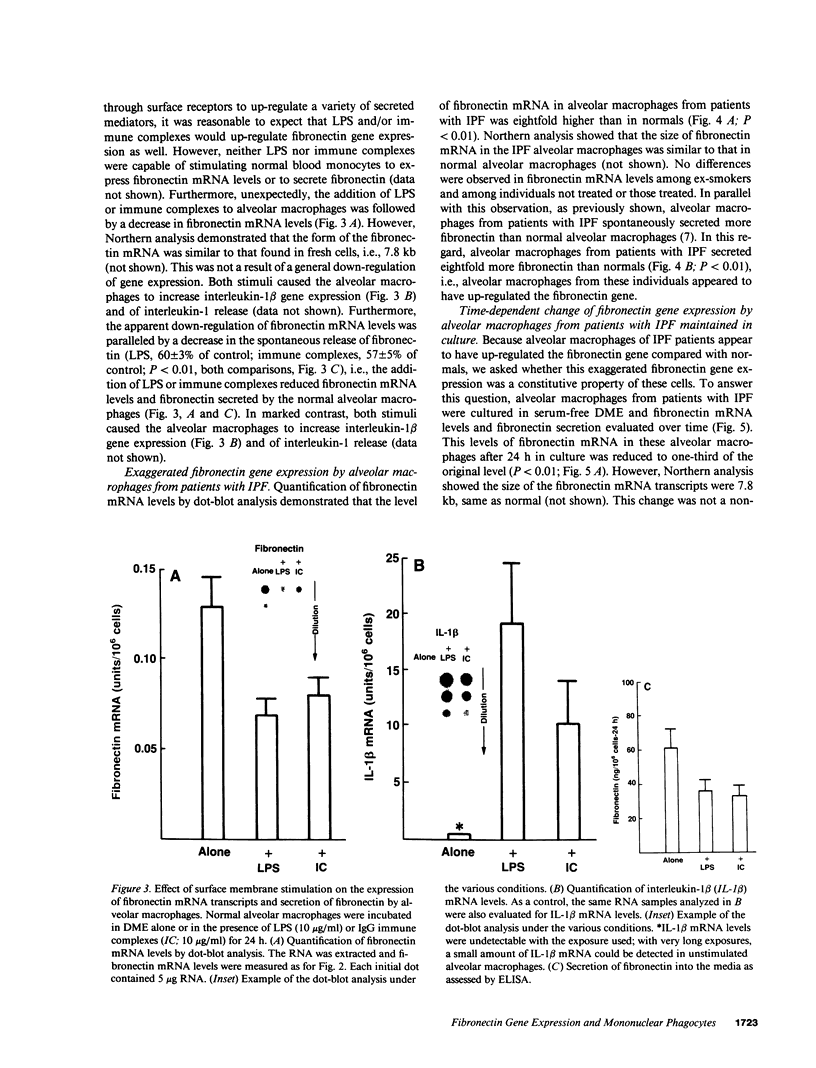
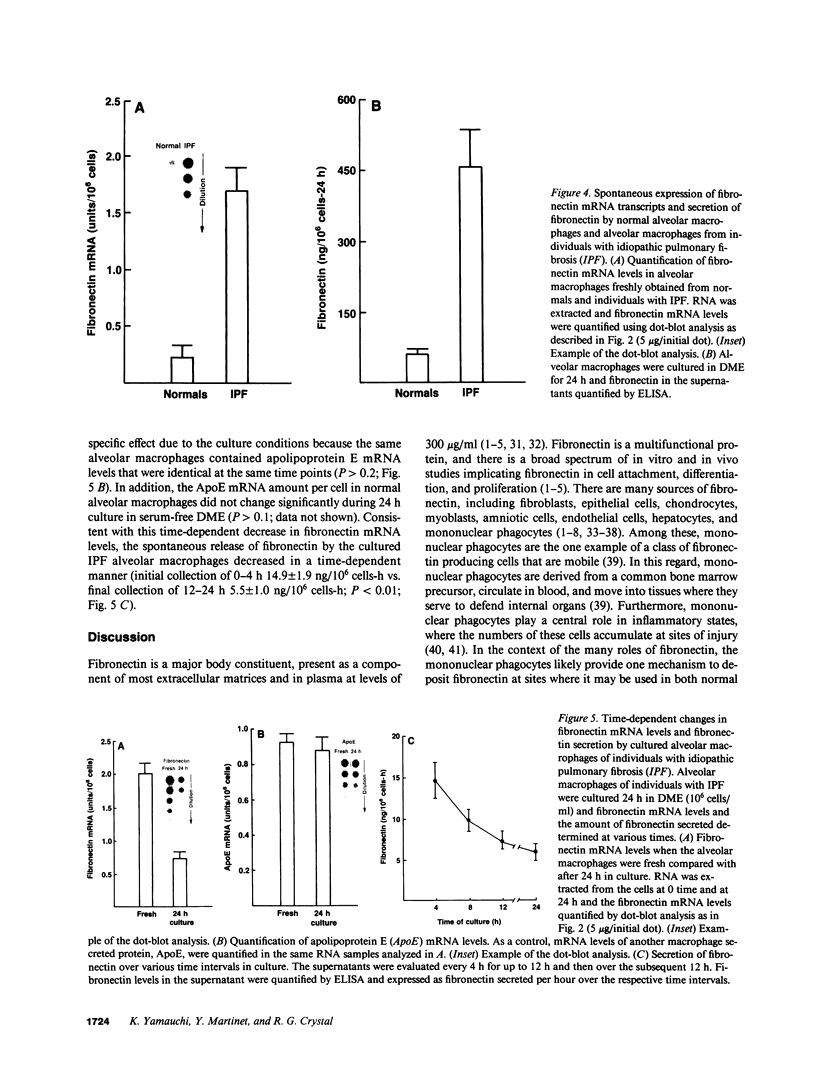
Abstract
Under some conditions, mononuclear phagocytes spontaneously synthesize and release fibronectin, an extracellular matrix glycoprotein with versatile effects on cell-matrix interactions. To gain insight into the processes that modulate the level of fibronectin secretion by these cells, we used monocytes, in vitro matured monocytes and alveolar macrophages as models to compare fibronectin mRNA levels and fibronectin secretion in a variety of circumstances. Using Northern analysis and dot-blot analysis with a 32P-labeled human fibronectin cDNA probe, we evaluated steady-state mRNA levels and a human fibronectin-specific ELISA was used to evaluate fibronectin secretion. In all cases the amounts of fibronectin secreted paralleled fibronectin mRNA levels. Specifically (a) when fibronectin mRNA was undetectable, as in the case of normal blood monocytes, no fibronectin was secreted, but whenever fibronectin mRNA was present, as in normal alveolar macrophages, fibronectin was secreted by the cells; (b) as monocytes matured into macrophages in vitro, the cells began to express fibronectin mRNA and the cells secreted fibronectin; (c) when alveolar macrophages were activated with surface stimuli such as lipopolysaccharide (LPS) or immune complexes, fibronectin mRNA levels decreased and in parallel, the cells secreted less fibronectin; (d) in idiopathic pulmonary fibrosis (IPF), alveolar macrophages contained severalfold more fibronectin mRNA transcripts that normal and the cells spontaneously secreted severalfold more fibronectin than normal; and (e) when IPF alveolar macrophages were placed in culture the fibronectin mRNA levels in the cells decreased with time, and concurrently the amounts of fibronectin produced per unit time continually decreased. The observation of a strict concordance of fibronectin mRNA levels and fibronectin release by mononuclear phagocytes suggests that, at least in many circumstances, fibronectin secretion by mononuclear phagocytes is controlled by steady-state levels of fibronectin mRNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anchors J. M., Gregg R. E., Law S. W., Brewer H. B., Jr ApoE deficiency: markedly decreased levels of cellular ApoE mRNA. Biochem Biophys Res Commun. 1986 Jan 29;134(2):937–943. doi: 10.1016/s0006-291x(86)80510-1. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Kolbe M., Weil D., Chu M. L. Human cellular fibronectin: comparison of the carboxyl-terminal portion with rat identifies primary structural domains separated by hypervariable regions. Biochemistry. 1985 May 21;24(11):2698–2704. doi: 10.1021/bi00332a016. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Adelberg S., Crystal R. G. Role of fibronectin as a growth factor for fibroblasts. J Cell Biol. 1983 Dec;97(6):1925–1932. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cofano F., Comoglio P. M., Landolfo S., Tarone G. Mouse immune interferon enhances fibronectin production of elicited macrophages. J Immunol. 1984 Dec;133(6):3102–3106. [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Davis W. B., Rennard S. I., Bitterman P. B., Crystal R. G. Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med. 1983 Oct 13;309(15):878–883. doi: 10.1056/NEJM198310133091502. [DOI] [PubMed] [Google Scholar]
- Gerdes J. S., Douglas S. D., Kolski G. B., Yoder M. C., Polin R. A. Decreased fibronectin biosynthesis by human cord blood mononuclear phagocytes in vitro. J Leukoc Biol. 1984 Jan;35(1):91–99. doi: 10.1002/jlb.35.1.91. [DOI] [PubMed] [Google Scholar]
- Goldstein C. S., Garrick R. E., Polin R. A., Gerdes J. S., Kolski G. B., Neilson E. G., Douglas S. D. Fibronectin and complement secretion by monocytes and peritoneal macrophages in vitro from patients undergoing continuous ambulatory peritoneal dialysis. J Leukoc Biol. 1986 Apr;39(4):457–464. doi: 10.1002/jlb.39.4.457. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman A., Yamada K. M., Kornblihtt A. Human fibronectin is synthesized as a pre-propolypeptide. FEBS Lett. 1986 Oct 20;207(1):145–148. doi: 10.1016/0014-5793(86)80029-1. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Schwarzbauer J. E., Tamkun J. W. Fibronectin: a versatile gene for a versatile protein. Ciba Found Symp. 1984;108:75–92. doi: 10.1002/9780470720899.ch6. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Johansson S., Rubin K., Hök M., Ahlgren T., Seljelid R. In vitro biosynthesis of cold insoluble globulin (fibronectin) by mouse peritoneal macrophages. FEBS Lett. 1979 Sep 15;105(2):313–316. doi: 10.1016/0014-5793(79)80637-7. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: cell specific alternative mRNA splicing generates polypeptide chains differing in the number of internal repeats. Nucleic Acids Res. 1984 Jul 25;12(14):5853–5868. doi: 10.1093/nar/12.14.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: molecular cloning evidence for two mRNA species differing by an internal segment coding for a structural domain. EMBO J. 1984 Jan;3(1):221–226. doi: 10.1002/j.1460-2075.1984.tb01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Isolation and characterization of cDNA clones for human and bovine fibronectins. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3218–3222. doi: 10.1073/pnas.80.11.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Miskulin M., Dalgleish R., Kluve-Beckerman B., Rennard S. I., Tolstoshev P., Brantly M., Crystal R. G. Human type III collagen gene expression is coordinately modulated with the type I collagen genes during fibroblast growth. Biochemistry. 1986 Mar 25;25(6):1408–1413. doi: 10.1021/bi00354a033. [DOI] [PubMed] [Google Scholar]
- Mornex J. F., Chytil-Weir A., Martinet Y., Courtney M., LeCocq J. P., Crystal R. G. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986 Jun;77(6):1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Mosher D. F. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Ruoslahti E. Evolution of the fibronectin gene. Exon structure of cell attachment domain. J Biol Chem. 1986 Feb 15;261(5):2113–2116. [PubMed] [Google Scholar]
- Paul J. I., Schwarzbauer J. E., Tamkun J. W., Hynes R. O. Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem. 1986 Sep 15;261(26):12258–12265. [PubMed] [Google Scholar]
- Raghow R., Lurie S., Seyer J. M., Kang A. H. Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest. 1985 Nov;76(5):1733–1739. doi: 10.1172/JCI112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Crystal R. G. Fibronectin in human bronchopulmonary lavage fluid. Elevation in patients with interstitial lung disease. J Clin Invest. 1982 Jan;69(1):113–122. doi: 10.1172/JCI110421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Paul J. I., Hynes R. O. On the origin of species of fibronectin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1424–1428. doi: 10.1073/pnas.82.5.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Schwarzbauer J. E., Hynes R. O. A single rat fibronectin gene generates three different mRNAs by alternative splicing of a complex exon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5140–5144. doi: 10.1073/pnas.81.16.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocci M. J., Hutchinson N. I., Cameron P. M., Kirk K. E., Norman D. J., Chin J., Rupp E. A., Limjuco G. A., Bonilla-Argudo V. M., Schmidt J. A. Expression in Escherichia coli of fully active recombinant human IL 1 beta: comparison with native human IL 1 beta. J Immunol. 1987 Feb 15;138(4):1109–1114. [PubMed] [Google Scholar]
- Torikata C., Villiger B., Kuhn C., 3rd, McDonald J. A. Ultrastructural distribution of fibronectin in normal and fibrotic human lung. Lab Invest. 1985 Apr;52(4):399–408. [PubMed] [Google Scholar]
- Tsukamoto Y., Helsel W. E., Wahl S. M. Macrophage production of fibronectin, a chemoattractant for fibroblasts. J Immunol. 1981 Aug;127(2):673–678. [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Tyagi J. S., Hirano H., Merlino G. T., Pastan I. Transcriptional control of the fibronectin gene in chick embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1983 May 10;258(9):5787–5793. [PubMed] [Google Scholar]
- Umezawa K., Kornblihtt A. R., Baralle F. E. Isolation and characterization of cDNA clones for human liver fibronectin. FEBS Lett. 1985 Jul 1;186(1):31–34. doi: 10.1016/0014-5793(85)81333-8. [DOI] [PubMed] [Google Scholar]
- Vibe-Pedersen K., Kornblihtt A. R., Baralle F. E. Expression of a human alpha-globin/fibronectin gene hybrid generates two mRNAs by alternative splicing. EMBO J. 1984 Nov;3(11):2511–2516. doi: 10.1002/j.1460-2075.1984.tb02165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibe-Pedersen K., Magnusson S., Baralle F. E. Donor and acceptor splice signals within an exon of the human fibronectin gene: a new type of differential splicing. FEBS Lett. 1986 Oct 27;207(2):287–291. doi: 10.1016/0014-5793(86)81506-x. [DOI] [PubMed] [Google Scholar]
- Villiger B., Broekelmann T., Kelley D., Heymach G. J., 3rd, McDonald J. A. Bronchoalveolar fibronectin in smokers and nonsmokers. Am Rev Respir Dis. 1981 Nov;124(5):652–654. doi: 10.1164/arrd.1981.124.5.652. [DOI] [PubMed] [Google Scholar]
- Villiger B. Function of pulmonary alveolar macrophage fibronectin. Curr Probl Clin Biochem. 1983;13:190–201. [PubMed] [Google Scholar]
- Villiger B., Kelley D. G., Engleman W., Kuhn C., 3rd, McDonald J. A. Human alveolar macrophage fibronectin: synthesis, secretion, and ultrastructural localization during gelatin-coated latex particle binding. J Cell Biol. 1981 Sep;90(3):711–720. doi: 10.1083/jcb.90.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]