Abstract
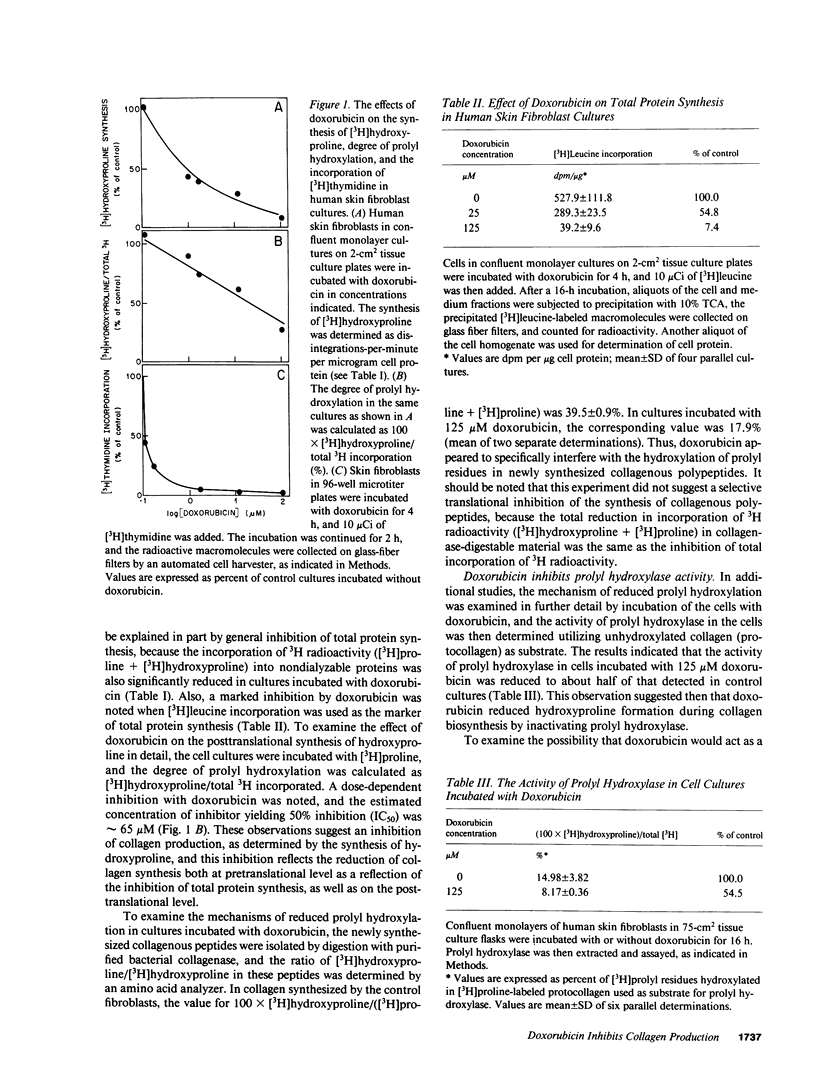
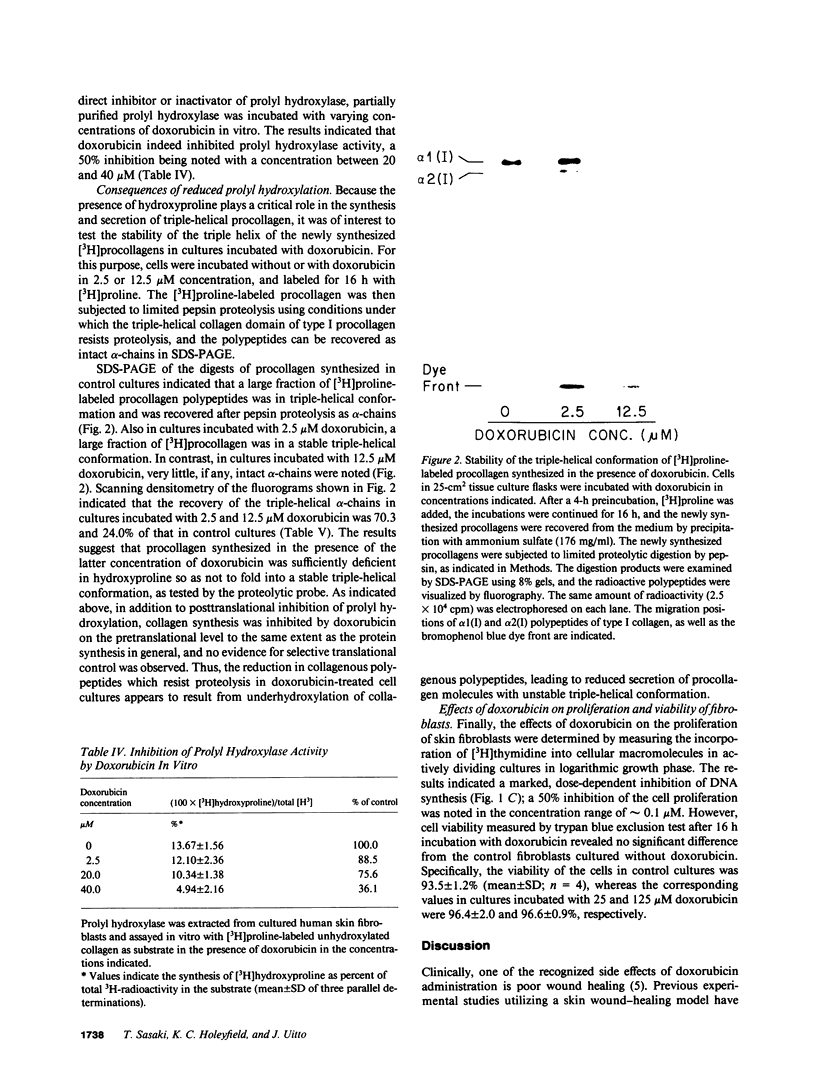
Previous clinical and experimental observations have indicated that wound healing is impaired as a result of treatment with doxorubicin, a chemotherapeutic agent. In this study, the effects of doxorubicin were examined in human skin fibroblast cultures with respect to collagen production and fibroblast proliferation. The results indicated that the synthesis of hydroxyproline as a marker of collagen production was markedly reduced, with an approximate concentration of inhibitor yielding 50% inhibition of 1 microM. This inhibition could be explained, in part, by generalized inhibition of total protein synthesis, but in addition, there was a significant inhibition of prolyl hydroxylation during collagen biosynthesis, as indicated by a reduction in the ratio of [3H]hydroxyproline/([3H]hydroxyproline + [3H]proline). The latter effect was shown to result from inhibition of prolyl hydroxylase by doxorubicin. As a consequence of reduced prolyl hydroxylation, the stability of newly synthesized procollagen triple helix was shown to be compromised. At the same time, doxorubicin significantly reduced fibroblast proliferation in vitro, as determined by [3H]thymidine incorporation. Thus, reduced collagen production and inhibition of fibroblast proliferation may explain the reduced wound healing in patients undergoing treatment with doxorubicin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham P. A., Perejda A. J., Carnes W. H., Uitto J. Marfan syndrome. Demonstration of abnormal elastin in aorta. J Clin Invest. 1982 Dec;70(6):1245–1252. doi: 10.1172/JCI110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Purification of (14C) protocollagen and its hydroxylation by prolyl-hydroxylase. Biochemistry. 1973 Aug 28;12(18):3395–3401. doi: 10.1021/bi00742a005. [DOI] [PubMed] [Google Scholar]
- Bland K. I., Palin W. E., von Fraunhofer J. A., Morris R. R., Adcock R. A., Tobin G. R., 2nd Experimental and clinical observations of the effects of cytotoxic chemotherapeutic drugs on wound healing. Ann Surg. 1984 Jun;199(6):782–790. doi: 10.1097/00000658-198406000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booth B. A., Polak K. L., Uitto J. Collagen biosynthesis by human skin fibroblasts. I. Optimization of the culture conditions for synthesis of type I and type III procollagens. Biochim Biophys Acta. 1980 Mar 28;607(1):145–160. doi: 10.1016/0005-2787(80)90228-2. [DOI] [PubMed] [Google Scholar]
- Booth B. A., Uitto J. Collagen biosynthesis by human skin fibroblasts. III. The effects of ascorbic acid on procollagen production and prolyl hydroxylase activity. Biochim Biophys Acta. 1981 Jun 11;675(1):117–122. doi: 10.1016/0304-4165(81)90076-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cohen S. C., Gabelnick H. L., Johnson R. K., Goldin A. Effects of cyclophosphamide and adriamycin on the healing of surgical wounds in mice. Cancer. 1975 Oct;36(4):1277–1281. doi: 10.1002/1097-0142(197510)36:4<1277::aid-cncr2820360413>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Devereux D. F., Thibault L., Boretos J., Brennan M. F. The quantitative and qualitative impairment of wound healing by adriamycin. Cancer. 1979 Mar;43(3):932–938. doi: 10.1002/1097-0142(197903)43:3<932::aid-cncr2820430322>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Devereux D. F., Triche T. J., Webber B. L., Thibault L. E., Brennan M. F. A study of adriamycin-reduced wound breaking strenght in rats. An evaluation by light and electron microscopy, induction of collagen maturation, and hydroxyproline content. Cancer. 1980 Jun 1;45(11):2811–2815. doi: 10.1002/1097-0142(19800601)45:11<2811::aid-cncr2820451116>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Prockop D. J., Berg R. A. Kinetics for the secretion of nonhelical procollagen by freshly isolated tendon cells. J Biol Chem. 1979 Apr 10;254(7):2234–2243. [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Majamaa K. Synthesis of collagen: chemical regulation of post-translational events. Ciba Found Symp. 1985;114:34–64. doi: 10.1002/9780470720950.ch4. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Post-translational processing of procollagens. Ann N Y Acad Sci. 1985;460:187–201. doi: 10.1111/j.1749-6632.1985.tb51167.x. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C. The connective tissue in scleroderma. Coll Relat Res. 1981 Apr;1(3):301–308. doi: 10.1016/s0174-173x(81)80007-6. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Hanauske-Abel H. M., Günzler V., Kivirikko K. I. The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for 2-oxoglutarate decarboxylation in a ligand reaction at the enzyme-bound ferrous ion. Eur J Biochem. 1984 Jan 16;138(2):239–245. doi: 10.1111/j.1432-1033.1984.tb07907.x. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Sasaki T., Uitto J. Inhibition of prolyl hydroxylation during collagen biosynthesis in human skin fibroblast cultures by ethyl 3,4-dihydroxybenzoate. J Invest Dermatol. 1987 Oct;89(4):405–409. doi: 10.1111/1523-1747.ep12471775. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Turpeenniemi-Hujanen T. M., Latipä P., Günzler V., Hanauske-Abel H. M., Hassinen I. E., Kivirikko K. I. Differences between collagen hydroxylases and 2-oxoglutarate dehydrogenase in their inhibition by structural analogues of 2-oxoglutarate. Biochem J. 1985 Jul 1;229(1):127–133. doi: 10.1042/bj2290127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Majamaa K., Uitto J. Reduction of collagen production in keloid fibroblast cultures by ethyl-3,4-dihydroxybenzoate. Inhibition of prolyl hydroxylase activity as a mechanism of action. J Biol Chem. 1987 Jul 5;262(19):9397–9403. [PubMed] [Google Scholar]
- Shamberger R. C., Devereux D. F., Brennan M. F. The effect of chemotherapeutic agents on wound healing. Int Adv Surg Oncol. 1981;4:15–58. [PubMed] [Google Scholar]
- Tuderman L., Prockop D. J. Procollagen N-proteinase. Properties of the enzyme purified from chick embryo tendons. Eur J Biochem. 1982 Jul;125(3):545–549. doi: 10.1111/j.1432-1033.1982.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Uitto J., Booth B. A., Polak K. L. Collagen biosynthesis by human skin fibroblasts. II. Isolation and further characterization of type I and type III procollagens synthesized in culture. Biochim Biophys Acta. 1980 Aug 21;624(2):545–561. doi: 10.1016/0005-2795(80)90095-1. [DOI] [PubMed] [Google Scholar]
- Uitto J., Murray L. W., Blumberg B., Shamban A. UCLA conference. Biochemistry of collagen in diseases. Ann Intern Med. 1986 Nov;105(5):740–756. doi: 10.7326/0003-4819-105-5-740. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Intracellular hydroxylation of non-helical protocollagen to form triple-helical procollagen and subsequent secretion of the molecule. Eur J Biochem. 1974 Apr 1;43(2):221–230. doi: 10.1111/j.1432-1033.1974.tb03403.x. [DOI] [PubMed] [Google Scholar]
- Uitto J., Ryhänen L., Tan E. M., Oikarinen A. I., Zaragoza E. J. Pharmacological inhibition of excessive collagen deposition in fibrotic diseases. Fed Proc. 1984 Oct;43(13):2815–2820. [PubMed] [Google Scholar]
- Uitto J., Santa Cruz D. J., Eisen A. Z. Connective tissue nevi of the skin. Clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980 Nov;3(5):441–461. [PubMed] [Google Scholar]



