Abstract
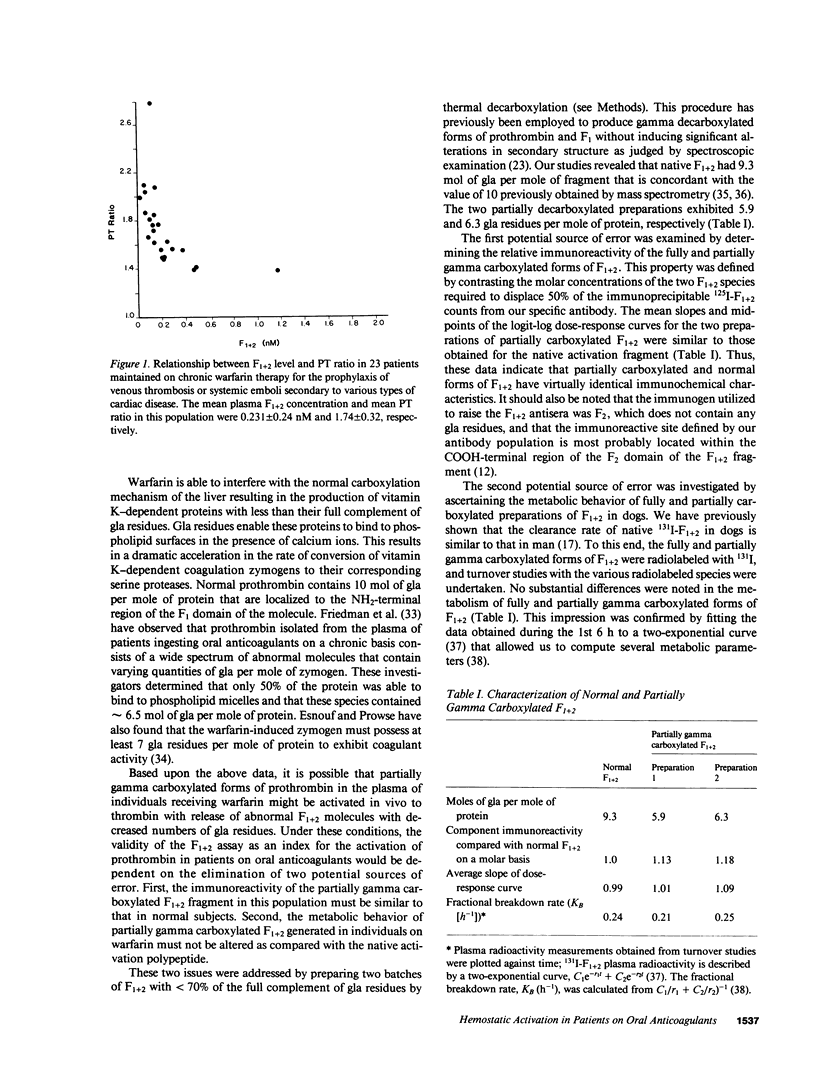
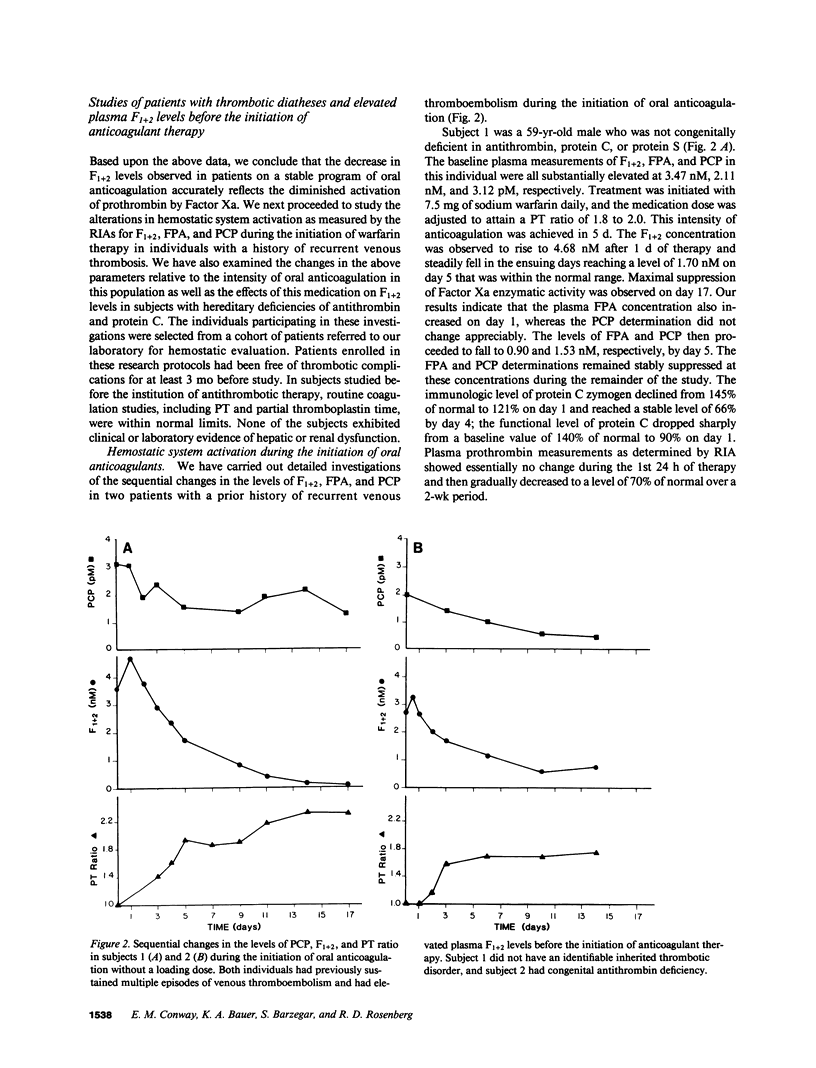
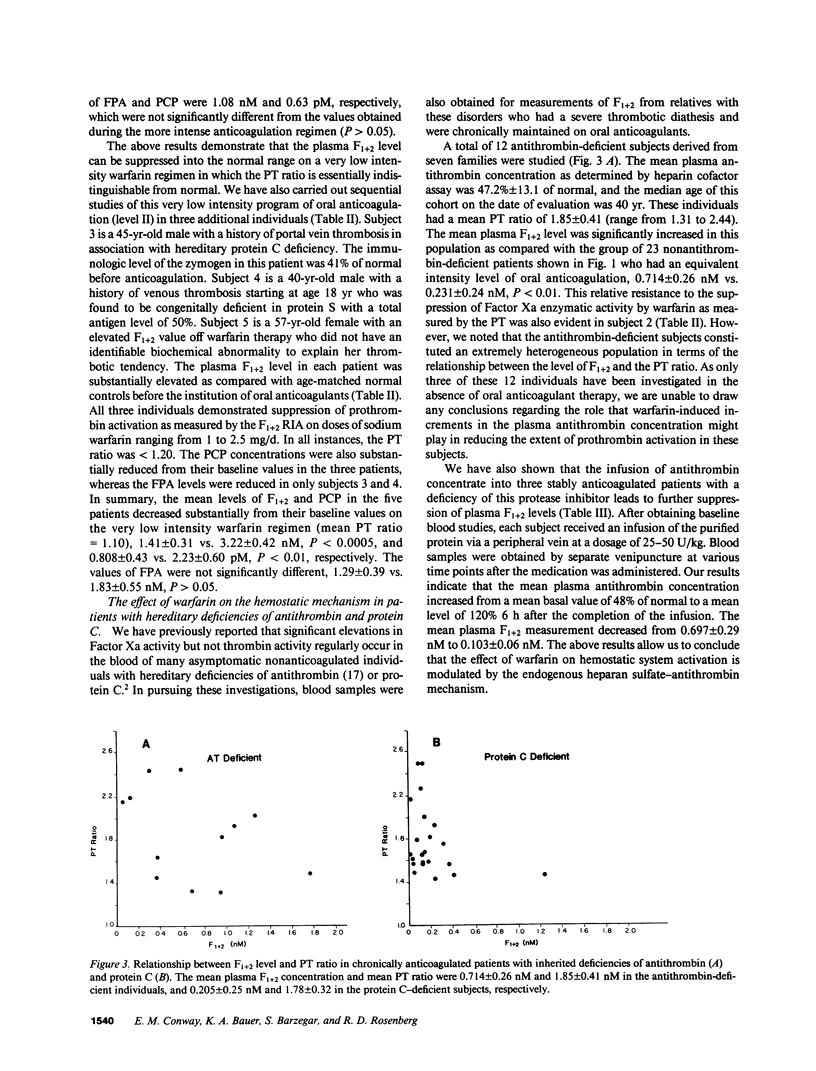
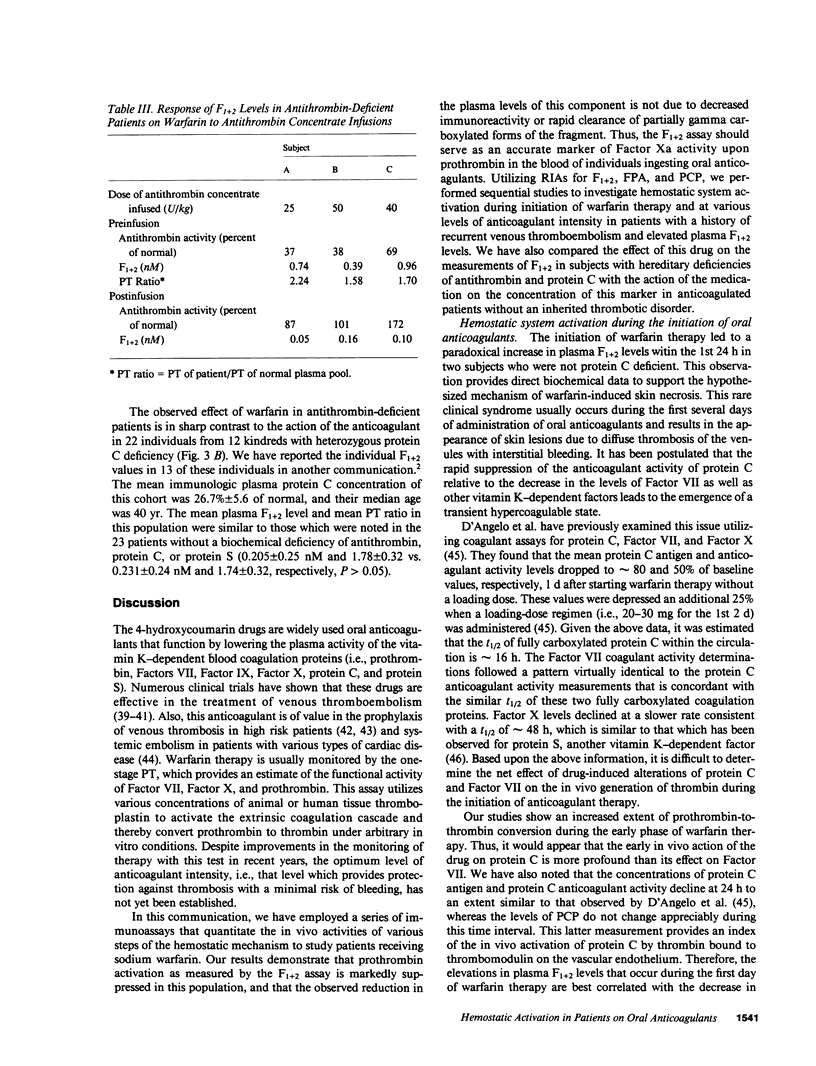
RIAs for hemostatic system activation were employed to study patients who were anticoagulated with warfarin. The mean prothrombin fragment F1 + 2 concentration in stably anticoagulated individuals without an inherited thrombotic diathesis (mean prothrombin time [PT] ratio [PT of patient/PT of normal plasma pool] = 1.74) was 0.231 nM as compared with a mean plasma F1 + 2 level of 1.68 nM for a nonanticoagulated control group (P less than 0.0001). The initiation of oral anticoagulants in two subjects who did not exhibit protein C deficiency led to a paradoxical increase in F1 + 2 levels during the first day of therapy. We have also shown that a relatively low intensity regimen of warfarin (PT ratio less than 1.2) may reduce elevated concentrations of F1 + 2 into the normal range in patients with a history of recurrent thromboembolism. The mean F1 + 2 level in antithrombin-deficient individuals on warfarin was significantly elevated (mean = 0.714 nM) as compared with that in anticoagulated subjects with protein C deficiency (mean = 0.205 nM) or in those without an inherited thrombotic disorder (P less than 0.01) at equivalent levels of intensity of oral anticoagulation. We therefore conclude that the effect of warfarin on hemostatic system activation is modulated by the endogenous heparan sulfate-antithrombin mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer K. A., Ashenhurst J. B., Chediak J., Rosenberg R. D. Antithrombin "Chicago": a functionally abnormal molecule with increased heparin affinity causing familial thrombophilia. Blood. 1983 Dec;62(6):1242–1250. [PubMed] [Google Scholar]
- Bauer K. A., Goodman T. L., Kass B. L., Rosenberg R. D. Elevated factor Xa activity in the blood of asymptomatic patients with congenital antithrombin deficiency. J Clin Invest. 1985 Aug;76(2):826–836. doi: 10.1172/JCI112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer K. A., Kass B. L., Beeler D. L., Rosenberg R. D. Detection of protein C activation in humans. J Clin Invest. 1984 Dec;74(6):2033–2041. doi: 10.1172/JCI111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer K. A., Weiss L. M., Sparrow D., Vokonas P. S., Rosenberg R. D. Aging-associated changes in indices of thrombin generation and protein C activation in humans. Normative Aging Study. J Clin Invest. 1987 Dec;80(6):1527–1534. doi: 10.1172/JCI113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertina R. M., Westhoek-Kuipers M. E., Alderkamp G. H. The inhibitor of prothrombin conversion in plasma of patients on oral anticoagulant treatment. Thromb Haemost. 1981 Jun 30;45(3):237–241. [PubMed] [Google Scholar]
- Bertina R. M., van Wijngaarden A., Reinalda-Poot J., Poort S. R., Bom V. J. Determination of plasma protein S--the protein cofactor of activated protein C. Thromb Haemost. 1985 Apr 22;53(2):268–272. [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Esnouf M. P., Prowse C. V. The gamma-carboxy glutamic acid content of human and bovine prothrombin following warfarin treatment. Biochim Biophys Acta. 1977 Feb 22;490(2):471–476. doi: 10.1016/0005-2795(77)90023-x. [DOI] [PubMed] [Google Scholar]
- Fernlund P., Stenflo J. Beta-hydroxyaspartic acid in vitamin K-dependent proteins. J Biol Chem. 1983 Oct 25;258(20):12509–12512. [PubMed] [Google Scholar]
- Fernlund P., Stenflo J., Roepstorff P., Thomsen J. Vitamin K and the biosynthesis of prothrombin. V. Gamma-carboxyglutamic acids, the vitamin K-dependent structures in prothrombin. J Biol Chem. 1975 Aug 10;250(15):6125–6133. [PubMed] [Google Scholar]
- Francis C. W., Marder V. J., Evarts C. M., Yaukoolbodi S. Two-step warfarin therapy. Prevention of postoperative venous thrombosis without excessive bleeding. JAMA. 1983 Jan 21;249(3):374–378. doi: 10.1001/jama.249.3.374. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V. Quantitative determination of gamma-carboxyglutamic acid in proteins. Anal Biochem. 1977 May 15;80(1):212–223. doi: 10.1016/0003-2697(77)90640-6. [DOI] [PubMed] [Google Scholar]
- Hirsh J. Effectiveness of anticoagulants. Semin Thromb Hemost. 1986 Jan;12(1):21–37. doi: 10.1055/s-2007-1003532. [DOI] [PubMed] [Google Scholar]
- Hull R., Delmore T., Carter C., Hirsh J., Genton E., Gent M., Turpie G., McLaughlin D. Adjusted subcutaneous heparin versus warfarin sodium in the long-term treatment of venous thrombosis. N Engl J Med. 1982 Jan 28;306(4):189–194. doi: 10.1056/NEJM198201283060401. [DOI] [PubMed] [Google Scholar]
- Hull R., Delmore T., Genton E., Hirsh J., Gent M., Sackett D., McLoughlin D., Armstrong P. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979 Oct 18;301(16):855–858. doi: 10.1056/NEJM197910183011602. [DOI] [PubMed] [Google Scholar]
- Hull R., Hirsh J., Jay R., Carter C., England C., Gent M., Turpie A. G., McLoughlin D., Dodd P., Thomas M. Different intensities of oral anticoagulant therapy in the treatment of proximal-vein thrombosis. N Engl J Med. 1982 Dec 30;307(27):1676–1681. doi: 10.1056/NEJM198212303072704. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Canfield W. M., Ericsson L. H., Davie E. W. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977 Dec 27;16(26):5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- Kisiel W. Human plasma protein C: isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Invest. 1979 Sep;64(3):761–769. doi: 10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H. K., Rosenberg J. S., Beeler D. L., Rosenberg R. D. The isolation and characterization of a specific antibody population directed against the prothrombin activation fragments F2 and F1 + 2. J Biol Chem. 1979 Sep 25;254(18):8751–8761. [PubMed] [Google Scholar]
- Marcum J. A., Atha D. H., Fritze L. M., Nawroth P., Stern D., Rosenberg R. D. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986 Jun 5;261(16):7507–7517. [PubMed] [Google Scholar]
- Marcum J. A., Fritze L., Galli S. J., Karp G., Rosenberg R. D. Microvascular heparin-like species with anticoagulant activity. Am J Physiol. 1983 Nov;245(5 Pt 1):H725–H733. doi: 10.1152/ajpheart.1983.245.5.H725. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., McKenney J. B., Rosenberg R. D. Acceleration of thrombin-antithrombin complex formation in rat hindquarters via heparinlike molecules bound to the endothelium. J Clin Invest. 1984 Aug;74(2):341–350. doi: 10.1172/JCI111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossel H. L., Ti M., Kaplan K. L., Spanondis K., Soland T., Butler V. P., Jr The generation of fibrinopeptide A in clinical blood samples: evidence for thrombin activity. J Clin Invest. 1976 Nov;58(5):1136–1144. doi: 10.1172/JCI108566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossel H. L., Yudelman I., Canfield R. E., Butler V. P., Jr, Spanondis K., Wilner G. D., Qureshi G. D. Measurement of fibrinopeptide A in human blood. J Clin Invest. 1974 Jul;54(1):43–53. doi: 10.1172/JCI107749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosslin B. Analysis of disappearance time-curves after single injection of labelled proteins. Ciba Found Symp. 1972;9:113–130. doi: 10.1002/9780470719923.ch7. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. A., Aggeler P. M. Studies on coumarin anticoagulant drugs. Initiation of warfarin therapy without a loading dose. Circulation. 1968 Jul;38(1):169–177. doi: 10.1161/01.cir.38.1.169. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T. Functional properties of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1981 Jun 10;256(11):5532–5535. [PubMed] [Google Scholar]
- Poser J. W., Price P. A. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979 Jan 25;254(2):431–436. [PubMed] [Google Scholar]
- Reeve E. B., Leonard B., Wentland S. H., Damus P. Studies with 131I-labelled antithrombin III in dogs. Thromb Res. 1980 Nov 15;20(4):375–389. doi: 10.1016/0049-3848(80)90277-7. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Lenox R. H., Wray H. L., Ramseth D. Statistical characterization of the random errors in the radioimmunoassay dose--response variable. Clin Chem. 1976 Mar;22(3):350–358. [PubMed] [Google Scholar]
- Rodbard D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem. 1974 Oct;20(10):1255–1270. [PubMed] [Google Scholar]
- SEVITT S., GALLAGHER N. G. Prevention of venous thrombosis and pulmonary embolism in injured patients. A trial of anticoagulant prophylaxis with phenindione in middle-aged and elderly patients with fractured necks of femur. Lancet. 1959 Dec 5;2(7110):981–989. doi: 10.1016/s0140-6736(59)91464-3. [DOI] [PubMed] [Google Scholar]
- Tans G., Govers-Riemslag J. W., van Rijn J. L., Rosing J. Purification and properties of a prothrombin activator from the venom of Notechis scutatus scutatus. J Biol Chem. 1985 Aug 5;260(16):9366–9372. [PubMed] [Google Scholar]
- Teitel J. M., Bauer K. A., Lau H. K., Rosenberg R. D. Studies of the prothrombin activation pathway utilizing radioimmunoassays for the F2/F1 + 2 fragment and thrombin--antithrombin complex. Blood. 1982 May;59(5):1086–1097. [PubMed] [Google Scholar]
- Tuhy P. M., Bloom J. W., Mann K. G. Decarboxylation of bovine prothrombin fragment 1 and prothrombin. Biochemistry. 1979 Dec 25;18(26):5842–5848. doi: 10.1021/bi00593a014. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Vigano D'Angelo S., Comp P. C., Esmon C. T., D'Angelo A. Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. J Clin Invest. 1986 Feb;77(2):416–425. doi: 10.1172/JCI112319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F. J. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J Biol Chem. 1981 Nov 10;256(21):11128–11131. [PubMed] [Google Scholar]


