Abstract
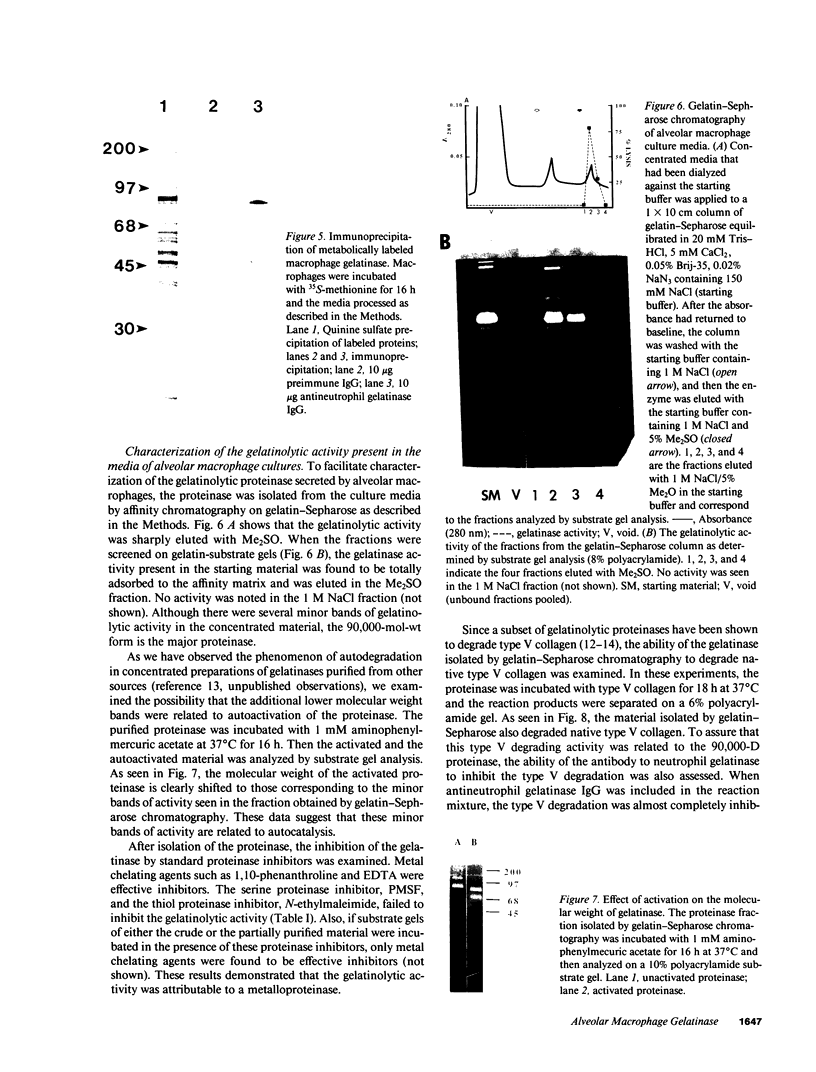
Human pulmonary alveolar macrophages obtained by bronchoalveolar lavage from both normal controls and smokers secreted in vitro a neutral proteinase that degraded denatured collagens. Optimal expression of the proteinase was detected after 3-5 d of culture. The proteinase could not be detected in the media of cultures that had been treated with 0.5 micrograms/ml of cycloheximide. The gelatinase had an Mr of 90,000 and was immunologically cross-reactive with human neutrophil gelatinase. When newly synthesized 35S-methionine-labeled proteins were analyzed, the proteinase appeared to be a major secretion product of alveolar macrophages. Chromatography on gelatin-Sepharose gave a single peak of activity that was predominantly composed of the 90,000-mol-wt proteinase. The proteolytic activity in the gelatin-Sepharose-purified material was inhibited by EDTA and 1,10-phenanthroline, but not by N-ethylmaleimide or phenylmethanesulfonyl fluoride, indicating that the proteinase was a metalloproteinase. The partially purified material was also capable of degrading native type V collagen and this degradation was inhibited in the presence of an antibody to neutrophil gelatinase. The data suggest that human alveolar macrophages in culture elaborate a metalloproteinase that degrades both native type V collagen and denatured collagens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Teitelbaum S. L., Stricklin G. P., Eisen A. Z., Kahn A. J., Welgus H. G. Differentiation of a human leukemia cell line and expression of collagenase inhibitor. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5380–5384. doi: 10.1073/pnas.82.16.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., White R. R., Senior R. M., Rodriguez R. J., Kuhn C. Receptor-mediated binding and internalization of leukocyte elastase by alveolar macrophages in vitro. J Clin Invest. 1979 Sep;64(3):824–833. doi: 10.1172/JCI109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Mainardi C. L., Seyer J. M., Kang A. H. Collagen-platelet interaction. Type V(A-B) collagen induced platelet aggregation. J Lab Clin Med. 1980 Jan;95(1):99–107. [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- GLIMCHER M. J., FRANCOIS C. J., RICHARDS L., KRANE S. M. THE PRESENCE OF ORGANIC PHOSPHORUS IN COLLAGENS AND GELATINS. Biochim Biophys Acta. 1964 Dec 9;93:585–602. doi: 10.1016/0304-4165(64)90342-3. [DOI] [PubMed] [Google Scholar]
- Garbisa S., Ballin M., Daga-Gordini D., Fastelli G., Naturale M., Negro A., Semenzato G., Liotta L. A. Transient expression of type IV collagenolytic metalloproteinase by human mononuclear phagocytes. J Biol Chem. 1986 Feb 15;261(5):2369–2375. [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Heterogeneity among human collagenases demonstrated by monoclonal antibody that selectively recognizes and inhibits human neutrophil collagenase. J Exp Med. 1984 May 1;159(5):1455–1463. doi: 10.1084/jem.159.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Secreted forms of human neutrophil collagenase. J Biol Chem. 1986 Apr 25;261(12):5645–5650. [PubMed] [Google Scholar]
- Hasty K. A., Jeffrey J. J., Hibbs M. S., Welgus H. G. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987 Jul 25;262(21):10048–10052. [PubMed] [Google Scholar]
- Herron G. S., Werb Z., Dwyer K., Banda M. J. Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J Biol Chem. 1986 Feb 25;261(6):2810–2813. [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Kang A. H., Mainardi C. L. Secretion of collagenolytic enzymes by human polymorphonuclear leukocytes. Coll Relat Res. 1984 Dec;4(6):467–477. doi: 10.1016/s0174-173x(84)80013-8. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Hibbs M. S., Postlethwaite A. E., Mainardi C. L., Seyer J. M., Kang A. H. Alterations in collagen production in mixed mononuclear leukocyte-fibroblast cultures. J Exp Med. 1983 Jan 1;157(1):47–59. doi: 10.1084/jem.157.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Louie J. S., Weiss J., Ryhänen L., Nies K. M., Rantala-Ryhänen S., Uitto J. The production of collagenase by adherent mononuclear cells cultured from human peripheral blood. Arthritis Rheum. 1984 Dec;27(12):1397–1404. doi: 10.1002/art.1780271210. [DOI] [PubMed] [Google Scholar]
- Mainardi C. L., Hibbs M. S., Hasty K. A., Seyer J. M. Purification of a type V collagen degrading metalloproteinase from rabbit alveolar macrophages. Coll Relat Res. 1984 Dec;4(6):479–492. doi: 10.1016/s0174-173x(84)80014-x. [DOI] [PubMed] [Google Scholar]
- McGowan S. E., Arbeit R. D., Stone P. J., Snider G. L. A comparison of the binding and fate of internalized neutrophil elastase in human monocytes and alveolar macrophages. Am Rev Respir Dis. 1983 Oct;128(4):688–694. doi: 10.1164/arrd.1983.128.4.688. [DOI] [PubMed] [Google Scholar]
- Miller E. J. The structure of fibril-forming collagens. Ann N Y Acad Sci. 1985;460:1–13. doi: 10.1111/j.1749-6632.1985.tb51152.x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Bretz U., Baggiolini M., Reynolds J. J. The latent collagenase and gelatinase of human polymorphonuclear neutrophil leucocytes. Biochem J. 1980 Nov 15;192(2):517–525. doi: 10.1042/bj1920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Galloway W. A., Barnes M. J., Bunning R. A., Mercer E., Reynolds J. J., Burgeson R. E. Metalloproteinases from rabbit bone culture medium degrade types IV and V collagens, laminin and fibronectin. Biochem J. 1981 Dec 1;199(3):807–811. doi: 10.1042/bj1990807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., McAlpine C. G., Poll C. T., Reynolds J. J. Purification and characterization of a bone metalloproteinase that degrades gelatin and types IV and V collagen. Biochim Biophys Acta. 1985 Sep 20;831(1):49–58. doi: 10.1016/0167-4838(85)90148-7. [DOI] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Bretz U., Baggiolini M. Partial purification of collagenase and gelatinase from human polymorphonuclear leucocytes. Analysis of their actions on soluble and insoluble collagens. Biochem J. 1982 Apr 1;203(1):209–221. doi: 10.1042/bj2030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes R. K., Miller E. J. Physicochemical characterization and molecular organization of the collagen A and B chains. Biochemistry. 1978 Aug 22;17(17):3442–3448. doi: 10.1021/bi00610a003. [DOI] [PubMed] [Google Scholar]
- Sellers A., Cartwright E., Murphy G., Reynolds J. J. Evidence that latent collagenases are enzyme-inhibitor complexes. Biochem J. 1977 May 1;163(2):303–307. doi: 10.1042/bj1630303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Bar-Shavit Z., Senior R. M., Teitelbaum S. L. Human alveolar macrophages produce a fibroblast-like collagenase and collagenase inhibitor. J Clin Invest. 1985 Jul;76(1):219–224. doi: 10.1172/JCI111949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Connolly N. L., Senior R. M. 12-o-Tetradecanoyl-phorbol-13-acetate-differentiated U937 cells express a macrophage-like profile of neutral proteinases. High levels of secreted collagenase and collagenase inhibitor accompany low levels of intracellular elastase and cathepsin G. J Clin Invest. 1986 May;77(5):1675–1681. doi: 10.1172/JCI112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]