. Patient and disease characteristics are summarized in Table 1. The supraglottic larynx was the subsite of origin in 125 patients (57%), and 93 patients (42%) had glottic or transglottic (involving both glottic and supraglottic areas) tumors. One-hundred forty-four patients (65%) had lymph-node-positive disease including 99 (79%) of those with supraglottic tumors and 44 (47%) of those with glottic/transglottic tumors.
Table 1.
Patient and disease characteristics
| Characteristic | No. of Patients (%) |
|---|---|
| Sex | |
| Female | 41 (19) |
| Male | 180 (81) |
| Ethnicity | |
| Asian/Pacific Islander | 1 (0.5) |
| Black/African-American | 44 (20) |
| Hispanic/Latino | 38 (17) |
| Other/unspecified | 2 (1) |
| White | 136 (61.5) |
| Initial Disease Site | |
| Glottic | 43 (19) |
| Subglottic | 3 (1) |
| Supraglottic | 125 (57) |
| Transglottic | 50 (23) |
| Nodal Status | |
| N+ | |
| N1 | 41 (19) |
| N2A | 6 (3) |
| N2B | 42 (19) |
| N2C | 39 (18) |
| N3 | 16 (7) |
| NX | 3 (1) |
| N0 | |
| N0 | 74 (33) |
| Smoking History at Diagnosis | |
| None | 8 (4) |
| Unknown or unspecified | 5 (2) |
| Positive | 208 (94) |
| History of GERD at Presentation | |
| Yes | 50 (23) |
| No | 89 (40) |
| Unknown or unspecified | 82 (37) |
| Dysphagia at Presentation | |
| None | 90 (41) |
| Unknown or unspecified | 20 (9) |
| Positive | 111 (50) |
| Vocal Cord Impairment at Presentation | |
| Impairment | 88 (40) |
| Paralysis | 73 (33) |
| Unknown or unspecified | 28 (13) |
| None | 32 (14) |
| Pathologic Grade* | |
| Moderately differentiated | 124 (56) |
| Poorly differentiated | 57 (26) |
| Unknown/unspecified | 24 (11) |
| Well differentiated | 16 (7) |
| Lymphovascular Space Invasion* | |
| None | 50 (23) |
| Unknown or unspecified | 133 (60) |
| Positive | 38 (17) |
| Perineural Invasion* | |
| None | 80 (36) |
| Unknown or unspecified | 102 (46) |
| Positive | 39 (18) |
| Vascular invasion* | |
| None | 71 (32) |
| Unknown or unspecified | 123 (56) |
| Positive | 27 (12) |
| Extracapsular nodal extension*§ | |
| None | 64 (29) |
| Unknown or unspecified | 100 (45) |
| Positive | 57 (26) |
| Subglottic extension*§ | |
| None | 87 (39) |
| Unknown or unspecified | 38 (17) |
| Positive | 96 (43) |
| Paraglottic space involvement*§ | |
| None | 54 (24) |
| Unknown or unspecified | 102 (46) |
| Positive | 65 (29) |
| Pre-epiglottic space involvement*§ | |
| None | 40 (18) |
| Unknown or unspecified | 84 (38) |
| Positive | 97 (44) |
| Hypopharyngeal involvement*§ | |
| Pyriform sinus | 62 (28) |
| Hypopharynx NOS | 15 (7) |
| None | 101 (46) |
| Post-cricoid involvement | 5 (2) |
| Posterior hypopharynx NOS | 5 (2) |
| Unknown or unspecified | 33 (15) |
| Cartilage invasion*§ | |
| Full thickness or greater erosion | 102 (46) |
| Minor/inner cortex erosion | 43 (19.5) |
| None | 50 (22.5) |
| Unknown or unspecified | 26 (12) |
Information obtained from pathology reports (for patients who had surgery)
Information obtained from medical records (pathology, imaging, or clinical reports)
Abbreviations: GERD, gastroesophageal reflux disease; NOS, not otherwise specified.
One hundred sixty-one patients (73%) were treated by total laryngectomy (TL) followed by post-operative RT (PORT); 30 of these patients (19%) received chemotherapy during the course of treatment. Sixty patients (27%) were treated by laryngeal preservation approach (LP) using definitive RT, and 51 of these patients (85%) also received chemotherapy. RT was delivered in a variety of fractionation schedules; the median BED2Gy for all patients was 72 Gy, with a median BED of 84 Gy for the RT group and 72 Gy for the TL-PORT group. Treatment characteristics are summarized in Table 2.
Table 2.
Treatment characteristics
| Primary Treatment Received | ||
|---|---|---|
| Laryngectomy and Adjuvant Radiotherapy | Radiotherapy | |
| No. of Patients (%) | No. of Patients (%) | |
| Nodal Status | ||
| N0 | 53 (24) | 21 (9.50) |
| N1 | 28 (13) | 13 (6) |
| N2A | 2 (1) | 4 (2) |
| N2B | 31 (14) | 11 (5) |
| N2C | 30 (13.5) | 9 (4) |
| N3 | 15 (7) | 1 (0.5) |
| NX | 2 (1) | 1 (0.5) |
| Chemotherapy | ||
| Induction+Concurrent | 4 (2) | 14 (6) |
| Concurrent | 24 (11) | 26 (12) |
| Induction | 2 (1) | 11 (5) |
| None | 129 (58) | 9 (4) |
| Unknown | 2 (1) | 0 (0) |
| Total Radiation Dose (Gy) | (mean 60.31± s.d. 5.7) | (mean 69.5± s.d. 8.3) |
| No. of Fractions Received | (mean 31.21± s.d. 4.4) | (mean 38± s.d. 12.3) |
| BED | (mean 72 ± s.d. 0.5) | (mean 82.9± s.d. 1.2) |
| Radiotherapy Technique | ||
| 2D/3D Conformal | 119 (54) | 33 (15) |
| IMRT | 42 (19) | 27 (12) |
| Radiation Beam Energy | ||
| 6 MV | 81 (37) | 42 (19) |
| 60Co | 79 (36) | 18 (8) |
| Unknown | 1 (0.5) | 0 (0) |
| Fractionation Schedules | ||
| Altered | ||
| Twice daily | 3 (1) | 9 (4) |
| Concomitant boost | 6 (3) | 9 (4) |
| Conventional | 152 (69) | 42 (19) |
Abbreviations: BED, biologically equivalent dose; IMRT, intensity-modulated radiotherapy
Survival Endpoint Analysis
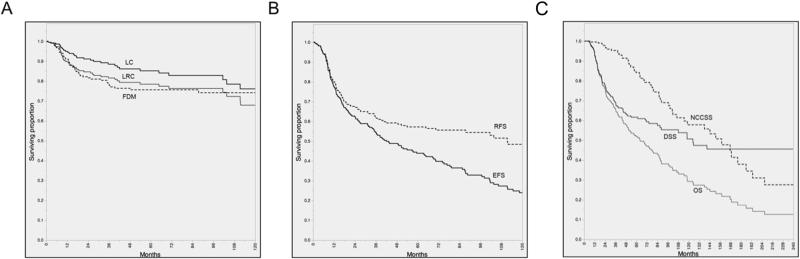
The median calendar follow-up time from initial diagnosis was 47 months (range 6-293 months) and 71 months (range 27-265 months) for patients who were alive at the time of analysis. The actuarial 5- and 10-year OS rates were 52% and 29%, and the corresponding DSS rates were 57% and 48%, respectively. For all patients, 5- and 10-year respective rates were: local control, 85% and 76%; locoregional control, 78% and 67%; freedom from distant metastasis/second primary disease was 76% and 74%. Figure 1 summarizes actuarial endpoints graphically.
Figure 1.
Kaplan-Meier curves depicting; local control (LC), locoregional control (LRC), freedom from distant disease (FDM) (A), recurrence free survival (RFS), cancer event free survival (EFS) (B), and overall survival (OS), disease specific survival (DSS), non-cancer cause specific survival (NCCSS) (C) calculated for all T4 larynx patients (n=221)
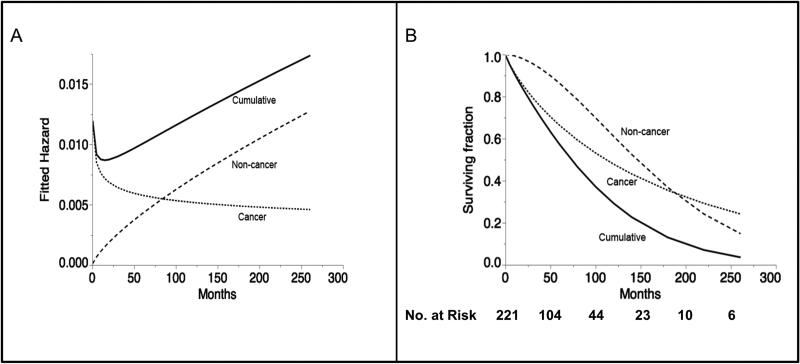
Competing risk assessment is demonstrated graphically with Weibull competing cause survival and hazard plots for all patients shown in Figure 2a and 2b respectively, illustrating the late predominance of non-cancer mortality after the initial post-therapy interval. The hazard ratio of non-cancer events predominates at approximately 7-years from diagnosis (Figure 2a), with non-cancer related death eclipsing disease-related mortality after 15-years post-diagnosis (Figure 1c and 2b).
Figure 2.
Weibull plot of competing risk of death over time. Figure 2a depicts the hazard ratio of death from cancer-specific, non-cancer specific and cumulative death hazard over the course of follow-up. Figure 2b depicts the Weibull fit of the competing causes cumulative survival, and iteratively omitting competing risk based on coded cause of death.
Correlates of Survival
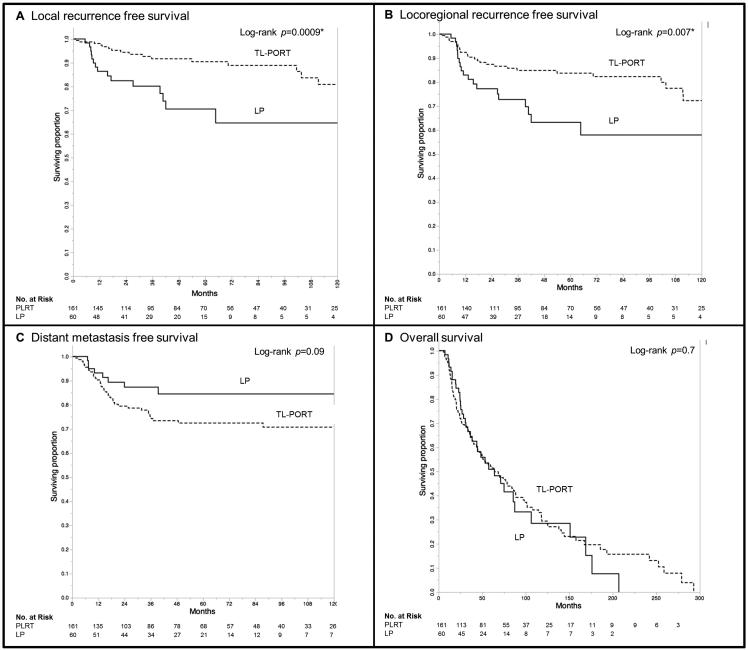
Median OS times were 64 months for both the TL-PORT and LP (95% confidence interval [CI] 47-48 months, and 38-87 months, respectively, P=0.7, Figure 3d). Five-year DSS rates were 60% for the TL-PORT group and 48.5% for the LP group (P=0.1). The TL-PORT group had comparatively better initial LRC (log-rank P<0.007), with 5- and 10-year LRC of 84% and 72%, respectively, for TL-PORT patients, compared to 63% and 58% in the LP cohort (Figure 3b). However, 63% (15) of patients with local and/or regional failure in the LP cohort received subsequent salvage surgery which maintained locoregional control (i.e. no second locoregional relapse) in 60% (9) of patients who underwent surgery. Consequently, the ultimate 5- and 10-year LRC for LP patients was 80% and 73%, respectively, which was not different when compared to TL-PORT group (log-rank P=0.5).
Figure 3.
Kaplan-Meier curves depicting; local recurrence free survival (A), locoregional recurrence free survival (B), distant metastasis free survival (C), and overall survival (D) for patients stratified by type of treatment received (LP=laryngeal preservation, TL-PORT=post-laryngectomy radiotherapy)
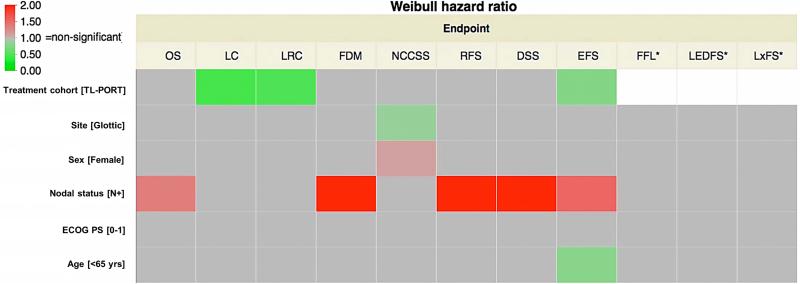
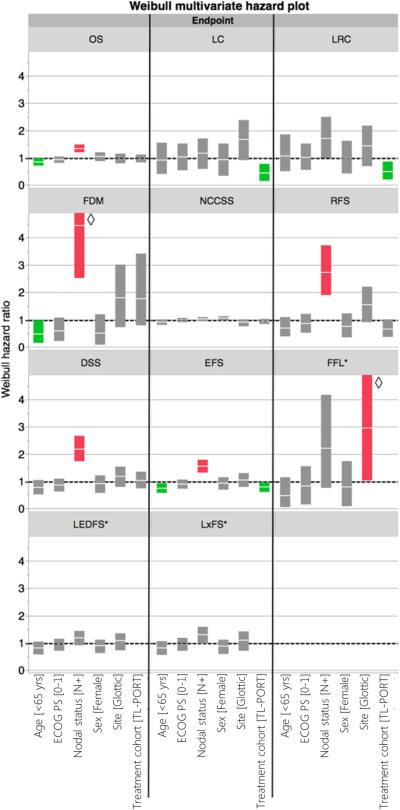
In both univariate (Figure 4) and multivariate analyses (Figure 5), node-positive disease at presentation was associated with overall mortality, (P<0.0001 for both); in multivariate analysis age > 65 was also associated with greater mortality. Figure 3 shows Kaplan-Meier curves of different survival endpoint for both TL-PORT and LP treatment cohorts. Additionally, results of listed treatment, disease and demographic variables on both univariate and multivariate analysis for LC, FDM, EFS, RFS, NCCSS are shown graphically. Broadly, on univariate analysis (Figure 4), nodal status was a significant correlate of event probability for FDM, RFS, EFS, and DSS, in addition to OS, with TL-PORT showing improved LC, LRC, and EFS, and age <65 years showing improved EFS. For multivariate models (Figure 5), nodal status and receipt of surgery remained significant for the same endpoints in univariate model, while age <65 years showed improved OS and FDM in addition to EFS. Regarding the initial disease sub-site, there were no significant risk differences between glottic or supraglottic site of origin for all endpoint except for reduction in the risk of NCCSS. Also in the multivariate analysis, no risk differences were detected in all endpoints. However, in the univariate analysis, Weibull hazard ratio for OS was of border line significance (p=0.06). Post-hoc Kaplan-Meier OS curves for both glottic and supraglottic sub-sites were plotted with log-rank test to compare survival curves. This showed a statistically significant superior median survival of 87 months (95%CI 45-259 months) for glottic patients compared to 61 months (95%CI 44-79 months) for supraglottic patients (log-rank p=0.037, see supplementary figure 1).
Figure 4.
Results of univariate Weibull hazards model. Statistical significance is indicated if the 95%CI hazard ratio does not encroach upon a risk ratio of 1.0; the more towards red color indicates increased risk of endpoint occurrence, while the more towards green color indicates risk reduction. OS, LC, LRC, FDD, RFS, EFS, DSS, and NCCSS proportional hazards were assessed for all patients (n=221). LEDFS, LxFS, and FFL hazard ratios (labeled with a “*”) were calculated only for those patients dispositioned to larynx preservation initially (n=60).
Figure 5.
Results of Weibull multivariate hazard model, showing hazard ratio as solid white stripe, with solid block representing 95%CI of hazard ratio. Statistical significance is indicated if the 95%CI hazard ratio boxplot does not encroach upon a risk ratio of 1.0; red boxplots represent increased probability of endpoint occurrence, while green boxplots indicate risk reduction. OS, LC, LRC, FDD, RFS, EFS, DSS, and NCCSS proportional hazards were assessed for all patients (n=221). LEDFS, LxFS, and FFL hazard ratios (labeled with a “*”) were calculated only for those patients dispositioned to larynx preservation initially (n=60). ◇ Upper limits of these boxplots are truncated for aesthetic considerations.
Functional Outcomes
Long-term functional outcomes are shown in Table 3. At any time during the follow-up period, patients in the LP group had a post-radiotherapy tracheostomy rate of 45% and any-event aspiration rate (recorded as any evidence or indication in follow-up or speech pathology notes) of 23%. Rates of dysphagia (defined as documentation in the record of difficulty or inability to eat a normal diet) at last follow-up were substantially worse for LP patients (P<0.01); however, degree of dysphagia was often not captured. Crude feeding tube rates at last contact were 17% for the LP group compared with 7% for the TL-PORT group (P<0.01). Because patients cannot aspirate after a laryngectomy, the number of aspiration events was, as anticipated, higher among the LP group than among the LPRT group (2-sided Fisher's exact χ2 test P<0.01).
Table 3.
Long-term functional sequelae according to treatment received
| Treatment Received | |||
|---|---|---|---|
| Laryngectomy and Adjuvant Radiotherapy | Radiotherapy | All Patients No. (%) | |
| Dysphagia at Last Contact | |||
| Not reported | 71 | 13 | 84 (38) |
| Unknown or unspecified | 11 | 3 | 14 (6) |
| Yes | 79 | 44 | 123 (56) |
| PEG /DHT Tube Present | |||
| at 6 Months | |||
| No | 129 | 35 | 164 (74) |
| Yes | 16 | 19 | 35 (16) |
| Unspecified | 15 | 6 | 21 (10) |
| at 12 Months | |||
| No | 122 | 38 | 160 (72) |
| Yes | 7 | 8 | 15 (7) |
| Unspecified | 17 | 8 | 25 ( 11) |
| at Last Contact | |||
| No | 136 | 46 | 182 (82) |
| Yes | 11 | 10 | 21 (10) |
| Unspecified | 14 | 4 | 18 ( 8) |
| Aspiration | |||
| No | 125 | 34 | 159 (72) |
| Yes | 12 | 14 | 26 (12) |
| Unspecified | 24 | 12 | 36 (16) |
| Tracheostomy Present | |||
| at baseline | |||
| No | 3 | 35 | 38 (17) |
| Yes | 141 | 24 | 165 (75) |
| Unspecified | 17 | 1 | 18 (8) |
| at 6 Months | |||
| No | 0 | 38 | 38 (17) |
| Yes | 160 | 15 | 175 (79) |
| Unspecified | 0 | 7 | 7 (3) |
| at 12 Months | |||
| No | 0 | 22 | 22 (10) |
| Yes | 146 | 12 | 158 (71) |
| Unspecified | 0 | 20 | 20 (9) |
| at Last Contact | |||
| No | 0 | 25 | 25 (11) |
| Yes | 161 | 25 | 186 (84) |
| Unspecified | 0 | 10 | 10 (5) |
Abbreviations: PEG, percutaneous endoscopic gastrostomy; DHT, dobhoff tube
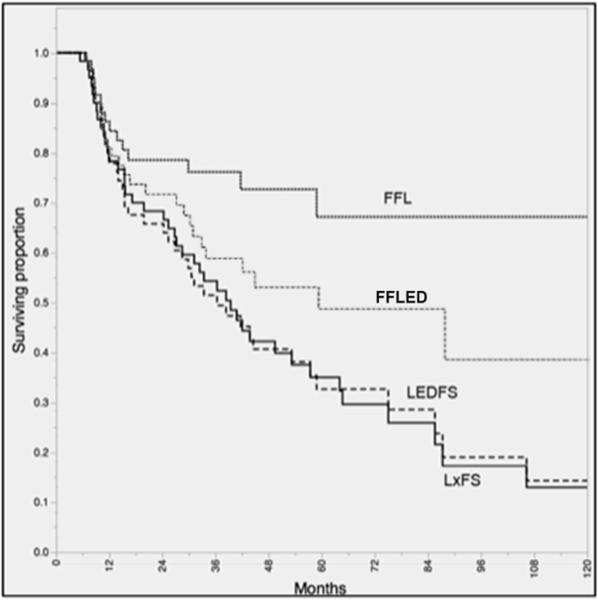
Figure 6 relates actuarial composite and functional endpoints for LP patients. The probability of being alive and without a laryngectomy (LxFS) was 33% at 5-years. Similarly, the observed rate for the LP cohort of being a “fully functional larynx cancer survivor” (i.e. alive, without recurrence, free from salvage total laryngectomy, tracheotomy and/or feeding tube placement/persistence) at 5-years was 32%, and at 10-years was 13%. The vast majority of late events among LP patients, however, were mortality-related; e.g. if deaths were censored, rates of freedom from laryngectomy/tracheotomy/gastrostomy (FFLED) were 50% and 40% at 5- and 10-years, respectively (Figure 6). By contrast, the observed rate of patients alive, without recurrence, and free from feeding tube placement/persistence in the TL-PORT cohort was 50% at 5-years and 29% at 10-years.
Figure 6.
Kaplan-Meier curves depicting laryngoesophageal dysfunction-free survival (LEDFS), laryngectomy-free survival (LxFS), actuarial freedom from laryngoesophageal dysfunction (FFLED), and actuarial freedom from laryngectomy (FFL) for patients dispositioned to larynx preservation (n=60).
For the LP patients, the calculated actuarial probability of freedom from salvage laryngectomy (FFL) was 67% at both 5- and 10-years. For survivors, no patient had a recorded laryngectomy or gastrostomy after 5-years of survival. On multivariate analysis, glottic sub-site was associated with a greater hazard of salvage laryngectomy (hazard ratio, 2.95; 95% CI 1.03-5.49)
DISCUSSION
The present series is the largest long-term study, of which we are aware, to include patients exclusively with T4 larynx cancer. We found that the potential for long-term (>10-year) survival among patients with T4 larynx cancer is significant, and that the choice of treatment is important for cancer control and functional outcomes. Patients treated by TL-PORT or LP approaches achieved the same median OS. Initial LRC interval was superior with upfront laryngectomy, compared to patients treated with LP, but ultimately both approaches had a comparable LRC as, remarkably, successful surgical salvage was accomplished in 38% of patients who developed LRF following LP. However, many of LP patients experienced functional impairments, as evidenced by an observed 5- and 10-year LEDFS rate of 32%, and 13%. Thus, even in our heavily screened institutional cohort, only around 1/3 of patients receiving LP strategies for T4 larynx cancer were alive with a functional larynx and gastrostomy feeding tube-free at 5-years after diagnosis.
The use of nonsurgical techniques as primary therapy for advanced larynx cancer has been asserted as associated with reductions in survival. Hoffman et al.3 reported a 3.4% drop in 5-year survival rates in 1994-1996 versus 1985-1990 in a National Cancer Database outcomes study.3” These results showed that patients with T4N0 cancer treated in 1994-1996 had a 1.3% improvement in 5-year survival compared with those treated in 1985-1990, but the gains were offset by a 2.4% decrease in survival for patients with T4N+ disease. The observation that survival is driven by a mixture of N and T status is consistent with our findings and those of other large T3/T4 series,14 in which nodal positivity represents the primary determinant of mortality.
Clinical staging in the absence of pathologic findings is challenging for laryngeal cancer. In a pathologic-radiologic comparison of cartilage invasion, Li et al.15 found that the value of CT for evaluating full thickness cartilage invasion was poor, with 47% of T4 assessments were downstaged to T3 disease after pathology review. These findings confirm the difficulty of clinical staging16, 17 and underscore the tendency for CT-based overstaging in nonsurgical series. Although we attempted to control for vagaries in staging, our retrospective series is also subject to this inherent weakness for the LP cohort.
Total laryngectomy has long been the cornerstone of therapy for T4 larynx cancer, as noted in a landmark 1975 study by Jesse et al.18 Several retrospective series have confirmed a survival benefit from the addition of post-laryngectomy radiotherapy especially for patients with node-positive disease19, 20.
The VA Larynx study1 investigated larynx preservation with an induction chemotherapy investigational arm. In this landmark study, only 85 of 332 enrolled patients (25%) had reported T4 disease, and of those, 56% required laryngectomy, as opposed to 29% with smaller primary disease21. Within this dataset, glottic primary disease and gross cartilage invasion were also associated with poorer outcomes. As a consequence, RTOG 91-11 excluded patients with full-thickness cartilage involvement,2 resulting in <10% of the series having T4 disease, precluding meaningful subset or failure analysis.4; therefore, for most T4 patients, laryngectomy followed by radiotherapy has remained the standard.
Building on the VA Larynx strategy, investigators have championed the response to induction chemotherapy to select patients with intermediate to advanced, including T4, larynx cancer for LP. For example, the University of Michigan has reported obtained a 58% laryngeal preservation rate, with 3-year OS and DSS of 78% and 80%, respectively22; likewise, the University of Chicago has reported T4 4-year LRC, LxFS, DFS, and OS of 71%, 86%, 67%, and 53%, respectively, with induction23. Nonetheless, despite these intriguing LP datasets, given lack of a large body of prospectively collected data, TL-PORT, with or without chemotherapy, remains the standard of care for patients with T4 larynx cancer24. Table 4 summarizes selected outcome of radiation therapy whether in the postoperative or the definitive setting for treatment of T4 laryngeal cancer reported in the literature.
Table 4.
Selected trials of radiation therapy for treatment of T4 laryngeal cancer
| First Author |
Year | T4 pts |
N+ (%) |
% TL- PORT |
% RT alone |
% chemo | Chemo used | OS % (yrs) |
CSS % (yrs) |
DFS % (yrs) |
DMFS % (yrs) |
LC % (yrs) | LRC % (yrs) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nishimura | 2007 | 8 | 100 | 100 | CDDP/5FU | 71 (5) | |||||||
| Hinerman | 2007 | 22 | 22 | 100 | 67 (5) | 87 (5) | 100 (5) | 82 (5) | 78 (5) | ||||
| Ampil | 2004 | 28 | 32 | 100 | 43 (7) | 30 (7) | |||||||
| Harwood | 1981 | 56 | 0 | 100 | 56 (5) | ||||||||
| Jesse | 1975 | 48 | 37 | 33 | 54 (4) | ||||||||
| Fagan | 1998 | 37 | 19 | 30 | 86 | ||||||||
| Yuen | 1984 | 57 | 16 | 35 | 63 (5) sx alone/94 (5) CMT | 68 sx alone/95 CMT/77 overall | |||||||
| Parsons | 1998 | 43 | 53 | 100 | 4 | CDDP/5FU | 37 (5) | 47 (5) | 52 (5); | ||||
| Mendenhall | 1998 | 30 | 100 | 89 (5) glottis/ 56 (5) supraglottis | |||||||||
| Paisley | 2002 | 12 | 0 | 100 | 39 (5) | 42 (58 after salvage sx) | |||||||
| Sykes | 2000 | 41 | 0 | 100 | 61 (5) | 73 (5) | 72 (5) | ||||||
| Karim | 1987 | 117 | 33 | 100 | 63 glottic xrt alone/70 supraglottic xrt alone 85 glottic xrt+salvage sx/ 84 supraglottic xrt+salvage sx | ||||||||
| Croll | 1989 | 28 | 100 | 75 (5) | |||||||||
| Adelstein | 1998 | 16 | 100 | 100 | 63 (2 - median) | ||||||||
| Guadagnolo | 2005 | 13 | 54 | 100 | 100 | induction - PF, TPF, TPFL, con - cis, doxetaxel | |||||||
| Nguyen-Tan | 2001 | 90 | majority | 17 | CDDP/5FU | 62 (5) overall/70 (5) sx+/− xrt/ 19 (5) xrt alone +/− salvage sx/ 36 (5) xrt+chemo | |||||||
| Knab | 2008 | 32 | 56 | 100 | 59 ind/100 concurrent | Paclitaxel/5FU/hydroxurea, carboplatin | 53 (4) | 67 (4) | 71 (4) | 83 (4) | 71 (4) | ||
| Henry | 1975 | 86 | 37 | 100 | 48 (3); 25(5) | ||||||||
| Kligerman | 1995 | 20 | 35 | 76 | 48 | ||||||||
| Worden | 2009 | 27 | 53 | 100 | 100 ind/75 concurrent | cis/carbo,5FU (induction), cis (concurrent) | 78 (3) | 80 (6) |
Abbreviations: TL-PORT, Post-Laryngectomy Radiation Therapy; RT or xrt, Radiation Therapy; OS, Overall Survival; CSS, Cause Specific Survival; DFS, Disease Free Survival; DMFS, Distant Metastases Free Survival; LC, Local Control; LRC, Loco-regional Control; CDDP, Cisplatin; 5FU, 5-Fluorouracil; sx, Surgery; PF, cisplatin 5FU; TPF, Docetaxel cisplatin 5-fluorouracil; ind, induction.
Despite advances in imaging, radiation planning and delivery, and combining chemotherapy with radiation, non-surgical larynx preservation efforts must be evaluated carefully in the context of functional integrity and disease control. Issues to be considered include freedom from aspiration (rarely evaluated or reported in literature), the need for tracheostomy and feeding tubes or dietary modifications, voice intelligibility, the potential for salvage surgery for failures after RT, and locoregional relapse-free survival as compared with initial laryngectomy. Notably, patients with T4 larynx cancers may present with an impaired airway or its protection, including loss of laryngeal sensation8, 9 or impaired swallowing function. Functional evaluation by trained speech pathologists with pre-therapy modified barium swallow and videostroboscopy is imperative25, 26. If pre-therapy larynx function is deranged (e.g. upper airway obstruction requiring tracheotomy, or insensate aspiration), attempts at LP would be hazardous. Because baseline functions generally worsen following RT7, 27, nonsurgical, RT-based treatment is ill-advised for most patients with significantly impaired function before treatment. Findings on functional outcomes from retrospective series (including this one) are limited by the lack of consistent and uniform data collection and the lack of instrumental swallowing evaluation and patient-reported outcomes with validated metrics. Although we searched our medical records for measures of functional outcome, we recognize that these measures (e.g. degree of dysphagia, and voice quality) may not have been recorded, perhaps because in earlier eras function was considered to be secondary to the goal of disease control.
This dataset spanning 3 decades, the 30-year interval encapsulating the advent of both chemoradiation and IMRT, suggests that long-term survival and local control outcomes are achievable with laryngectomy followed by adjuvant (chemo)radiation. While there was no observed survival difference for patients with non-surgical initial management for T4 disease, locoregional control and functional outcomes suggest caution is warranted in the selection of patients for larynx preservation approaches in this population. At our facility, patients were selected for LP versus TL-PORT using our standard tertiary cancer center institutional model, which includes initial evaluation in a shared clinic setting by a head and neck surgery, medical oncology, radiation oncology, diagnostic radiology, pathology, and speech pathology faculty (including pre-therapy modified barium swallow and videostroboscopic evaluation to ensure intact, protected airway and adequate swallowing at baseline). This is followed by multidisciplinary tumor board discussion of all patients by this panel of head and neck experts. Broadly, our institutional practice is for most patients with T4 larynx cancer to get TL-PORT.
LP is reserved for the minority of highly selected patients who present with smaller volume cancers, and who have intact airway protection and swallowing function, no requirement for tracheotomy or feeding tube, limited cartilage destruction (i.e. enough residual laryngeal cartilage to afford a high likelihood of mechanical stability and/or post-therapy regeneration), and who are candidates for chemotherapy-based LP regimens (e.g. induction and/or concurrent chemoradiation). We also favor induction chemotherapy, and then re-evaluation for local therapy selection based on cancer response and functional reassessment.
CONCLUSIONS
Long term survival is achievable for T4 larynx cancer patients with nodal status being the most predictive correlate of mortality. Total laryngectomy followed by postoperative RT produced longer locoregional relapse-free intervals and better functional outcomes than did initial nonsurgical radiation-based treatment, yet the patients treated with initial laryngectomy had no ultimate locoregional control or overall survival benefit. Total laryngectomy followed by radiation therapy remains the standard treatment for the majority of patients with T4 larynx cancer, and represents the preferred therapy strategy. Nonsurgical therapy should be reserved for carefully selected T4 patients with more limited disease who are expected to have a high probability of achieving locoregional control with preserved airway/airway protection, and adequate, safe swallowing.
Supplementary Material
Supplementary Figure 1. Kaplan-Meier curves depicting overall survival for patients stratified by sub-site of disease origin. Solid lines represent Kaplan-Meier curves while shaded colors represent 95% confidence intervals. * is significant log-rank p-value.
Precis.
Total laryngectomy followed by postoperative radiation therapy can produce long-term cancer control and survival for patients with T4 larynx cancer. Selection of patients for nonsurgical initial treatment should be done carefully because of significant rates of functional impairment and locoregional failure.
Acknowledgements
We thank C. Floyd Holsinger, MD, Nebil Ark, MD, and Cidney Hulett, MD, MPH, for their assistance with data collection, and Christine Wogan for her editorial support.
Funding sources and financial disclosures: This work was supported in part by National Institutes of Health Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. Dr. Radwan received salary support from the Union for International Cancer Control /American Cancer Society International Fellowships for Beginning Investigators (UICC/ACSBI) mechanism. Dr. Fuller received/receives grant support from the National Institutes of Health/National Cancer Institute's Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06) and Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award: an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant: the Center for Radiation Oncology Research at MD Anderson Cancer Center: and the MD Anderson Institutional Research Grant Program. These listed funders/supporters played no role in the study design, collection, analysis, interpretation of data, manuscript writing, or decision to submit the report for publication.
REFERENCES
- 1.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1–13. doi: 10.1097/01.mlg.0000236095.97947.26. [DOI] [PubMed] [Google Scholar]
- 4.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg. 2003;129:44–49. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 5.Gleich LL, Ryzenman J, Gluckman JL, Wilson KM, Barrett WL, Redmond KP. Recurrent advanced (T3 or T4) head and neck squamous cell carcinoma: is salvage possible? Arch Otolaryngol Head Neck Surg. 2004;130:35–38. doi: 10.1001/archotol.130.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Kraus DH, Pfister DG, Harrison LB, et al. Salvage laryngectomy for unsuccessful larynx preservation therapy. Ann Otol Rhinol Laryngol. 1995;104:936–941. doi: 10.1177/000348949510401204. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal J, Dutta D, Palwe V, et al. Prospective subjective evaluation of swallowing function and dietary pattern in head and neck cancers treated with concomitant chemo-radiation. J Cancer Res Ther. 6:15–21. doi: 10.4103/0973-1482.63563. [DOI] [PubMed] [Google Scholar]
- 8.Aviv JE, Liu H, Parides M, Kaplan ST, Close LG. Laryngopharyngeal sensory deficits in patients with laryngopharyngeal reflux and dysphagia. Ann Otol Rhinol Laryngol. 2000;109:1000–1006. doi: 10.1177/000348940010901103. [DOI] [PubMed] [Google Scholar]
- 9.Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112:338–341. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 83:554–568. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Withers HR, Taylor JM, Maciejewski B. Treatment volume and tissue tolerance. Int J Radiat Oncol Biol Phys. 1988;14:751–759. doi: 10.1016/0360-3016(88)90098-3. [DOI] [PubMed] [Google Scholar]
- 12.Kohler HF, Kowalski LP. A critical appraisal of different survival techniques in oral cancer patients. Eur Arch Otorhinolaryngol. 2012;269:295–301. doi: 10.1007/s00405-011-1601-3. [DOI] [PubMed] [Google Scholar]
- 13.Carroll KJ. On the use and utility of the Weibull model in the analysis of survival data. Controlled Clinical Trials. 2003;24:682–701. doi: 10.1016/s0197-2456(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen-Tan PF, Le QT, Quivey JM, et al. Treatment results and prognostic factors of advanced T3--4 laryngeal carcinoma: the University of California, San Francisco (UCSF) and Stanford University Hospital (SUH) experience. Int J Radiat Oncol Biol Phys. 2001;50:1172–1180. doi: 10.1016/s0360-3016(01)01538-3. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Bobinski M, Gandour-Edward R, Farwell DG, Chen AM. Overstaging of cartilage invasion by multidetector CT scan for laryngeal cancer and its potential effect on the use of organ preservation with chemoradiation. Br J Radiol. 2010 doi: 10.1259/bjr/66700901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal DI, Asper JA, Barker JL, Jr., et al. Importance of patient examination to clinical quality assurance in head and neck radiation oncology. Head Neck. 2006;28:967–973. doi: 10.1002/hed.20446. [DOI] [PubMed] [Google Scholar]
- 17.Loevner LA, Sonners AI, Schulman BJ, et al. Reinterpretation of cross-sectional images in patients with head and neck cancer in the setting of a multidisciplinary cancer center. AJNR Am J Neuroradiol. 2002;23:1622–1626. [PMC free article] [PubMed] [Google Scholar]
- 18.Jesse RH. The evaluation of treatment of patients with extensive squamous cancer of the vocal cords. Laryngoscope. 1975;85:1424–1429. doi: 10.1288/00005537-197509000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kao J, Lavaf A, Teng MS, Huang D, Genden EM. Adjuvant radiotherapy and survival for patients with node-positive head and neck cancer: an analysis by primary site and nodal stage. Int J Radiat Oncol Biol Phys. 2008;71:362–370. doi: 10.1016/j.ijrobp.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 20.Groome PA, O'Sullivan B, Irish JC, et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the Surveillance, Epidemiology, and End Results areas of the United States. J Clin Oncol. 2003;21:496–505. doi: 10.1200/JCO.2003.10.106. [DOI] [PubMed] [Google Scholar]
- 21.Forastiere AA. Larynx preservation and survival trends: should there be concern? Head Neck. 32:14–17. doi: 10.1002/hed.21295. [DOI] [PubMed] [Google Scholar]
- 22.Worden FP, Moyer J, Lee JS, et al. Chemoselection as a Strategy for Organ Preservation in Patients with T4 Laryngeal Squamous Cell Carcinoma with Cartilage Invasion. Laryngoscope. 2009;119:1510–1517. doi: 10.1002/lary.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knab BR, Salama JK, Solanki A, et al. Functional organ preservation with definitive chemoradiotherapy for T4 laryngeal squamous cell carcinoma. Annals of Oncology. 2008;19:1650–1654. doi: 10.1093/annonc/mdn173. [DOI] [PubMed] [Google Scholar]
- 24.Groome PA, O'Sullivan B, Irish JC, et al. Glottic cancer in Ontario, Canada and the SEER areas of the United States. Do different management philosophies produce different outcome profiles? J Clin Epidemiol. 2001;54:301–315. doi: 10.1016/s0895-4356(00)00295-x. [DOI] [PubMed] [Google Scholar]
- 25.Meleca RJ, Dworkin JP, Kewson DT, Stachler RJ, Hill SL. Functional outcomes following nonsurgical treatment for advanced-stage laryngeal carcinoma. Laryngoscope. 2003;113:720–728. doi: 10.1097/00005537-200304000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Hutcheson KA, Lewin JS. Functional Assessment and Rehabilitation How to Maximize Outcomes. Otolaryngologic Clinics of North America. 2013;46:657. doi: 10.1016/j.otc.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauloski BR, Rademaker AW, Logemann JA, Colangelo LA. Speech and swallowing in irradiated and nonirradiated postsurgical oral cancer patients. Otolaryngol Head Neck Surg. 1998;118:616–624. doi: 10.1177/019459989811800509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Kaplan-Meier curves depicting overall survival for patients stratified by sub-site of disease origin. Solid lines represent Kaplan-Meier curves while shaded colors represent 95% confidence intervals. * is significant log-rank p-value.