Abstract
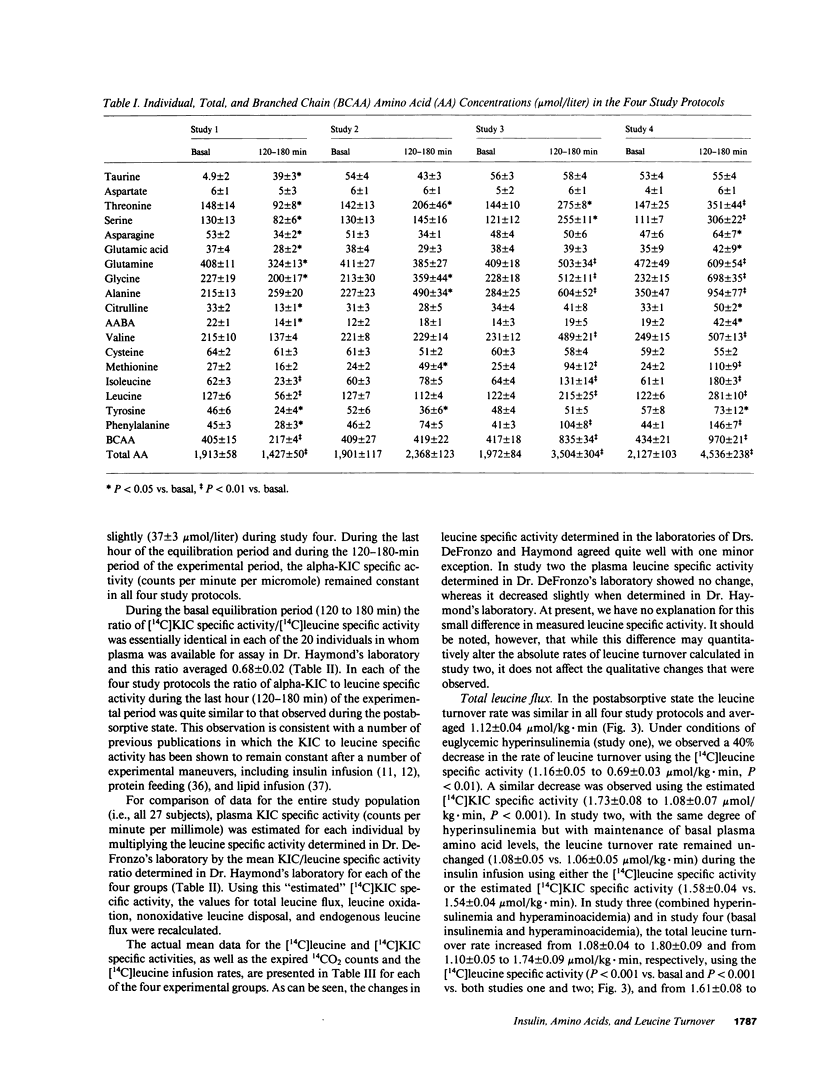
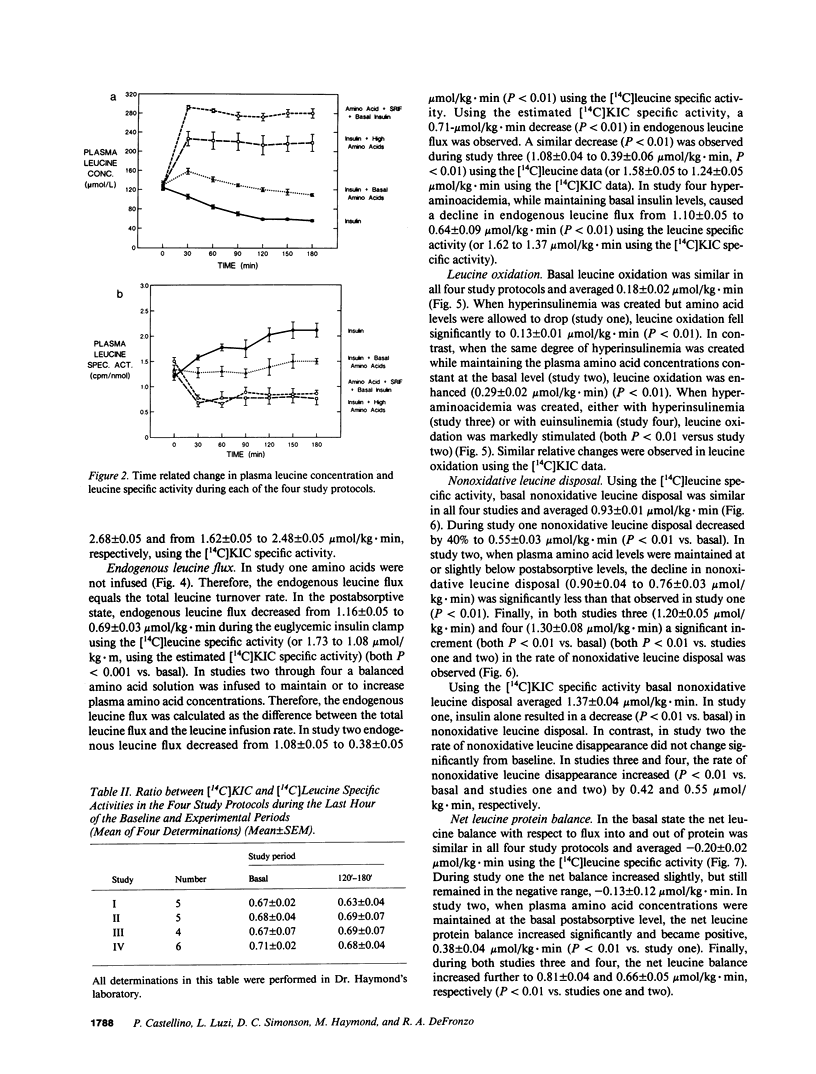
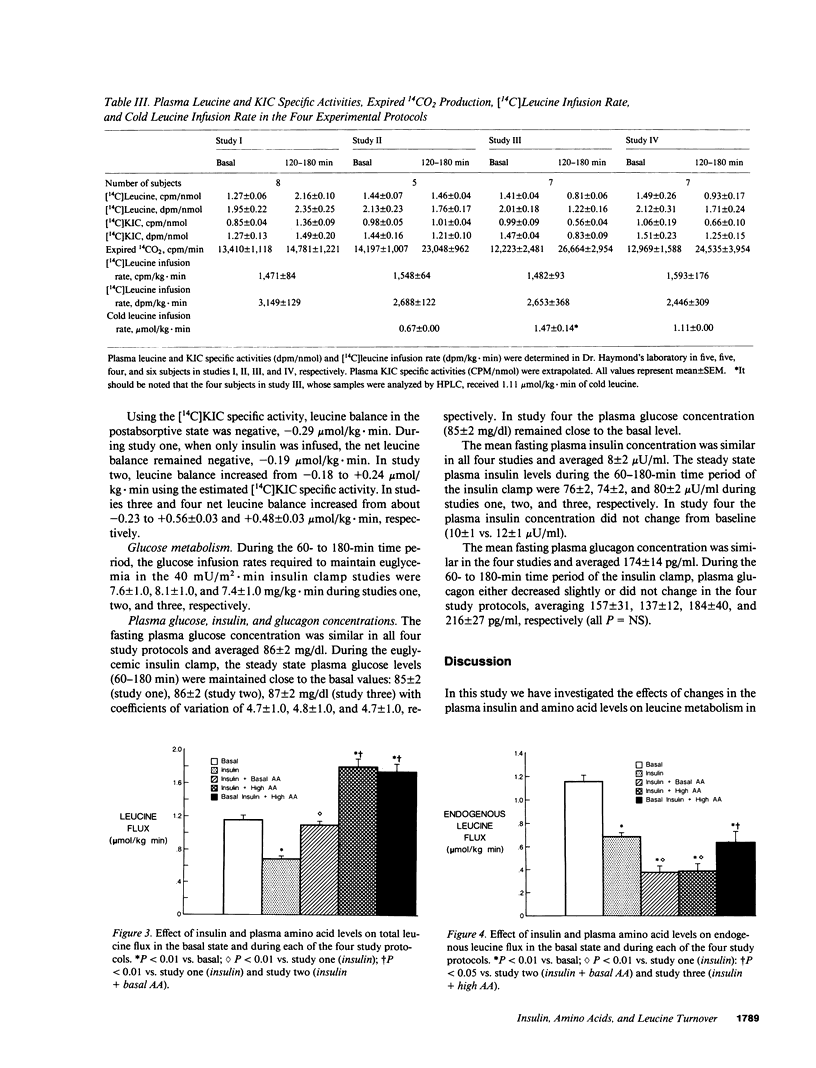
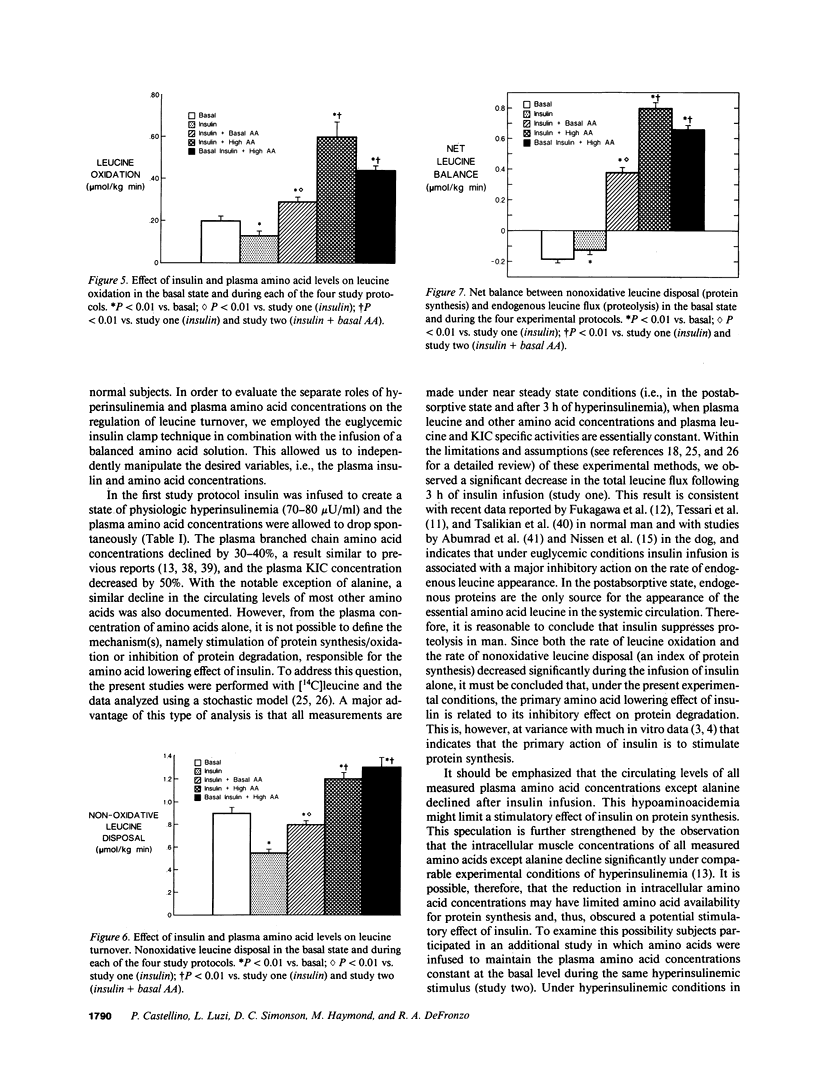
We examined the effect of insulin and plasma amino acid concentrations on leucine kinetics in 15 healthy volunteers (age 22 +/- 2 yr) using the euglycemic insulin clamp technique and an infusion of [1-14C]leucine. Four different experimental conditions were examined: (a) study one, high insulin with reduced plasma amino acid concentrations; (b) study two, high insulin with maintenance of basal plasma amino acid concentrations; (c) study three, high insulin with elevated plasma amino acid concentrations; and (d) study four, basal insulin with elevated plasma amino acid concentrations. Data were analyzed using both the plasma leucine and alpha-ketoisocaproate (the alpha-ketoacid of leucine) specific activities. In study one total leucine flux, leucine oxidation, and nonoxidative leucine disposal (an index of whole body protein synthesis) all decreased (P less than 0.01) regardless of the isotope model utilized. In study two leucine flux did not change, while leucine oxidation increased (P less than 0.01) and nonoxidative leucine disposal was maintained at the basal rate; endogenous leucine flux (an index of whole body protein degradation) decreased (P less than 0.01). In study three total leucine flux, leucine oxidation, and nonoxidative leucine disposal all increased significantly (P less than 0.01). In study four total leucine flux, leucine oxidation, and nonoxidative leucine disposal all increased (P less than 0.001), while endogenous leucine flux decreased (P less than 0.001). We conclude that: (a) hyperinsulinemia alone decreases plasma leucine concentration and inhibits endogenous leucine flux (protein breakdown), leucine oxidation, and nonoxidative leucine disposal (protein synthesis); (b) hyperaminoacidemia, whether in combination with hyperinsulinemia or with maintained basal insulin levels decreases endogenous leucine flux and stimulates both leucine oxidation and nonoxidative leucine disposal.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Jefferson L. S., Rannels S. R., Williams P. E., Cherrington A. D., Lacy W. W. Role of insulin in the regulation of leucine kinetics in the conscious dog. J Clin Invest. 1982 Nov;70(5):1031–1041. doi: 10.1172/JCI110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Beaufrere B., Tessari P., Cattalini M., Miles J., Haymond M. W. Apparent decreased oxidation and turnover of leucine during infusion of medium-chain triglycerides. Am J Physiol. 1985 Aug;249(2 Pt 1):E175–E182. doi: 10.1152/ajpendo.1985.249.2.E175. [DOI] [PubMed] [Google Scholar]
- Clark W. A., Jr, Zak R. Assessment of fractional rates of protein synthesis in cardiac muscle cultures after equilibrium labeling. J Biol Chem. 1981 May 25;256(10):4863–4870. [PubMed] [Google Scholar]
- Davey P. J., Manchester K. L. Isolation of labelled aminoacyl transfer RNA from muscle. Studies of the entry of labelled amino acids into acyl transfer RNA linkage in situ and its control by insulin. Biochim Biophys Acta. 1969 May 20;182(1):85–97. doi: 10.1016/0005-2787(69)90523-1. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Defronzo R. A. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979 Dec;28(12):1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Deibert D. C., DeFronzo R. A. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980 Mar;65(3):717–721. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett A. W., Prior G., Zak R. Equilibration of leucine between the plasma compartment and leucyl-tRNA in the heart, and turnover of cardiac myosin heavy chain. Biochem J. 1981 Jan 15;194(1):365–368. doi: 10.1042/bj1940365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J., Sherwin R., Palaiologos G. Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med. 1977 Apr;137(4):507–513. [PubMed] [Google Scholar]
- Forlani G., Vannini P., Marchesini G., Zoli M., Ciavarella A., Pisi E. Insulin-dependent metabolism of branched-chain amino acids in obesity. Metabolism. 1984 Feb;33(2):147–150. doi: 10.1016/0026-0495(84)90127-6. [DOI] [PubMed] [Google Scholar]
- Fukagawa N. K., Minaker K. L., Rowe J. W., Goodman M. N., Matthews D. E., Bier D. M., Young V. R. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985 Dec;76(6):2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Golden M. H., Waterlow J. C. Total protein synthesis in elderly people: a comparison of results with [15N]glycine and [14C]leucine. Clin Sci Mol Med. 1977 Sep;53(3):277–288. doi: 10.1042/cs0530277. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz B., Jr, Paul P., Miller H. I., Bortz W. M. Oxidation of plasma FFA in lean and obese humans. Metabolism. 1968 Jan;17(1):62–73. doi: 10.1016/s0026-0495(68)80008-3. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- KIPNIS D. M., NOALL M. W. Stimulation of amino acid transport by insulin in the isolated rat diaphragm. Biochim Biophys Acta. 1958 Apr;28(1):226–227. doi: 10.1016/0006-3002(58)90466-9. [DOI] [PubMed] [Google Scholar]
- Kelley J., Stirewalt W. S., Chrin L. Protein synthesis in rat lung. Measurements in vivo based on leucyl-tRNA and rapidly turning-over procollagen I. Biochem J. 1984 Aug 15;222(1):77–83. doi: 10.1042/bj2220077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANCHESTER K. L., YOUNG F. G. The effect of insulin on incorporation of amino acids into protein of normal rat diaphragm in vitro. Biochem J. 1958 Nov;70(3):353–358. doi: 10.1042/bj0700353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. F., Rabinowitz M., Blough R., Prior G., Zak R. Measurements of half-life of rat cardiac myosin heavy chain with leucyl-tRNA used as precursor pool. J Biol Chem. 1977 May 25;252(10):3422–3429. [PubMed] [Google Scholar]
- Matthews D. E., Schwarz H. P., Yang R. D., Motil K. J., Young V. R., Bier D. M. Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982 Nov;31(11):1105–1112. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Motil K. J., Matthews D. E., Bier D. M., Burke J. F., Munro H. N., Young V. R. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol. 1981 Jun;240(6):E712–E721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Garrow J. S., Ford C., Mahler R. F., Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983 Nov;25(5):400–403. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- Nissen S. L., Van Huysen C., Haymond M. W. Measurement of branched chain amino acids and branched chain alpha-ketoacids in plasma by high-performance liquid chromatography. J Chromatogr. 1982 Oct 8;232(1):170–175. doi: 10.1016/s0378-4347(00)86021-1. [DOI] [PubMed] [Google Scholar]
- Nissen S., Haymond M. W. Changes in leucine kinetics during meal absorption: effects of dietary leucine availability. Am J Physiol. 1986 Jun;250(6 Pt 1):E695–E701. doi: 10.1152/ajpendo.1986.250.6.E695. [DOI] [PubMed] [Google Scholar]
- Nissen S., Van Huysen C., Haymond M. W. Measurement of plasma alpha-ketoisocaproate concentrations and specific radioactivity by high-performance liquid chromatography. Anal Biochem. 1981 Jan 15;110(2):389–392. doi: 10.1016/0003-2697(81)90208-6. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Moldawer L. L., Young V. R., Blackburn G. L. The influence of intravenous nutrition on protein dynamics following surgery. Metabolism. 1981 Dec;30(12):1150–1158. doi: 10.1016/0026-0495(81)90034-2. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Beaufrere B., Koziet J., Desjeux J. F., Bier D. M., Young V. R., Lestradet H. Whole body de novo amino acid synthesis in type I (insulin-dependent) diabetes studied with stable isotope-labeled leucine, alanine, and glycine. Diabetes. 1985 Jan;34(1):67–73. doi: 10.2337/diab.34.1.67. [DOI] [PubMed] [Google Scholar]
- Schneible P. A., Airhart J., Low R. B. Differential compartmentation of leucine for oxidation and for protein synthesis in cultured skeletal muscle. J Biol Chem. 1981 May 25;256(10):4888–4894. [PubMed] [Google Scholar]
- Schwenk W. F., Beaufrere B., Haymond M. W. Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol. 1985 Dec;249(6 Pt 1):E646–E650. doi: 10.1152/ajpendo.1985.249.6.E646. [DOI] [PubMed] [Google Scholar]
- Tessari P., Nosadini R., Trevisan R., De Kreutzenberg S. V., Inchiostro S., Duner E., Biolo G., Marescotti M. C., Tiengo A., Crepaldi G. Defective suppression by insulin of leucine-carbon appearance and oxidation in type 1, insulin-dependent diabetes mellitus. Evidence for insulin resistance involving glucose and amino acid metabolism. J Clin Invest. 1986 Jun;77(6):1797–1804. doi: 10.1172/JCI112504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud D., Jacot E., DeFronzo R. A., Maeder E., Jequier E., Felber J. P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982 Nov;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- WOOL I. G., KRAHL M. E. Incorporation of C14-amino acids into protein of isolated diaphragms: an effect of insulin independent of glucose entry. Am J Physiol. 1959 May;196(5):961–964. doi: 10.1152/ajplegacy.1959.196.5.961. [DOI] [PubMed] [Google Scholar]


