Abstract
Studies indicate that improving sleep decreases reported pain in patients with knee osteoarthritis (OA), but it is unclear if this association extends to experimentally-induced pain responses. A community-based sample of 88 African-American and 52 non-Hispanic white adults (45-76y) with knee OA completed the Insomnia Severity Index and the arousal subscale of the Sleep Hygiene and Practices Scale. Participants underwent quantitative sensory testing including measures of pain sensitivity and facilitation at the knee, and pain inhibition. Outcomes were analyzed with multiple Tobit, hierarchical regression models, with adjustment for relevant covariates. Ethnicity and sex by sleep interactions were also entered into the models. After covariate adjustment, main associations were not observed. However, sex interacted with insomnia severity to predict greater temporal summation of heat and punctate pressure pain among women and lower heat temporal summation among men. Men and women who engaged in frequent arousal-associated sleep behaviors demonstrated higher and lower heat temporal summation, respectively. Non-Hispanic whites with greater insomnia severity displayed lower pressure pain thresholds and pain inhibition. Our findings are the first to demonstrate that disrupted sleep is associated with altered pain processing differentially by sex and ethnicity/race among people with knee OA.
Keywords: quantitative sensory testing, knee osteoarthritis, insomnia, sleep, ethnicity
Introduction
Osteoarthritis (OA) is the most common form of arthritis and the leading cause of disability and work limitations in the United States,10 with the knee being the most frequently affected joint. More than half of patients with OA experience pain during the night,20 and suffer from some form of sleep disruption including poor sleep quality, sleep fragmentation, and frequent shifts between sleep stages.29,31,39,53,61 Importantly, sleep disruption is also associated with significant daytime fatigue,26and reduced quality of life.62 Further, about a third of OA sufferers report both clinically significant pain and insomnia.35 Hence, comorbid insomnia and pain in OA represent a major deterrent to patient well-being.
Interactions between pain and sleep disturbance in pain populations were previously thought to be cyclical in nature.24,25,51,52,58 However, a recent review suggests that sleep disruption may be a more consistent predictor of the incidence and augmentation of pain severity as opposed to pain causing difficulties in sleep.21 A possible explanation for these observations is sleep disturbance may engage multiple pain modulatory circuits within the central nervous system through inflammatory mediators or N-methyl-D-aspartate receptor activation.13,54 Consequently, activation of these mechanisms are associated with reductions in pain thresholds, pain inhibition and enhanced temporal summation of pain (i.e., enhanced pain in response to repeated noxious stimuli) as measured with laboratory-based quantitative sensory testing (QST). However, the nature of the association between sleep disruption and pain modulation is unclear among individuals with knee OA. The first paper in this area reported that among persons with knee OA, those with diagnosed insomnia, relative to those without insomnia, display significantly greater increases in IL-10 evoked by QST procedures suggesting support for the inflammatory mediation hypothesis.42
To better understand the relationship between sleep disruption and pain modulation, additional QST studies are needed. QST is a clinically-relevant method of pain assessment because the responses produced are related to clinical pain reports and treatment outcomes,3,32,F34 and it provides mechanistic information on abnormal pain processing that can be used for more accurate diagnosis and tailored treatment.11,18 QST responses are also associated with moderate to severe knee OA-related symptoms in comparison to patients with mild symptom severity and persons with no knee OA.27 The substantial relationship between QST measures and clinical pain reports among persons with knee OA is likely related to the finding that increases in clinical pain and QST procedures evoke enhanced activity in the same brain regions (i.e., thalamus, cingulate cortex, amygdala).28 It should also be noted that among persons with knee OA, ethnic differences between African Americans and non-Hispanic whites in self-reports of pain intensity disappear after covariates (e.g., body mass index, socioeconomic status) are controlled.1,12 However, ethnic differences on QST pain measures remain significant after these covariates are entered in analyses.12,27 Thus, QST measures are particularly preferable to self-reports of pain in studies of ethnic differences in pain.
Only a few studies have implemented QST to examine whether sleep disruption is associated with pain facilitation in samples of relatively healthy persons and persons with chronic pain.25,49 Schuh-Hofer and colleagues found that one night of sleep deprivation was unrelated to changes in temporal summation in healthy participants,49 whereas Haack and colleagues reported lower temporal summation of heat pain in participants with insomnia compared to participants without insomnia.25 Despite these mixed findings, there is reliable evidence that experimental sleep deprivation and poor sleep efficiency are related to reduced pain inhibition in healthy persons and those with chronic pain.17,30,40,51 Thus, it is possible that sleep disruption is associated with increased pain sensitivity and enhanced pain facilitation in addition to reduced pain inhibition in persons with chronic pain such as knee OA.
Identifying the relationships between sleep and pain in knee OA is important because sleep is a highly modifiable behavior that may potentially alter pain. Evidence from a community-based study of patients with heterogeneous pain conditions revealed that patients with comorbid sleep disturbance engaged in more maladaptive behaviors for pain and sleep management (e.g., catastrophizing and activity avoidance) that, in turn, were associated with greater comorbid disease severity.33 Alterations in maladaptive sleep-related behaviors are the cornerstone of cognitive-behavioral interventions for insomnia. Additionally, a recent outcome study of a cognitive-behavioral intervention for insomnia revealed improvements in sleep and decreases in clinical pain severity among elderly patients with comorbid insomnia and OA.60 However, this study did not address whether specific, maladaptive sleep behaviors are associated with experimental pain responses in persons with knee OA.
The aim of the present study was to determine the relationships of self-reported insomnia severity and maladaptive sleep behaviors on QST measures of pain sensitivity, inhibition, and facilitation among persons with knee OA. We hypothesized that reports of greater insomnia severity and maladaptive sleep behaviors would be associated with lower pain thresholds and inhibition, and greater temporal summation of pain. Furthermore, both clinical pain and QST pain responses are known to differ by ethnicity/race and sex, such that ethnic minorities and women report heightened pain experiences.8,9,12,14-16,19,45 There are also known sex and ethnic/racial differences in reports of sleep disruption 47,66; however, these differences have not been investigated among persons with knee OA. Therefore, perceived sleep disturbance may be related to abnormal pain processing differentially by sex and ethnicity/race. As an exploratory aim to the study, we investigated the interactions of ethnicity/race and sex with the sleep parameters on pain outcomes. Given the exploratory nature of this aim, no directional hypotheses were stated.
Methods
Study Design and Sample
This study was part of a multi-site investigation at the University of Florida and the University of Alabama at Birmingham aimed at ascertaining ethnic/racial differences in knee OA-related pain sensitivity and limitations in African American and non-Hispanic white middle-aged to older adults (Understanding Pain and Limitations in OsteoArthritic Disease; UPLOAD Study). Participants with knee pain were recruited from the community through flyers, radio and print media ads, and word-of-mouth referral. Participants were eligible to participate in the study if they were between 45 and 85 years of age, self-reported race/ethnicity as African American or non-Hispanic white, and had unilateral or bilateral symptomatic knee OA based upon American College of Rheumatology clinical criteria.2 Participants were excluded if they self-identified as belonging to any other ethnic group, if they had concurrent medical or arthritic conditions that could confound testing procedures, or coexisting disease that could preclude successful completion of the protocol. These conditions included systemic rheumatologic disease, a history of surgical knee replacement to both knees, uncontrolled hypertension (>150/95mmHg), decreased peripheral sensitivity to tuning fork vibration, peripheral neuropathy, recent acute myocardial infarction, heart failure, serious psychiatric disorder or active suicidal ideation, diminished cognitive function (as measured by Mini Mental Status Exam (MMSE) with score ≤ 22), drug or alcohol abuse, and daily opioid use. Participants were also excluded if they had a Kellgren-Lawrence score of four for knee OA severity. Nearly all of the participants had Kellgren-Lawrence scores of two while a small number of participants scored a three. All procedures were reviewed and approved by the institutional review boards at the University of Florida and University of Alabama at Birmingham. Participants provided written consent and were compensated for their participation.
Procedure
Participants completed two in-person visits. The first visit was a health assessment session designed to verify eligibility criteria and collect baseline information. This session consisted of a comprehensive medical history, physical examination by a rheumatologist or nurse practitioner, radiographic exam of the affected knee(s), anthropomorphic measures, cognitive status exam, review of current medications, vital signs, and a self-reported sleep assessment. The second visit was a QST session, which occurred within two to four weeks of the first visit. QST was comprised of basal responses to heat and mechanical (punctate and pressure) pain assessments, temporal summation as well as assessment of conditioned pain modulation. The order of heat and mechanical testing was randomized. The cold pain and conditioned pain modulation testing always occurred last. Participants were asked to withhold any opioid (taken as needed or PRN) or benzodiazepine medication for one week prior to the QST testing. Over-the-counter pain medications were permitted.
Sleep Measures
A subsample of participants (n = 140) completed two self-report sleep questionnaires up to seven days prior to the QST session: the Insomnia Severity Index (ISI) and the Sleep Hygiene and Practices Scale (SHPS). The subsample occurred because the questionnaires were introduced to the UPLOAD study in Year 3 of the investigation.
The ISI measures outcomes of perceived sleep difficulties and insomnia severity over the past two weeks.37 The ISI has 7 items each measured on a Likert scale from 0 to 4 (range: 0-28). The validity and reliability of the ISI has been demonstrated,5,38 and it has been used effectively across multiple populations with comorbid disease.58 A clinical cut-off of 10 is sensitive and specific to differentiating normal sleepers from those with clinically significant insomnia among persons with medical conditions.38
The SHPS is a 30-item multifactorial scale designed to measure the practice of sleep habits over the past month likely to negatively impact sleep.64 The scale has a four-factor structure of the following sleep habit subscales: a) behaviors interfering with sleep scheduling and timing; b) arousal-associated behaviors; c) eating/drinking habits; and d) environmental interferences.64 Exploratory and confirmatory factor analyses have supported the validity of these subscales in normal sleepers, insomnia patients, and obstructive sleep apnea patients.63,64 Total scores for each subscale are summed. The arousal-associated behaviors subscale (SHPS-A, range: 6-42) was the only subscale used for analysis because the behaviors (i.e., engaging in sleep-irrelevant activities in bed, pondering about unresolved matters, and worry about not being able to fall asleep) identified in this subscale are more highly related to sleep disturbance for both persons with insomnia and normal sleepers.64 Considering the wide variation in behaviors within this subscale, the Cronbach's α coefficient of this subscale for the study sample was in the moderate range (0.63) which is acceptable for use.50
Quantitative Sensory Testing (QST)
QST was performed using standardized methodology that is common in the field, as previously reported.12,27
Thermal heat testing was conducted to assess pain threshold, pain tolerance, and temporal summation (5 pulses each at 44°C, 46°C, 48°C) with a computer-controlled Medoc Thermal Sensory Analyzer (Ramat Yishai, Israel) at three sites on the most symptomatic knee. Pain intensity ratings (0-100) were collected for the temporal summation procedure. Temporal summation involved administering five brief (700 msec) heat pulses at inter-stimulus intervals of 2.5 seconds, and participants rated the pain intensity experienced after each pulse. Temporal summation was calculated as the difference in pain intensity ratings between the first heat pulse and the maximum rating from any of the four subsequent heat pulses, as in previous studies.23,27
Pressure pain threshold was assessed three times each at two sites on the knee (medial and lateral joint lines). Order of sites was randomized and counterbalanced. Pressure was applied using a digital, handheld algometer (Medoc, Sollentuna Sweden) at a constant rate of 30 kilopascals (kPa)/second. The average amount of pressure required to evoke the first sensation of pain (kPa) was assessed and pressure pain threshold was calculated by averaging the three trials for each site.
Temporal summation of punctate mechanical pain was assessed twice at the knee. For the procedure, a 300g nylon monofilament probe was applied to the skin for one second and then applied over a series of 10 taps. Participants rated the intensity of pain (0-100 scale) of the first tap and following the 10th tap for both trials. To measure temporal summation, pain intensity ratings were averaged for the single and 10th taps and then the difference was calculated (i.e., 10th tap – single tap). The greater the difference score, the greater the temporal summation that occurred.
Cold sensitivity was measured after the thermal and mechanical tests with a modified cold pressor test. Participants were asked to immerse their right hand up to their wrist into a cold-water bath (Thermo Scientific Refrigerated Bath) three times each for one-minute at temperatures set at 16, 12, and 8°C, respectively. Participants were asked to verbalize when the cold sensation ‘first became painful’ (i.e., time of report represents pain threshold) as well as to report separate ratings of pain intensity and unpleasantness when pain tolerance occurred or at the end of the maximum 1-minute immersion period on a scale from 0 (no pain/unpleasantness) to 100 (the most intense/unpleasant pain sensation imaginable).
Conditioned pain modulation (CPM)
We used CPM as a marker of endogenous pain inhibition. CPM was assessed by determining the ability of a conditioning stimulus, immersion of the right hand into cold water, to diminish the experience of pain from a test stimulus (temporal summation of thermal heat) applied to the opposite ventral forearm. The temperatures of the water bath and the heat stimulus were tailored for each participant to achieve a stimulus that would produce moderate pain (i.e. pain intensity rating of 40-60 on the 0-100 scale). First, baseline heat pain ratings were initially assessed on the left ventral forearm. Next, participants immersed their right hand in the cold water bath for a maximum of 60 seconds. Immediately after removing their hand from the cold water bath, the heat stimulus was again applied to the left ventral arm and pain ratings obtained. In order to operationalize CPM, the average heat pain rating following cold water immersion was subtracted from the average pre-immersion heat pain rating, such that higher scores reflected greater pain inhibition.
Other Measures
Depressive symptomatology was measured with the Center for Epidemiologic Studies Depression Scale (CES-D, range: 0-60), a well-validated and reliable measure of current frequency of depressive symptoms.43 Clinical pain was measured with the Western Ontario and McMaster Universities Arthritis Index (WOMAC), a well-validated measure of symptoms of clinical pain, stiffness and physical functioning over the preceding 48 hours among patients with osteoarthritis.6 The WOMAC has demonstrated high construct validity and test-retest reliability.7 In the present study the total score of all items was used as the main outcome measure (range: 0-96). Higher scores indicate greater symptoms of clinical pain, stiffness, and poor physical functioning.
Statistical Analysis
Descriptive statistics of measures of central tendency and dispersion were calculated for all study variables. Ethnicity/race and sex comparisons were conducted with t tests for continuous variables and Chi square tests of independence for categorical variables. Likely confounding variables considered in the association between sleep and pain responses included sociodemographic information such as age, sex, ethnicity/race (African American or non-Hispanic white), and education (≤ high school degree vs. some college or more). Health factors often related to sleep disturbance were also considered including body mass index (BMI), depressive symptomatology, and clinical pain levels.
The distributions for all experimental pain outcomes were not normally distributed with the exception of CPM. Temporal summation scores, in particular, are often subject to difference scores of 0 (i.e., no temporal summation occurred). A temporal summation difference score of zero occurred among 62.5%, 41.4%, 30.2%, and 7.5% of the participants at the heat testing temperatures of 44°C, 46°C, and 48°C, and the punctate mechanical test, respectively. Censoring at the upper limit of mechanical threshold (600.1 kPA) at the lateral and medial knee joint lines occurred among 7.4% and 6.6% of the participants respectively. Censoring at the upper limit for cold pain threshold (60.1 seconds of hand immersion) at 16°C, 12°C, and 8°C occurred among 22.5%, 7.2%, and 1.5% of participants, respectively. Censoring at the upper limit for cold pain unpleasantness (100 on a scale from 0 to 100) at 16°C, 12°C, and 8°C occurred among 5.1%, 24.1%, and 32.6% of participants, respectively. Censoring at the upper limit for cold pain intensity (100 on a scale from 0 to 100) at 16°C, 12°C, and 8°C occurred among 4.4%, 19.9%, and 29.5% of participants, respectively. For these reasons all experimental pain outcomes, with the exception of CPM, were explored with hierarchical Tobit regression models to estimate their relationships with the sleep measures controlling for the covariates above.48,55 Tobit regression models allow for left or right censoring of the dependent variable. The error distributions of the models were examined to determine level of heteroscedasticity. Variables were entered into the model in the following sets:
Model 1 included all sociodemographic and health-related information
Model 2 = Model 1 + SHPS-A score
Model 3 = Model 2 + ISI scores
Model 4a = Model 3 without ethnicity/race + separate interactions between ethnicity/race and SHPS-A and ISI scores
Model 4b = Model 3 without sex + separate interactions between sex and SHPS-A and ISI scores.
SHPS-A scores were entered into the model prior to ISI scores because ISI scores were hypothesized to mediate the association between arousal-associated sleep behaviors and pain responses. The correlation between the two variables was r = 0.48, hence the risk of multicollinearity was low. Prior to the creation of the interaction terms, the sleep parameters were centered. An alpha level of p < 0.05 was considered statistically significant. In all, eighteen models were constructed to address each QST outcome separately as has been previously done.8,12,27 The Tobit regression analyses were conducted using the PROC QLIM statement in SAS 9.4 (SAS Institute, Inc., Cary, North Carolina). Significant main effects or interactions with insomnia severity on any of the outcomes were further explored with rank analysis of covariance to test whether these relationships differentiated between participants with and without clinically significant insomnia severity (cutoff of 10 on the ISI).41
Results
Sample Characteristics
Table 1 presents descriptive characteristics of the total sample (n = 140) and each ethnic group. On average, the sample was middle-aged, had BMI > 30, and had subclinical levels of depression symptomatology (M < 16). Fifty percent of the sample were experiencing clinical levels of insomnia severity (ISI score ≥10). The sample was balanced by sex and represented diverse levels of education. About 60% of the total sample was African-American (n = 88). WOMAC scores ranged from 0 to 87 with an average score of 37.0, indicating moderate OA symptoms.27 In addition, the median duration of knee pain experienced by the participants was 72 months (Interquartile Range: 36 - 180). Compared to non-Hispanic whites, African-Americans were significantly younger, less educated, reported greater levels of clinical pain and disability on the WOMAC, and engaged more frequently in arousal-associated sleep behaviors. Men and women did not differ on any of the descriptors. Missing data were minimal for all variables (<4%).
Table 1.
Descriptive Characteristics of the Total Sample and by Ethnicity and Sexa
| Variable | Total Sample (n=140) | African Americans (n=88) | Non-Hispanic Whites (n=52) | t or X2 | p | Males (n=68) | Females (n=72) | t or X2 | p |
|---|---|---|---|---|---|---|---|---|---|
| Age | 56.2 (7.3) | 55.1 (7.1) | 58.1 (7.3) | 2.3 | .02 | 56.2 (6.9) | 56.2 (7.8) | −.02 | .98 |
| Sex (n,% women) | 72 (51.4) | 48 (54.5) | 24 (46.2) | 0.9 | .34 | -- | -- | -- | -- |
| Race (n,% AA) | 88 (62.9) | -- | -- | -- | -- | 40 (58.8) | 48 (66.7) | 0.9 | .34 |
| Education (n,%) | 25.4 | <.001 | 4.2 | .24 | |||||
| < HS Degree | 14 (10.1) | 12 (13.8) | 2 (3.8) | 7 (10.4) | 7 (9.7) | ||||
| HS Degree | 58 (41.7) | 44 (50.6) | 14 (26.9) | 33 (49.3) | 25 (34.7) | ||||
| 2-4 Yr College | 51 (36.7) | 29 (33.3) | 22 (42.3) | 19 (28.4) | 32 (44.4) | ||||
| > 4 Yr College | 16 (11.5) | 2 (2.3) | 14 (26.9) | 8 (11.9) | 8 (11.9) | ||||
| BMI | 32.1 (7.2) | 32.5 (7.3) | 31.4 (7.1) | −0.9 | .39 | 31.2 (7.4) | 32.9 (7.1) | 1.4 | .17 |
| WOMAC | 37.0 (19.8) | 39.6 (20.9) | 32.7 (17.3) | −2.0 | .046 | 36.6 (20.0) | 37.4 (19.8) | 0.2 | .82 |
| CES-D | 11.0 (8.7) | 11.3 (8.9) | 10.3 (8.5) | −0.7 | .51 | 11.5 (8.4) | 10.5 (9.0) | −0.7 | .52 |
| SHPS-A | 24.1 (5.9) | 25.1 (5.7) | 22.4 (5.8) | −2.7 | .008 | 23.6 (5.6) | 24.5 (5.9) | 0.9 | .35 |
| ISI | 9.8 (6.6) | 10.4 (6.9) | 8.8 (5.8) | −1.4 | .16 | 10.9 (7.0) | 8.8 (5.9) | −1.9 | .056 |
means and standard deviations are presented unless otherwise noted.
AA = African Americans; BMI = Body mass index; CES-D = Center for Epidemiological Studies - Depression scale; ISI = Insomnia Severity Index; SHPS-A = Sleep Hygiene and Practices Scale arousal associated behaviors subscale; WOMAC = Western Ontario and McMaster Universities Arthritis Index
Heat Pain Outcomes
Multiple, hierarchical Tobit regression analyses were conducted to evaluate how well the sleep parameters predicted heat pain sensitivity (Supplementary Table 1) and facilitation (see Table 2). There were no significant relationships between any of the sleep parameters and heat pain threshold and tolerance (Supplementary Table 1). After adjustment for variables in Model 1 (i.e., sociodemographic information, BMI, WOMAC score, and CES-D score), scores on the SHPS-A and ISI were not significantly related to temporal summation of heat pain at any of the temperature levels in Models 2 and 3, respectively (Table 2).
Table 2.
Tobit Hierarchical Regression Analysis of Association of Sleep measures and Temporal Summation of Heat Pain at Affected Kneea
| 44°C |
46°C |
48°C |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | t | Pseudo-R2 | Estimate | t | Pseudo-R2 | Estimate | t | Pseudo-R2 | |
| Model 1 | 0.09 | 0.10 | 0.09 | |||||||
| Age | 0.29 | 0.79 | 0.46 | 1.46 | 0.73* | 2.35 | ||||
| Sex | −3.76 | −1.04 | −4.72 | −1.08 | −7.21‡ | −1.67 | ||||
| Ethnicity/Race | 2.65 | 0.39 | 9.50 | 1.90‡ | 6.97 | 1.40 | ||||
| Education | −2.43 | −1.19 | −1.06 | −0.55 | −0.46 | −0.24 | ||||
| CES-D | 0.84* | 3.10 | 0.74 | 2.74* | 0.42 | 1.49 | ||||
| WOMAC | −0.10 | −0.79 | −0.09 | −0.72 | −0.13 | −1.05 | ||||
| Model 2 | 0.09 | 0.09 | 0.09 | |||||||
| SHPS-A | 0.07 | 0.17 | 0.37 | 0.88 | 0.02 | 0.04 | ||||
| Model 3 | 0.09 | 0.10 | 0.10 | |||||||
| ISI | −0.27 | −0.57 | −0.36 | −0.77 | −0.54 | −1.17 | ||||
| Model 4a | 0.09 | 0.16 | 0.12 | |||||||
| Ethnicity/Race × SHPS-A | 0.34 | 0.40 | −1.51‡ | −1.73 | −1.07 | −1.24 | ||||
| Ethnicity/Race × ISI | −0.59 | −0.65 | −0.28 | −0.30 | −0.15 | −0.17 | ||||
| Model 4b | 0.09 | 0.09 | 0.23 | |||||||
| Sex × SHPS-A | −0.19 | −0.22 | 0.77 | 0.87 | 3.06* | 3.75 | ||||
| Sex × ISI | −0.57 | −0.66 | −2.10* | −2.33 | −3.41* | −4.26 | ||||
p < 0.05
p < 0.1
Variables in Models 2-4 are centered.
CES-D = Center for Epidemiologic Studies – Depression Scale; Eth = Ethnicity/Race; SHPS-A = Sleep Hygiene & Practices Scale, arousal-associated behaviors subscale; WOMAC = Western Ontario and McMasters Universities Arthritis Index
Mechanical Pain Outcomes
A multiple, hierarchical Tobit regression analysis was conducted to evaluate how well the sleep parameters predicted temporal summation of punctate pressure pain at the knee (Table 3). After adjustment for variables in Model 1, scores on the SHPS-A and ISI were not significantly related to temporal summation of punctate pressure pain in Models 2 and 3, respectively.
Table 3.
Tobit Hierarchal Regression Models of Sleep and Mechanical Pain Outcomesa
| Temporal Summation at Knee |
Pain Threshold at Knee Lateral Joint Line |
Pain Threshold at Knee Medial Joint Line |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | t | Pseudo-R2 | Estimate | t | Pseudo-R2 | Estimate | t | Pseudo-R2 | |
| Model 1 | 0.16 | 0.27 | 0.30 | |||||||
| Age | 0.19 | 0.76 | −2.01 | −1.06 | 0.36 | 0.19 | ||||
| Sex | −10.9* | −3.24 | 140.12* | 5.11 | 146.62* | 5.51 | ||||
| Ethnicity/Race | 8.86* | 2.30 | −53.92‡ | −1.71 | −43.58 | −1.43 | ||||
| Education | 2.03 | 1.34 | 15.76 | 1.26 | −8.49 | −0.70 | ||||
| CES-D | 0.32 | 1.50 | −2.85 | −1.67 | −3.30* | −2.01 | ||||
| WOMAC | 0.16 | 1.62 | −0.38 | −0.49 | −1.27‡ | −1.72 | ||||
| BMI | −0.14 | −0.60 | −4.08 | −2.12 | −2.89 | −1.57 | ||||
| Model 2 | 0.16 | 0.28 | 0.32 | |||||||
| SHPS-A | −0.22 | −0.65 | 0.28 | 0.11 | 1.96 | 0.77 | ||||
| Model 3 | 0.18 | 0.29 | 0.32 | |||||||
| ISI | 0.52 | 1.51 | −3.43 | −1.28 | −3.38 | −1.30 | ||||
| Model 4a | 0.18 | 0.35 | 0.39 | |||||||
| Ethnicity × SHPS-A | −0.23 | −0.32 | −4.52 | −0.85 | −5.26 | −1.03 | ||||
| Ethnicity × ISI | 0.17 | 0.25 | 17.13* | 3.33 | 17.73* | 3.63 | ||||
| Model 4b | 0.22 | 0.29 | 0.33 | |||||||
| Sex × SHPS-A | 0.57 | 0.83 | −1.79 | −0.32 | 0.94 | 0.18 | ||||
| Sex × ISI | −1.56* | −2.50 | 4.02 | 0.83 | 0.91 | 0.19 | ||||
p < 0.05
p < 0.1
Variables in Models 2-4 are centered.
BMI = body mass index; CES-D = Center for Epidemiologic Studies - Depression Scale; Eth = Ethnicity/Race; SHPS-A = Sleep Hygiene & Practices Scale, arousal-associated behaviors subscale; WOMAC = Western Ontario and McMasters Universities Arthritis Index
Pressure Pain Thresholds at the Knee
Multiple, hierarchical Tobit regression analyses were conducted to evaluate how well the sleep parameters predicted pressure pain thresholds at the medial and lateral knee joint line (Table 3). After adjustment for variables in Model 1 (i.e., sociodemographic information, BMI, WOMAC score, and CES-D score), scores on the SHPS-A and ISI were not significantly related to pressure pain thresholds at either knee site in Models 2 and 3, respectively.
Cold Sensitivity
Multiple hierarchical regression analyses were conducted to evaluate how well the sleep parameters predicted measures of cold sensitivity. Neither of the sleep parameters were significantly associated with any of the cold sensitivity measures (Supplementary Tables 2-4).
Conditioned Pain Modulation
A hierarchical regression analysis was conducted to evaluate how well the sleep parameters predicted changes in CPM (Table 4). There were no main associations between the sleep parameters and CPM.
Table 4.
Tobit Hierarchical Regression Analysis of Sleep measures and Conditioned Pain Modulationa
| Variable | Estimate | t | R 2 | |
|---|---|---|---|---|
| Model 1 | 0.03 | |||
| Age | −0.13 | −0.75 | ||
| Sex | −1.56 | −0.64 | ||
| Ethnicity/Race | −3.10 | −1.11 | ||
| Education | −0.84 | −0.77 | ||
| CES-D | −0.14 | −0.95 | ||
| WOMAC | 0.08 | 1.19 | ||
| Model 2 | 0.04 | |||
| SHPS-A | 0.23 | 0.96 | ||
| Model 3 | 0.04 | |||
| ISI | −0.07 | −0.03 | ||
| Model 4a | 0.09 | |||
| Ethnicity/Race × SHPS-A | −0.33 | −0.67 | ||
| Ethnicity/Race × ISI | 1.29* | 2.65 | ||
| Model 4b | 0.04 | |||
| Sex × SHPS-A | −0.32 | −0.62 | ||
| Sex × ISI | 0.29 | 0.65 |
p < 0.05
‡ p < 0.1
Variables in Models 2-4 are centered.
CES-D = Center for Epidemiologic Studies – Depression Scale; SHPS-A = Sleep Hygiene & Practices Scale, arousal-associated behaviors subscale; WOMAC = Western Ontario and McMasters Universities Arthritis Index
Exploratory Interactions with Sex and Ethnicity/Race
Heat Pain Outcomes
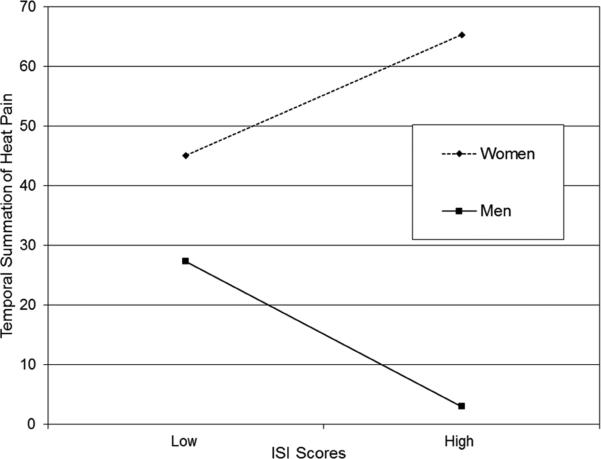
There were no significant interactions between any of the sleep parameters and ethnicity/race for any of the temperature levels. However, there were significant interactions between ISI score and sex on temporal summation at 46°C (p = 0.02) and 48°C (p < 0.001). There also was a significant interaction between SHPS-A and sex at 48°C (p < 0.001). In Figure 1, the simple slopes were plotted, but not the true intercepts on the original temporal summation response scale (1-100) because the sleep parameters and the interaction terms were centered. Figure 1a represents the sex by ISI score interaction for temporal summation at 46°C. Men demonstrated a borderline significant (negative) relationship between ISI score (greater insomnia severity) and lower temporal summation at 46°C (simple slope = −1.16, SE = 0.59, t = −1.97, p = 0.052) but women did not (p = 0.20). A rank analysis of covariance indicated a strong but non-significant trend for the interaction between sex and clinical levels of insomnia severity (i.e., ISI score cutoff of 10), F(1,125) = 2.92, p = 0.09. Figure 1b represents the sex by ISI score for temporal summation at 48°C. Among men, greater insomnia severity was associated with lower temporal summation (simple slope = −1.89, SE = 0.52, t = −3.63, p = 0.0004), whereas for women greater insomnia severity was associated with higher temporal summation (simple slope = 1.51, SE =0.67, t = 2.28, p = 0.025). A rank analysis of covariance indicated a significant interaction between sex and clinical levels of insomnia severity such that men and women with clinically significant insomnia severity differed significantly in temporal summation compared to those within their sex that did not have insomnia, F(1,121) = 3.93, p = 0.05. Figure 1c displays the sex by SHPS-A subscale score interaction for temporal summation at 48°C. Among men, frequent arousal-associated sleep behaviors were associated with greater temporal summation (simple slope= 1.82, SE = 0.62, t = 2.92, p = 0.004), whereas among women, these behaviors were associated with lower temporal summation (simple slope = −1.24 SE = 0.55), t = −2.28, p = 0.025).
Figure 1a.
Gender by ISI Scores Interaction on Temporal Summation of Heat Pain at 46°C
Figure 1b.
Gender by ISI Scores Interaction on Temporal Summation of Heat Pain at 48°C
Figure 1c.
Gender by SHPS-A Scores Interaction on Temporal Summation of Heat Pain at 48°C
To understand which arousal-associated behaviors were dominant in these relationships, correlations by item were conducted for both sexes. For men, watching television/listening to music (r = .26, p = 0.04), or exercising vigorously before bed (r = .36, p = 0.003) were associated with greater temporal summation. For women, frequently pondering about unresolved matters in bed was related to less temporal summation (r = −.28, p = 0.02).
Mechanical Pain Outcomes
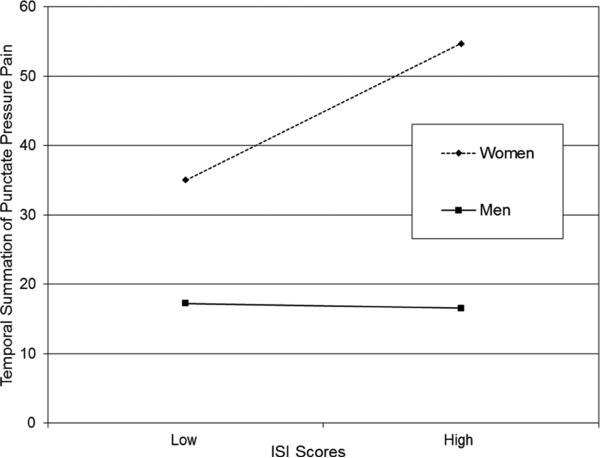
There were no significant interactions between any of the sleep parameters and ethnicity/race. However, there was a significant interaction between ISI score and sex (p = 0.01). Figure 2a displays a significant association between greater insomnia severity and temporal summation of punctate pressure pain for women (simple slope = 1.50, SE = 0.51, t = 2.95, p = 0.004) but not men (p = 0.88). A rank analysis of covariance indicated a strong but non-significant trend for the interaction between sex and clinical levels of insomnia severity, F(1,129) = 3.86, p = 0.052. In other words, temporal summation was differentiated between women with clinically significant insomnia compared to women without insomnia.
Figure 2a.
Gender by ISI Scores Interaction on Temporal Summation of Punctate Pressure Pain
Pressure Pain Thresholds at the Knee
There were no significant interactions between any of the sleep parameters and sex for either knee site. However, there were significant interactions between ISI score and ethnicity/race for pressure pain thresholds at both the medial and lateral joint lines (p's < 0.001). In Figure 2, the simple slopes for these interactions are plotted. In both Figures 2b and 2c, the simple slopes indicate significant associations between greater insomnia severity and lower pressure pain thresholds at both knee sites for non-Hispanic whites (Lateral joint line: simple slope = −15.95, SE = 4.57), t = −3.49, p = 0.007; Medial joint line: simple slope = −16.32, SE = 4.35, t = −3.76, p = 0.0003) but not for African-Americans (Lateral joint line: p = 0.69; Medial joint line: p = 0.61). A rank analysis of covariance indicated significant interactions between ethnicity/race and clinical levels of insomnia severity for pressure pain threshold at the lateral joint line (F(1,127) = 7.86, p = 0.006), and at the medial joint line, F(1,128) = 13.76, p = < 0.001, such that pressure pain thresholds were significantly different between non-Hispanic whites with clinically significant insomnia severity compared to non-Hispanic whites without insomnia.
Figure 2b.
Ethnicity by ISI Scores Interaction on Pressure Pain Threshold at the Patella
Figure 2c.
Ethnicity by ISI Scores Interaction on Pressure Pain Threshold at the Tibial Tuberoscity
Conditioned Pain Modulation
After adjustment for variables in Models 1, 2, and 3, there was a significant interaction between insomnia severity and ethnicity/race on CPM (p = 0.009). Lower pain inhibition was associated with greater insomnia severity in non-Hispanic whites (n = 52, simple slope: −2.31 (0.88), t = −2.62, p = 0.009) but not African Americans (n = 88, simple slope: −1.02 (2.75), t = −0.37, p = 0.71) (Figure 3). A rank analysis of covariance indicated there was no significant interaction between ethnicity/race and clinical levels of insomnia severity, F(1,128) = 1.11, p = 0.29. No significant interactions between the sleep parameters and sex were observed.
Figure 3.
Ethnicity by ISI Scores Interaction on Conditioned Pain Modulation
Discussion
In a sample of middle-aged to older adults with knee OA, self-reported insomnia severity is associated with altered pain processing and central sensitization. The associations varied across multiple stimuli, and were moderated either by sex or ethnicity/race. Among women, greater insomnia severity was related to greater temporal summation of thermal and mechanical pain, whereas for men, greater insomnia severity was related to less summation of thermal pain and was unrelated to mechanical pain summation. Among non-Hispanic whites, greater insomnia severity was related to lower pressure pain thresholds as well as diminished pain inhibition in response to the CPM task, which was not true of their African American counterparts. Contrary to our expectations, frequent arousal-associated sleep behaviors were related to greater temporal summation of thermal pain among men, and lower thermal pain summation among women. None of the sleep parameters was related to cold and thermal pain threshold, tolerance or sensitivity. Overall, the study hypotheses predicting disturbed sleep would be associated with greater pain facilitation, sensitivity, and lower pain inhibition were partially confirmed in certain subgroups and for specific pain stimuli.
Insomnia Severity, Gender, and Temporal Summation of Pain
Greater insomnia severity was associated with greater thermal (at 46°C and 48°C) and mechanical temporal summation for women. The likely reason for the lack of an association between insomnia severity and temporal summation at 44°C was the perceived painfulness at this stimulus level was low. The majority (75%) of the pain ratings for each heat pulse were mild (i.e. pain rating of < 40). This finding of greater insomnia severity associated with greater temporal summation is consistent with evidence that adult women with insomnia report more pain and somatic symptoms than women without insomnia and men with and without insomnia.65 On the other hand, men with greater insomnia severity showed less thermal temporal summation though there was no relationship with mechanical temporal summation. Similarly, Haack and colleagues,25 reported that persons with insomnia showed decreases in temporal summation of heat pain compared to healthy, good sleeping controls. They hypothesized that persons with insomnia may have a constantly activated pain inhibition system. This constant activation may counteract heightened responses to noxious stimuli. In general, activation of the pain inhibition system decreases with age and tends to diminish in persons with knee OA.4,44 Activation of this system may remain more intact for men than women with knee OA. Previous reports do find that men demonstrate less thermal temporal summation than women.19 Thus, men with knee OA and sleep disturbance tend to exhibit lower temporal summation, which is then coupled with a chronically activated pain inhibition system, possibly leading to the present findings. This explanation, however, does not reveal why greater insomnia severity was related to heat temporal summation but not mechanical temporal summation in men. Heat temporal summation is mediated by responses of dorsal horn neurons to c-fiber input, whereas punctate mechanical pain depends primarily on a-fiber nociceptor input.67 The relationship between insomnia severity and temporal summation among men may be pronounced only for c-fiber mediated pain responses, which may be more strongly influenced by endogenous pain inhibition.
Insomnia Severity, Ethnicity/Race, and Pain Sensitivity and Inhibition
Among African Americans insomnia severity was unrelated to pain inhibition and pressure pain thresholds, while for to non-Hispanic whites high insomnia severity predicted poorer pain inhibition and lower pressure pain thresholds. Thus, insomnia may confer increased mechanical sensitization among whites but not African Americans with knee OA. The mechanisms underlying this finding are not clear; however, this suggests that alternative mechanisms may contribute to pressure pain sensitivity in African Americans. For example, we previously reported that African Americans showed significantly lower vitamin D levels compared to non-Hispanic whites, and these lower levels of vitamin D mediated the greater pressure pain sensitivity of African Americans.22 Alternatively, if pain were driving insomnia, this effect might differ for African Americans and non-Hispanic whites. That is, insomnia may be more strongly related to knee pain among non-Hispanic whites, which would also be associated with lower pressure pain thresholds. In contrast, insomnia may be driven by other factors in African Americans, such as environmental conditions (e.g. noise, temperature) or other health-related factors, rather than knee pain. Regarding the pain inhibition results, African Americans tended to show pain facilitation rather than inhibition, which may have created a floor effect preventing insomnia from reducing pain inhibition. However, in non-Hispanic whites, the presence of higher insomnia predicted pain facilitation similar to their African American counterparts.
Maladaptive Sleep Behaviors and Pain
A major aim of the present study was to determine if a set of maladaptive sleep behaviors would significantly predict pain responses. Additionally, we hypothesized that maladaptive sleep behaviors would mediate the association between insomnia severity and responses to painful stimuli. However, this scenario did not occur for any of the models analyzed. It is possible the great diversity of arousal-associated sleep behaviors presented in the SHPS-A may have weakened the true nature of the relationship, accounting for the lack of results in the sample. Further, the SHPS-A has not been previously validated in this population.
In our exploratory analyses, however, a significant interaction emerged between the SHPS-A and sex on heat temporal summation. This relationship indicated frequent maladaptive behaviors in men were related to greater temporal summation, yet less temporal summation among women. This finding is in opposition to the results of the insomnia severity and sex interaction on heat temporal summation. Logic would suggest that both greater insomnia severity and maladaptive sleep behaviors among men would predict less temporal summation. Therefore, these results should be interpreted with caution. To understand this relationship more clearly we analyzed each item of the SHPS-A by sex. Greater temporal summation among men was most associated with vigorous exercise before bed. Vigorous exercise before bed among men with knee OA may lead to poorer sleep, particularly if the exercise activates knee joint nociceptors, such as running or playing sports (weight-bearing on the knee joint) versus swimming that is gentle on the knee joint. Such knee-demanding exercises may lead to increased clinical knee pain along with greater central sensitization of pain through activation of pro-inflammatory cytokines or N-methyl-D-aspartate (NMDA) receptors. One study found that strenuous, aerobic exercise was related to increases in heat temporal summation in persons with fibromyalgia, effects that were opposite to those observed in the no pain controls.57 No data on this relationship among individuals with knee OA are available; however, increased temporal summation after exercise may generalize to other pain conditions including knee OA.
In contrast to the positive relationship between insomnia severity and SHPS-A scores in the present sample, and specifically among women (data not presented), heightened temporal summation was not related to frequent maladaptive sleep behaviors despite its relationship with insomnia severity. Female participants with greater insomnia severity produced higher pain ratings to the first heat pulse than those with less insomnia severity (data not presented). Hence, the relationship between SHPS-A scores and temporal summation was likely attenuated because there was less of a range in pain ratings to report as the temporal summation procedure continued. This likely created the observed inverse relationship.
Strengths and Limitations
Our study has notable strengths that contribute to the current literature. This study is one of only a few that have explored the associations between disrupted sleep, sleep-related behaviors, and responses to QST in people with knee OA. Our sample also had greater ethnic/racial diversity than previous studies evaluating this pain population. Much of the sample was recruited from the community and had comorbidities similar to that of the general population with the exception of severe or acute conditions. Therefore, the results may be generalizable to the broader knee OA population.
There are several limitations within this study that are worth discussing. First, sleep disruption was measured via retrospective self-report with the ISI. The ISI is limited in scope because it does not assess many other sleep factors that might be related to pain modulation such as objective short sleep duration, which, coupled with insomnia has been associated with numerous poor health outcomes.56 To facilitate a greater understanding of the role of disturbed sleep and central pain processing, prospective assessment of sleep with both objective and subjective measures of sleep (i.e., polysomnography, actigraphy, sleep diaries, and questionnaires) is needed in this patient population. Second, chronicity of insomnia severity was not determined. The biological consequences of the stress from short-term vs. chronic insomnia symptoms on pain responses likely differ.36 Third, the SHPS-A subscale was not previously validated in this population. Although the intra-class coefficient in the present sample was similar to the validation sample, the coefficient was in the acceptable range likely reflecting the diversity of arousal-associated sleep behaviors that persons with sleep disturbance tend to engage in. Fourth, we performed a large number of statistical tests without correction, which increases the risk of obtaining significant findings due to chance alone. Last, the present study was cross-sectional and does not present data on the causality of the relationship between sleep and pain.
Conclusions
The severity of sleep disruption appears to be associated with altered pain processing and central sensitization depending on the pain stimulus used as well as the sex and ethnicity/race of participants. These data, taken with compelling evidence indicating poor sleep is predictive of future pain provide further support that sleep interventions for individuals with knee OA-related pain are warranted. Study of potential mechanisms for this relationship such as neuroendocrine and pro-inflammatory mediators as indicated by Quartana and colleagues,42 psychosocial factors, and endogenous systems involving dopamine, opioid, serotonin and noradrenaline would greatly enrich the literature and inform the refinement of interventions that improve sleep and influence associated mechanisms of action. Cognitive behavioral therapies specifically focused on sleep may have the greatest potential to make an impact. These therapies have already been found to initially improve sleep in patients with comorbid insomnia and OA, and those initial improvements predicted long-term reductions in clinical pain severity, sleep disruption, and fatigue.59 These types of interventions should be systematically evaluated for their effects on responses to QST among knee OA patients with clinical and subclinical insomnia.
Supplementary Material
Perspective.
This article presents the association between insomnia severity, maladaptive sleep behaviors, and experimentally-induced pain responses among people with knee osteoarthritis. Disrupted sleep was associated with altered pain processing by sex and ethnicity/race. Offering sleep interventions may help ameliorate pain, but treatment may need to be tailored by sex and ethnicity/race.
Highlights.
We studied the link between sleep and experimental pain in knee osteoarthritis
Sleep disruption was related to altered pain processing by sex and ethnicity/race
Disrupted sleep was related to greater heat and pressure pain facilitation in women
Compared to African Americans, whites with poor sleep had less pain inhibition
Whites with poor sleep had less pressure pain thresholds than African Americans.
Acknowledgments
Support provided by the National Institutes of Health/National Institute on Aging (grant number R01AG033906) and the University of Florida Clinical and Translational Science Institute (grant number UL1TR000064), R. Fillingim, Primary Investigator and the University of Alabama at Birmingham Clinical and Translational Science Institute (grant number UL1TR000165).
Dr. Petrov received support from the Agency for Healthcare Research and Quality (AHRQ; 5 T32 HS013852-09) and the National Institute on Minority Health and Health Disparities (NIMHD: 3 P60 MD000502-08S1). Dr. Glover received support from the John A. Hartford Foundation (2011-2013) as a Building Academic Geriatric Nursing Capacity Scholar and a Mayday Fund grantee (grant number AAN 11-116). Dr. Sibille received support from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23AR062099). Dr. Emily J. Bartley is supported by NINDS training grant T32NS045551 to the University of Florida Pain Research and Intervention Center of Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures.
COI: None
References
- 1.Allen KD, Helmick CG, Schwartz TA, DeVellis RF, Renner JB, Jordan JM. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: The Johnson County Osteoarthritis Project. Osteoarthritis and Cartilage. 2009;17:1132–1136. doi: 10.1016/j.joca.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S, III, Mankin H, McShane DJ, Medsger T, Jr, Meenan R, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 3.Aranda-Villalobos P, Fernández-de-las-Peñas C, Navvaro-Espigares JL, Hernández-Torres E, Villalobos M. Arendt-Nielsen L, Arroyo-Morales M. Normalization of widespread pressure pain hypersensitivity after total hip replacement in patients with hip osteoarthritis is associated with clinical and functional improvements. Arthritis Rheum. 2013;65:1262–1270. doi: 10.1002/art.37884. [DOI] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy N. Pain assessment in osteoarthritis: experience with the WOMAC osteoarthritis index. Semin Arthritis Rheum. 1989;18:14–17. doi: 10.1016/0049-0172(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 8.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GK, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls (DNIC). J Pain. 2008;9:759–766. doi: 10.1016/j.jpain.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Racial/ethnic differences in the prevalence and impact of doctor-diagnosed arthritis--United States, 2002. MMWR Morb Mortal Wkly Rep. 2005;54:119–123. [PubMed] [Google Scholar]
- 11.Cruz-Almeida Y, Fillingim R. Can Quantitative Sensory Testing Move Us Closer to Mechanism-Based Pain Management? Pain Medicine. 2014;15:61–72. doi: 10.1111/pme.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz-Almeida Y, Sibille KT, Goodin BR, Petrov ME, Bartley EJ, Riley JL, 3rd, King CD, Glover TL, Sotolongo A, Herbert MS, Schmidt JK, Fessler BJ, Staud R, Redden D, Bradley LA, Fillingim RB. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. 2014;66:1800–10. doi: 10.1002/art.38620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DelVentura JL, Terry EL, Bartley EJ, Rhudy JL. Emotional modulation of pain and spinal nociception in persons with severe insomnia symptoms. Ann Behav Med. 2014;47:303–315. doi: 10.1007/s12160-013-9551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Edwards CL, Fillingim RB, Keefe FJ. Race, ethnicity and pain: a review. Pain. 2001;94:133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. European Journal of Pain. 2009;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards RR, Sarlani E, Wesselman U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114:315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 20.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, Campbell CM, Haythornwaite JA, Edwards RR, Smith MT. Discordance between pain and radiographic severity in knee osteoarthritis. Findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65:363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glover TL, Goodin BR, Horgas AL, Kindler LL, King CD, Sibille KT, Peloquin CA, Riley JL, III, Staud R, Bradley LA, Fillingim RB. Vitamin D, race, and experimental pain sensitivity in older adults with knee osteoarthritis. Arthritis Rheum. 2012;64:3926–3935. doi: 10.1002/art.37687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, Sotolongo A, Sibille KT, Cruz-Almeida Y, Staud R, Fessler BJ, Redden DT, Bradley LA, Fillingim RB. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: ethnic differences. Psychosom Med. 2014;76:302–310. doi: 10.1097/PSY.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Mullington JM. Pain sensitivity and modulation in primary insomnia. Euro J Pain. 2012;16:522–533. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawker GA, French MR, Waugh EJ, Gignac MAM, Cheun C, Murray BJ. The multidimensionality of sleep quality and its relationship to fatigue in older adults with painful arthritis. Osteoarthritis Cartilage Cart. 2010;18:1365–1371. doi: 10.1016/j.joca.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.King CD, Sibille K, Goodin BR, Cruz-Almeida Y, Glover TL, Bradley LA, Fillingim RB. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1243–1252. doi: 10.1016/j.joca.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, Boyle Y, El-Deredy W, Jones AKP. Arthritic pain is processed in brain areas concerned with emotion and fear. Arthritis Rheum. 2007;56:1345–1354. doi: 10.1002/art.22460. [DOI] [PubMed] [Google Scholar]
- 29.Lamberg L. Chronic pain linked with poor sleep: Exploration of causes and treatment. JAMA. 1999;281:691–692. [PubMed] [Google Scholar]
- 30.Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, Solomon DH, Karlson EW. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum. 2013;65:59–68. doi: 10.1002/art.37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leigh TJ, Hindmarch I, Bird HA, Wright V. Comparison of sleep in osteoarthritic patients and age and sex matched healthy controls. Ann Rheum Dis. 1988;47:40–42. doi: 10.1136/ard.47.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundblad H, Kreicbergs A, Jansson KÅ. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg. 2008;90-B:166–171. doi: 10.1302/0301-620X.90B2.19640. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald S, Linton SJ, Jansson-Fröjmark M. Avoidant safety-behaviors and catastrophizing: shared cognitive-behavioral processes and consequences in co-morbid pain and sleep disorders. Int J Behav Med. 2008;12:201–210. doi: 10.1080/10705500802222675. [DOI] [PubMed] [Google Scholar]
- 34.Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg. 2007;105:815–821. doi: 10.1213/01.ane.0000278091.29062.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCurry SM, Von Korff M, Vitiello MV, Saunders K, Balderson BH, Moore AL, Rybarczyk BD. Frequency of comorbid insomnia, pain, and depression in older adults with osteoarthritis: predictors of enrollment in a randomized treatment trial. J Psychosom Res. 71:296–2992011. doi: 10.1016/j.jpsychores.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metab Clin Exp. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Morin CM. Insomnia: psychological assessment and management. Guilford Press; New York: 1993. [Google Scholar]
- 38.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311–314. doi: 10.1097/00002508-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Paul-Savoie E, Marchand S, Morin M, Bourgault P, Brissette N, Rattanavong V, Cloutier C, Bissonnette A, Potvin S. Is the deficit in pain inhibition in fibromyalgia influence by sleep impairments? Open Rheum J. 2012;6:296–302. doi: 10.2174/1874312901206010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quade D. Rank analysis of covariance. J Am Stat Assoc. 1967;62:1187–1200. [Google Scholar]
- 42.Quartana PJ, Finan PH, Page GG, Smith MT. Effects of insomnia disorder and knee osteoarthritis on resting and pain-evoked inflammatory markers. Brain Behav, Immun. 2015 doi: 10.1016/j.bbi.2014.12.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:384–401. [Google Scholar]
- 44.Riley JL, 3rd, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010;150:153–160. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley JL, 3rd, Robinson ME Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 46.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–51. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 47.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8:246–259. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 48.Schnedler W. Likelihood estimation for censored random vectors. Econom Rev. 2005;24:195–217. [Google Scholar]
- 49.Schuh-Hofer S, Wodarski R, Pfau DB, Caspani O, Magerl W, Kennedy JD, Treede R. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154:1613–1621. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 50.Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res. 1998;7:301–317. doi: 10.1177/096228029800700306. [DOI] [PubMed] [Google Scholar]
- 51.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 52.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 53.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23:1–13. doi: 10.1023/a:1005444719169. [DOI] [PubMed] [Google Scholar]
- 54.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Reports. 2009;13:447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 55.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26:24–36. [Google Scholar]
- 56.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 58.Viitanen J, Ronni S, Ala-Peijari S, Uoti-Reilama K, Kautiainen H. A comparison of self-estimated symptoms and impact of disease in fibromyalgia and rheumatoid arthritis. J Musculoskelet Pain. 2000;8:21–33. [Google Scholar]
- 59.Vitiello MV, McCurry SM, Shortreed SM, Baker LD Rybarczyk BD, Keefe FJ, Von Korff M. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. 2014;155:1547–1554. doi: 10.1016/j.pain.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–362. [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Amer Geria Soc. 2000;48:1241–1251. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 62.Wolfe F, Michaud K, Li T. Sleep disturbance in patients with rheumatoid arthritis: evaluation by medical outcomes study and visual analog sleep scales. J Rheumatol. 2006;33:1942–1951. [PubMed] [Google Scholar]
- 63.Yang CM, Liao YS, Lin SM, Chou SL, Wang EN. Psychological and behavioral factors in patients with comorbid obstructive sleep apnea and insomnia. J Psychosom Res. 2011;70:355–361. doi: 10.1016/j.jpsychores.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Yang CM, Lin SC, Hsu SC, Chen CP. Maladaptive sleep hygiene practices in good sleepers and patients with insomnia. J Health Psychol. 2010;15:147–155. doi: 10.1177/1359105309346342. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Lam S-P, Li SX, Tang NL, Yu MWM, Li AM, Wing Y-K. Insomnia, sleep quality, pain and somatic symptoms: sex differences and shared genetic components. Pain. 2012;153:666–673. doi: 10.1016/j.pain.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Zhang B, Wing Y. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 67.Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122:2245–2257. doi: 10.1093/brain/122.12.2245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.