Abstract
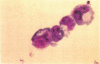
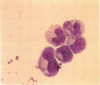
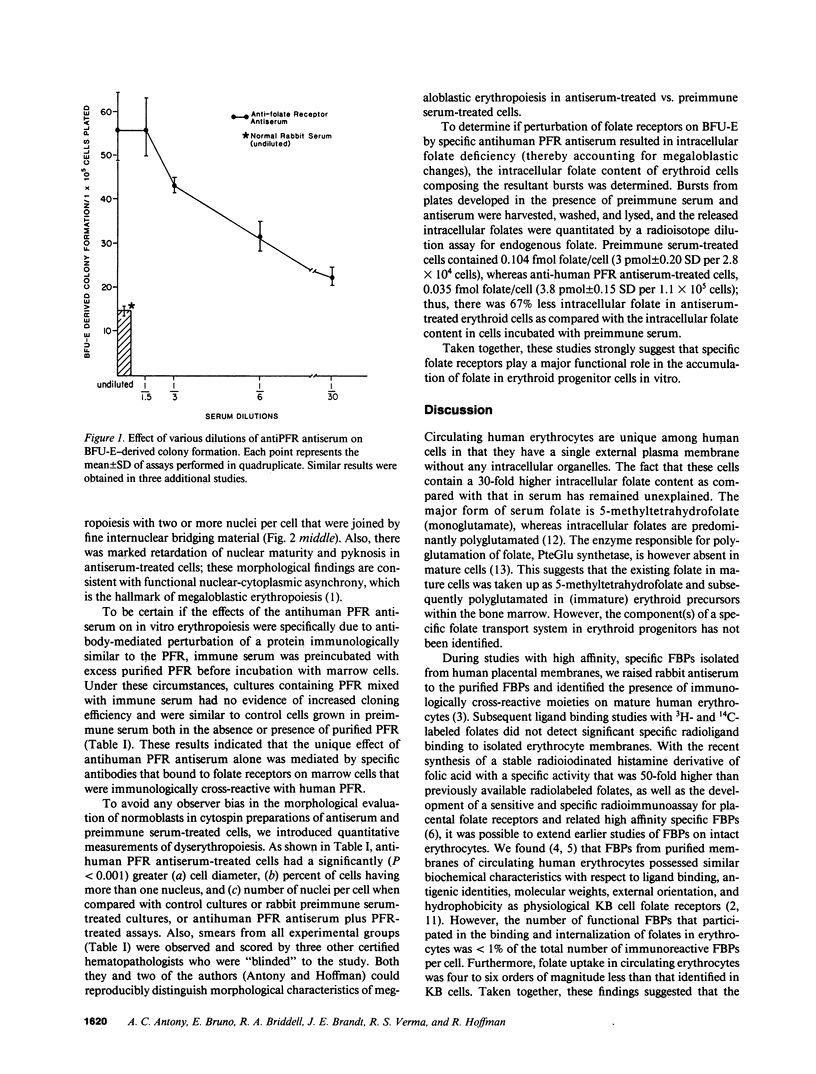
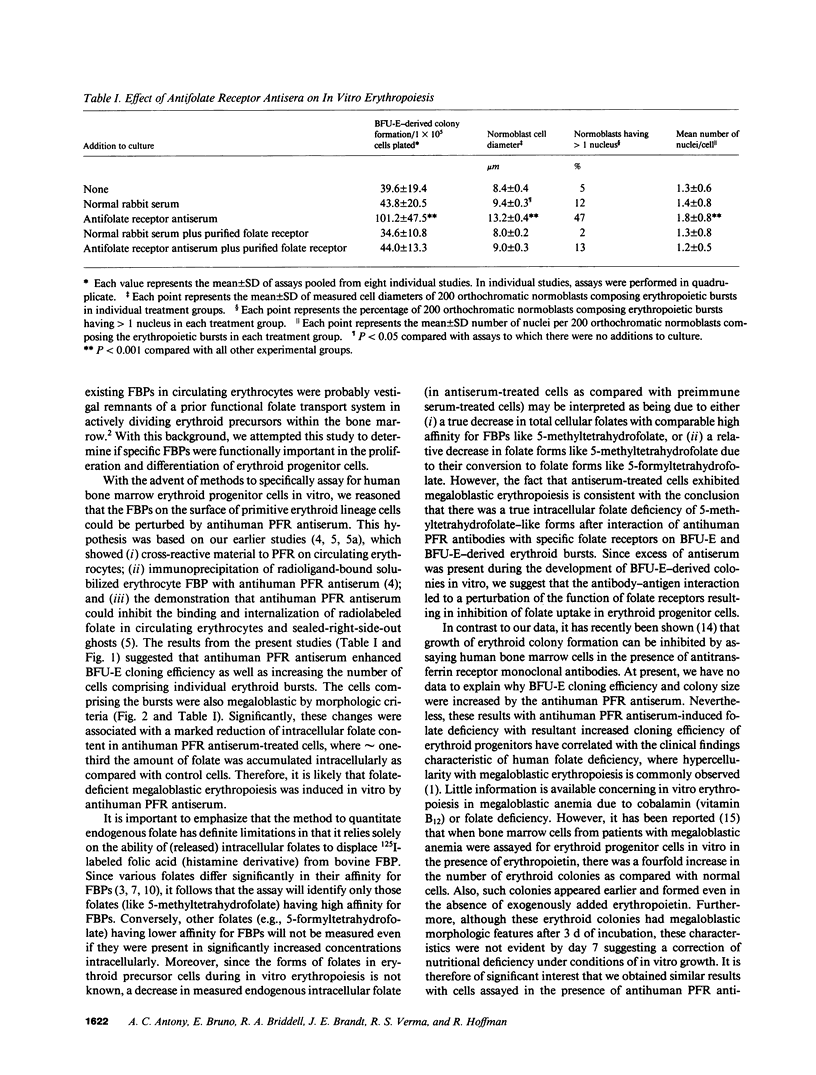
Although antisera to specific placental folate receptors inhibits the uptake of 5-methyltetrahydrofolate into cultured malignant human cells, little is known of the functional significance of folate receptors in normal human cells. Human bone marrow cells were therefore assayed for erythropoietic burst-forming units in the presence of an antihuman placental folate receptor serum and preimmune serum to determine the role of such a receptor in erythroid differentiation. When marrow cells were assayed in the presence of anti-receptor antiserum, there was (i) a threefold increase in erythropoietic burst formation and a twofold increase in the number of cells per erythroid burst; (ii) morphological evidence for nuclear/cytoplasmic dissociation of orthochromatic normoblasts composing erythroid bursts (megaloblastic erythropoiesis); (iii) intracellular folate deficiency with a 70% reduction of intracellular folate in antiserum treated cells as compared with control cells; and (iv) complete reversal of antiserum-induced changes on preincubation of antiserum with purified human placental folate receptor. These data support the conclusion that folate receptors on marrow cells provide an important function in the cellular uptake of folates during in vitro erythropoiesis. This process of folate uptake also appears to play a pivotal role in the differentiation and proliferation of erythroid progenitor cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony A. C., Kane M. A., Portillo R. M., Elwood P. C., Kolhouse J. F. Studies of the role of a particulate folate-binding protein in the uptake of 5-methyltetrahydrofolate by cultured human KB cells. J Biol Chem. 1985 Dec 5;260(28):14911–14917. [PubMed] [Google Scholar]
- Antony A. C., Kincade R. S., Verma R. S., Krishnan S. R. Identification of high affinity folate binding proteins in human erythrocyte membranes. J Clin Invest. 1987 Sep;80(3):711–723. doi: 10.1172/JCI113126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Utley C. S., Marcell P. D., Kolhouse J. F. Isolation, characterization, and comparison of the solubilized particulate and soluble folate binding proteins from human milk. J Biol Chem. 1982 Sep 10;257(17):10081–10089. [PubMed] [Google Scholar]
- Antony A. C., Utley C., Van Horne K. C., Kolhouse J. F. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981 Sep 25;256(18):9684–9692. [PubMed] [Google Scholar]
- Antony A. C., Verma R. S., Kincade R. S. Development of a specific radioimmunoassay for the placental folate receptor and related high-affinity folate binding proteins in human tissues. Anal Biochem. 1987 Apr;162(1):224–235. doi: 10.1016/0003-2697(87)90031-5. [DOI] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Hultin M. B. Modulation of thrombin-mediated activation of factor VIII:C by calcium ions, phospholipid, and platelets. Blood. 1985 Jul;66(1):53–58. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Kolhouse J. F. The interrelationship of the soluble and membrane-associated folate-binding proteins in human KB cells. J Biol Chem. 1986 Nov 25;261(33):15625–15631. [PubMed] [Google Scholar]
- Kane M. A., Portillo R. M., Elwood P. C., Antony A. C., Kolhouse J. F. The influence of extracellular folate concentration on methotrexate uptake by human KB cells. Partial characterization of a membrane-associated methotrexate binding protein. J Biol Chem. 1986 Jan 5;261(1):44–49. [PubMed] [Google Scholar]
- Porter P. N., Ogawa M. Characterization of human erythroid burst-promoting activity derived from bone marrow conditioned media. Blood. 1982 Jun;59(6):1207–1212. [PubMed] [Google Scholar]
- Shannon K. M., Larrick J. W., Fulcher S. A., Burck K. B., Pacely J., Davis J. C., Ring D. B. Selective inhibition of the growth of human erythroid bursts by monoclonal antibodies against transferrin or the transferrin receptor. Blood. 1986 Jun;67(6):1631–1638. [PubMed] [Google Scholar]
- Waxman S., Schreiber C. Determination of folate by use of radioactive folate and binding proteins. Methods Enzymol. 1980;66:468–483. doi: 10.1016/0076-6879(80)66490-8. [DOI] [PubMed] [Google Scholar]