Abstract
The medical research and training enterprise in the United States is complex in both its scope and implementation. Accordingly, adaptations to the associated workforce needs present particular challenges. This is particularly true for maintaining or expanding national needs for physician-scientists where training resource requirements and competitive transitional milestones are substantial. For the individual, these phenomena can produce financial burden, prolong the career trajectory, and significantly influence career pathways. Hence, when national data suggest that future medical research needs in a scientific area may be met in a less than optimal manner, strategies to expand research and training capacity must follow. This article defines such an exigency for research and training in nonneoplastic hematology and presents potential strategies for addressing these critical workforce needs. The considerations presented herein reflect a summary of the discussions presented at 2 workshops cosponsored by the National Heart, Lung, and Blood Institute and the American Society of Hematology.
Introduction
The research conducted by investigators in the field of nonmalignant hematology has been very impactful for more than 3 decades and continues to be evermore impactful. Despite this, the number of investigators submitting and being awarded National Institutes of Health (NIH) grants has declined dramatically over the past decade. To address this paradoxical situation, the Division of Blood Diseases and Resources (DBDR) of the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the American Society of Hematology (ASH) convened a series of 2 overlapping working groups (June 8th, 2012, at the NIH Neuroscience Building in Rockville, Maryland,1 and June 18, 2013, at the Lister Hill Center Auditorium, Bethesda, Maryland) to discuss the exigency of a perceived declining physician clinical and research workforce in hematology (note: in this article, we define the term hematology as specific for nonmalignant or nonneoplastic hematology for both pediatric and adult subspecialties). A group of invited guests, including academic representatives from the hematology and pediatric hematology/oncology community, joined hematologists and other scientists from ASH and government agencies including the NIH (extramural and intramural programs in the NHLBI and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Science Foundation, the Health Resources and Services Administration, the Centers for Disease Control and Prevention, and the US Department of Defense to constitute the panels for these two 1-day meetings. This forum article focuses on the research and training aspects of these discussions, with a special focus on how to support the research activities of physician-scientists and their optimal training. Companion documents will address the clinical nonneoplastic hematologic workforce. Table 1 enumerates strategies that could enhance both clinical care and research in hematology.
Table 1.
Suggested strategies for future enhancement of hematology in the United States
| 1. The hematologist of the future would be |
| • Expert in |
| o Malignant and nonmalignant disorders of the hematopoietic, hemostatic, and lymphatic systems, and disorders of the interaction between blood and blood vessel wall |
| o Hematopoietic stem cell transplantation, stem cell biology, and cellular therapies; genetics, genomics, and gene therapy; transfusion and laboratory medicine; and coagulation and vascular biology |
| o The statistical and computational methods of epidemiology, quality assessment, and comparative effectiveness research applied to hematology |
| • Facilitated by |
| o A multidisciplinary strategy for the scientific and clinical integration of hematology with other medical and surgical fields |
| o Innovative and flexible training programs |
| o Board certification and recertification policies that encourage research careers |
| • Supported by |
| o Expanded and innovative roles in the hospital health system and laboratory |
| o Funding opportunities from governmental and nongovernmental sources for |
| ■ Institutional and individual training of junior physicians |
| ■ Training of physician-scientists |
| ■ Mentoring |
| ■ Retraining |
| ■ Generation and analysis of data to support this mission |
| 2. The recruitment of more physicians into the field of hematology requires |
| • Introducing the excitement of hematologic practice and discovery to high school students through NOVA-type programming |
| • Broadly demonstrating to medical students, in the classroom and hospital/clinic settings, the wide range of hematologic practice and the high impact of hematologic discoveries |
| • Developing mechanisms to provide early experience in hematology research to medical students |
| • Providing incentives through secure mentored research training programs that are sufficiently long to increase the likelihood of successful research funding in hematologic research |
| • Providing a diverse choice of career options for residents and fellows who are considering careers in the field, including |
| o Teaching the field |
| o Service line specialization |
| o Epidemiology and Comparative Effectiveness Research/Hospital Quality Reporting research career alternatives |
| 3. The primary elements of Hematology Center of Research and Training Excellence would include the following |
| • Hematology research and training partnerships that would provide outstanding |
| o Multidisciplinary, mentored research opportunities |
| o Mechanisms to allow trainees to acquire a broad range of expertise through training opportunities at several institutional partners |
| o Balanced training in malignant and nonmalignant hematology disorders |
| o Opportunities for cross-training in medical and pediatric hematology for lifecycle care |
| o Training in hematologic disease related to global health |
| o Physical proximity between clinical and research training venues, allowing the trainee to move easily between clinic and laboratory |
| o Maximized integration of hematology with other medical, surgical, and basic research disciplines to allow for |
| ■ Generalized practice opportunities for those desiring a clinical career pathway |
| ■ Consultative specialization |
| ■ Clinical laboratory medicine and blood-banking expertise |
| ■ Cross-disciplinary research opportunities with opportunities to collaborate with PhD scientists |
| o Sustained and integrated NIH support for the training pathway |
| 4. Potential partners and strategies ensuring the future hematology workforce include |
| • ASH and allied professional societies |
| • Integrated support from all federal agencies including the Health Resources and Services Administration, US Food and Drug Administration, Centers for Disease Control and Prevention, Centers for Medicare and Medicaid Services, and Agency for Healthcare Research and Quality |
| • Patient-interest organizations that would encourage |
| o Harnessing the collective advocacy power of patients with rare diseases |
| o Partnership with genetics colleagues |
| • Mobilization within the profession to |
| o Advocate on behalf of itself with the support of multidisciplinary colleagues by |
| ■ Capitalizing on advocacy training through ASH |
| ■ Increasing national awareness through initiatives focused on national standards of hematologic health |
| ■ Creating a congressional caucus on hematology |
| ■ Engaging state governments and public health agencies |
| o Lead the way in developing fields such as regenerative and personalized medicine |
Delineating the challenges
Several challenges to the United States biomedical enterprise were identified: (1) the declining NIH budget and the corresponding reduction in application success rates for R01 and other investigator-initiated research grants; (2) the declining level of government-funded research and development as a proportion of gross domestic product at a time when other countries are expanding their investments; and (3) the declining share of biopharmaceutical patents granted to inventors, mirrored by a growing share of such patents granted in other countries. MD scientists face additional obstacles as the physician-discoverer model that served specialties like hematology so well in the last century has become harder to sustain. Potential causes for this include the prolonged gestation periods for clinical medicine and basic investigation, changes in requirements for specialty training and board certification, the outsourcing of research to PhD scientists, and a medical school curriculum that creates an intellectual conservatism and risk aversion that does not encourage discovery.
Concerns regarding the adequacy of the workforce in hematology are in part congruent with concerns reported more broadly across medical research.2,3 Recently, the director of the NIH, Francis Collins, established a working group on the physician-scientist workforce (PSW).2 This group was charged to develop approaches that can inform decisions about the PSW in the United States, to analyze the size of the PSW, to assess the needs and career opportunities for physician-scientist trainees, and to identify incentives and barriers to entering the PSW. All of these charges overlap with the aims for research and training for the hematologic workforce discussed herein. Similarly, the NIH group and the group represented in these 2 workshops are utilizing evolving workforce data to define national needs and to strategize about how to meet these needs. An added concern for the hematology workforce is that hematology focuses on a multitude of relatively rare diseases and overlaps with many other scientific disciplines. Hence, the future of hematologic research must embrace team- and integrated-science models. Similarly, hematologists must be trained in these overlapping areas.
As an example, the explication of the biological interface between blood, vasculature, and other human organ systems has begun to transform medical practice in areas such as traumatic injury and sepsis.4,5 Because these medical advancements require a fundamental understanding of blood science, bending the attrition curve of the hematologic workforce is essential for the advancement of health, both in traditional and nontraditional areas.
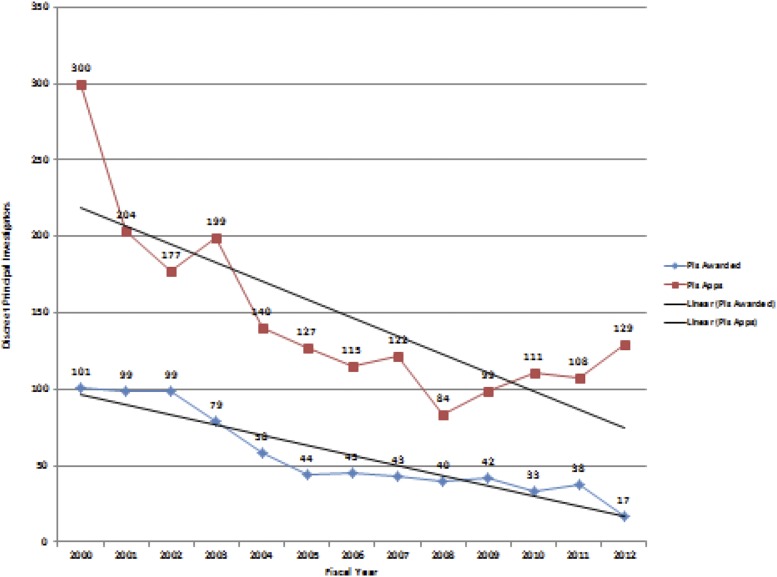
What is the evidence of such attrition in research hematology and what is unique about it among other research medical subspecialties? The number of funded grants in each of the divisions of the NHLBI has mirrored this downward trend in overall NIH funding. Accordingly, the total number of investigator-initiated (R01) grants that the NHLBI DBDR funds (almost all of which have nonmalignant hematologic science as their emphasis) has declined over the past decade, as have the number of unique R01 investigators (Figure 1). Total spending has declined less dramatically because of initiative funding. Moreover, in certain subspecialty areas such as erythrocyte or leukocyte biology, the number of R01 principal investigators who are funded by the DBDR has declined even more dramatically. Other NIH institutes (eg, the NIDDK) also fund in these areas, which has helped to reduce the impact on these focused scientific areas. Although decreases in funding are similar across the NIH and NHLBI, the fact that the DBDR is the smallest of the NHLBI program divisions accentuates the impact on the hematologic scientific capacity.
Figure 1.
New Investigator–initiated R01 principal investigator awards. Shown are the numbers of principal investigators applying to (PIs Apps, red) and funded by (PIs Awarded, blue) the NHLBI DBDR beginning in fiscal year (FY) 2000 and ending September 30, 2013 (FY 2013). Each funded principal investigator is included only in the first year during which he or she applied or was funded. This includes new and established principal investigators who were funded prior to FY 2000. The graph may be said to represent the “steady-state” of new or reentering hematologic PIs funded by the NHLBI DBDR over the represented time period.
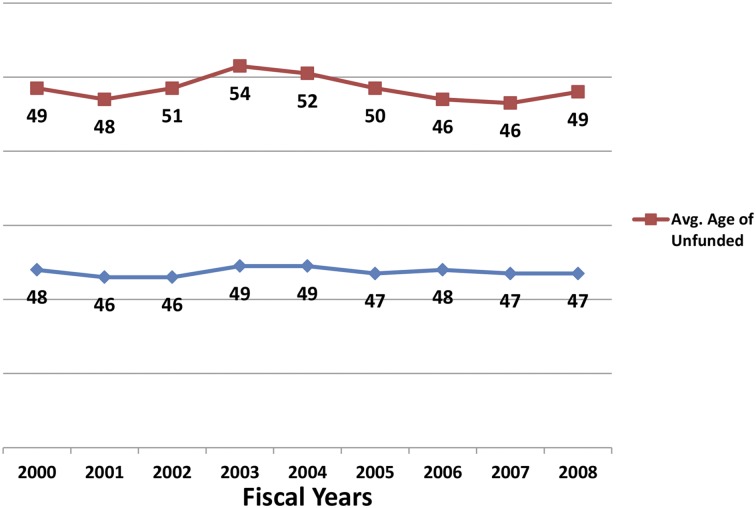
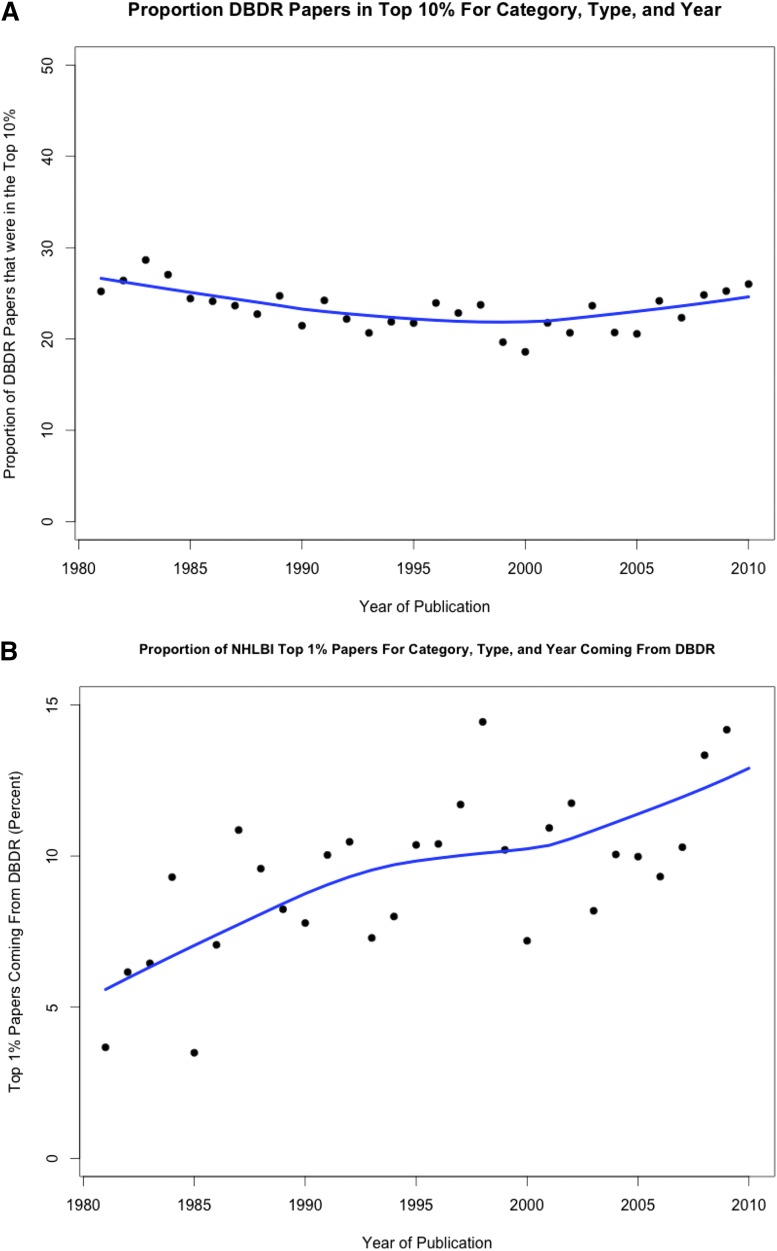
Despite the decline in unique DBDR R01 investigators for most of the last decade, their mean age has remained constant, at least through 2008 (Figure 2). This data suggest that young hematology investigators continue to compete successfully for R01 grants, potentially aided by special consideration under Early Stage Investigator funding. The quality of hematology research has been very high for many decades and shows evidence of becoming even higher as judged by the overrepresentation of papers from hematology grantees that are highly cited: ∼25% from this group from 1980-2010 have consistently been in the top 10% of cited papers in the years 1980-2010 (Figure 3A). During the same time period, the percentage in the top 1% of highly cited papers increased from ∼5% to 14% (Figure 3B). These data support the contention that augmenting the PSW in hematology is likely to yield high-quality and impactful scientific discoveries.
Figure 2.
Average age of principal investigators. Graph plots the average age of principle investigators unfunded (red) and funded (blue) by the NHLBI DBDR between the years 2000 and 2008.
Figure 3.
Ranking of published papers authored by investigators funded by the NHLBI DBDR in the years 1980-2010. For ranking purposes, all articles cited in Web of Science are evaluated by a proprietary algorithm developed by Thompson Reuters. The denominator is the total of top 10% (A) or top 1% (B) of papers published by any investigator funded by NHLBI in that year.
Exploring ways to bend the attrition curve of hematology researchers
There was consensus among workshop participants that the role of the physician-scientist is critical to the long-term societal success of hematologic research because the bench-to-bedside-to-bench intellectual engine needs such individuals for ignition. Further, the link between clinical and basic science has always represented one of hematology’s strongest assets. Because defining health needs is a crucial part of translational research, it is important that physician-scientists continue to participate in the clinical care of patients. Moreover, the movement to develop “learning healthcare systems” requires rigorous scientific assessment of all phases of health care delivery. Stated more succinctly, the central goal of medical education should be to develop compassionate physician-discoverers6,7 and to inculcate the principle that physicians have a responsibility to make discoveries about patients and to share these discoveries with the rest of the world. With this in mind, the stages from research career training to academic advancement were discussed.
Recruiting the next generation of hematology physician-scientists
Introducing more opportunities for discovery into medical school training should not only result in the higher likelihood of producing physician-scientists but also enhance the attractiveness of medical specialties such as hematology, which permit rapid and clinical and tissue correlation (because blood and marrow represent human tissues that can be collected relatively noninvasively). Such opportunities help to emphasize the essential role that scientific inquiry plays in optimal medical practice. Achieving this aim nationally will likely require partnering with entities in the public and private sectors. For example, the National Science Foundation takes a comprehensive approach to investing in the development of future scientists and engineers that includes aligning funding with goals, leveraging investments that support both training and research, and developing partnerships. The following paragraphs contain ideas that were presented for training scientists and engineers in general and how they may be adapted to attract individuals, including PhD, MD/PhD, and MD scientists to careers in hematology. Although the discussions focused on scientific and training programs in the United States, similar principles will likely apply to Canada, Europe, and elsewhere, given the global nature of hematology research and the many collaborations among investigators internationally.
Specialty-specific strategies to enhance the early hematologist pipeline.
Possible strategies that have been or are being pursued include the following: identifying promising young people early in their career for summer internships; offering travel awards to hematology meetings (a strategy that ASH has pioneered for hematology undergraduates, residents, and fellows); targeting educational opportunities for students from diverse backgrounds who have demonstrated an interest and aptitude for science or medicine (another ASH initiative); and upgrading the medical school Introduction to Hematology course at medical schools around the country. Traditionally, medical schools in the United States offer formal courses in hematology during the second year of instruction. More recently, however, a number have now chosen to teach blood-related science earlier in the curriculum and often in association with other disciplines to allow quicker entry by students into clinical rotations. This approach may have the undesirable effect of diluting the student experience in hematology, which may secondarily reduce the likelihood of their choosing the discipline for enhanced training during elective rotations; conversely, if this approach is integrated with the concepts of discovery and an emphasis on the excitement and importance of research, it could enhance the likelihood of the recruitment of physician-scientists. In this regard, it is important that hematology researchers remain active participants in curriculum decision making and in teaching at their institutions.
Partnering with the pharmaceutical industry and its collective drug development effort offers another possible opportunity. Industry and academia share the requisite knowledge pipeline for the workforce, and in many instances, translational and clinical trials initiating within the pharmaceutical industry also provide learning and teaching opportunities for trainees in the academic setting.
An additional avenue is to introduce predoctoral students to the field of hematology and to hematologic physician-scientists through mentored laboratory experiences. The NHLBI and NIDDK are planning a pilot R25 short-term grant program using funded hematologic core laboratory programs for up to 6 months of mentored laboratory experiences in scientific Centers of Excellence for predoctoral, as well as postdoctoral, trainees. These grants will fund travel and support interim living expenses for these students who will be able to acquire additional skills at convenient times during their training.
Strategies to expose physician trainees to hematology research during residency and fellowship.
Clinical residencies in the United States are required for medical licensure almost universally. The ever-expanding knowledge required to practice a medical specialty demands a more than full-time commitment to learning from clinical practice situations. This makes the incorporation of research opportunities or experiences into the training curriculum challenging at best. For individuals who have elected subspecialty training beyond clinical residency, research is required in most cases; at a minimum, it is encouraged. However, for residency trainees with little or no prior research exposure, inspiring a passion for a future career that is research focused is less likely. Mentorship by committed clinician scientists during residency may overcome this research naïveté. Yet, a more proactive approach is to formally incorporate a research perspective into residency training.
Also, the certification process for physicians in the United States, overseen by the Accreditation Council for Graduate Medical Education, has a major impact on trainees’ choice of medical specialty. In the broader arena of medical workforce enhancement, Drs David Goodman and Russell G. Roberson have proposed broad educational reform of graduate medical education in their recent article, “Accelerating physician workforce transformation through competitive graduate medical education funding.”8 It is their hypothesis that if medical teaching/training programs were forced to compete in a peer-reviewed process for funding based on data-documented innovation, the quality of training would improve. Such an approach could be complementary to many of the strategies proposed herein.
With regard to the importance of hematologic certification to the viability of clinical care, training, and research in the discipline, it is helpful to differentiate adult and pediatric certifications. The American Board of Internal Medicine (ABIM) through its Hematology Subspecialty Board certifies adult hematologists. Similarly, the American Board of Pediatrics (ABP) through its Pediatric Hematology-Oncology Subspecialty Board certifies pediatric hematologists. Among pediatric hematologists/oncologists who recently took the subspecialty board recertification examination, only 3.7% self-identified as research scientists and only 9% characterized their practice as primarily pediatric hematology, academic or otherwise. Despite the small number of individuals identifying themselves as being engaged in hematologic care, in 2014, the ABP plans to offer 3 hematology/oncology options for maintenance of certification: hematology/oncology, hematology only, and oncology only.
ABIM has a standing invitation for those accredited training programs that would like to offer innovative training options to submit pilot-project applications. In addition, ABIM allows exceptions to its certification requirements to programs that submit petitions on behalf of trainees who can complete training in less than the required minimum time. This flexibility offers opportunities for creative programs of training, including new pilot pathways that join clinical and research training requirements without prolongation of training time. Such alternative pathways would, of necessity, have frequent built-in “exit ramps” that would mitigate the time penalties for individuals who undertake a combined clinical and research trajectory but who subsequently choose to forego the research path for a career in clinical hematology. It was emphasized that such creative pilot career paths would not only need to protect trainees who undertake them but also require data collection on all participants’ career outcomes to ascertain the success or failure of the pilot.
Training curriculum expansion for preparing fellows for a greater breadth of both practice and research competencies was discussed in the context of whether such alterations would fit within the potential ABIM and ABP flexibilities cited earlier. One example is to collaborate with other specialty fields such as surgery, anesthesiology, and obstetrics/gynecology to design training curricula in hematology with more elements devoted to critical care (eg, vascular injury, acute hemostatic management, and so forth). There are examples from other countries that could serve as a template for such innovation. Another opportunity may lie in the creation of a specialized training path for lifelong hematologic chronic diseases. Because there are combined residency programs in medicine and pediatrics, there may be an opportunity to focus and compress in time a fellowship path that includes clinical research across the age spectrum for individuals wishing a career in chronic hematologic disease management. If successful, such pilots could serve as a rationale for codifying such training within certification guidelines of both ABIM and ABP.
It was noted that for innovative training pilots to transition to viable expanded hematologic career options, there must be a parallel effort to employ data to justify reimbursement of these capabilities by the health care system. Otherwise, no one will seek this expanded training.
There was agreement among participants that greater fiscal support for scientific mentoring by established investigative clinician scientists is another approach to enhance training. National, institutional, or foundation funding for supporting a substantial commitment to mentoring may prove attractive to potential trainees and create an institutional resource for recruitment of the best and brightest to programs undertaking such an effort. Such an approach would, of course, also apply more broadly to the mentoring of physician-scientists in multiple disciplines.
An over-arching theme of the training discussion was the inextricable link between clinical and basic science that is critical to maintain across medical specialties in order for the United States to remain competitive internationally. Due to many of the factors discussed in these workshops, hematology is particularly vulnerable to a decline because this clinical-basic science link is jeopardized by multiple challenges.
Consistent with this recognition, it behooves hematology to take advantage of new opportunities in computational biology. For research training, vast amounts of scientific data are accessible for query and analysis. These data from the NIH and other sources are available to hematologists (those in training and established investigators throughout their academic career). Other medical research specialties have exploited these opportunities more consistently. It may be worthwhile for the NIH and ASH to systematically inform training directors of hematology programs about the data and biological specimens available for generating and testing thoughtful research hypotheses. These data and specimens may furnish the basis for joint ASH-NIH educational initiatives to introduce computational skills into hematology training programs and for more intense short-course experiences for those wanting a deeper understanding of the methodology. The NHLBI-funded Biologic Specimen and Data Repository Information Coordinating Center is but 1 example of these available resources. Further, these computational tools can also be exploited to enhance the research capabilities of epidemiology and clinical studies.
Improving the academic advancement of hematology physician-scientists and their transition to independence.
A novel strategy to enhance the later research physician-scientist pipeline is to support K12 or K23 clinical research training grants that emphasize specialized training in clinical trial design, comparative/cost effectiveness study methodology, epidemiology, and implementation research for young physician-scientist trainees interested in clinical, but not laboratory-based basic or translational, research. ASH, through its Clinical Research Training Institute program for senior fellows and beginning assistant professors, is an important partner. The impact of such specialized training on health outcomes in aggregate could be assessed by how effective their research is in impacting such clinical parameters as use of blood products and anticoagulants or intensive care unit length of stay. ASH and the European Hematology Association cosponsor a similar training program to mentor early translational researchers (Translational Research Training in Hematology). An alternative strategy could include funding of K12 grants to supplement Clinical and Translational Science Award Programs, with requisite mentoring by senior hematologists with R01 support until and even after the junior investigator successfully receives R01 funding. Achieving this end for hematologists without PhD training will likely require 8 years of mentored research support to prepare them to compete with PhD and MD/PhD candidates who have 8 to 10 years of mentored graduate and postdoctoral research experience. One potential avenue forward would be for the NIH to create an 8-year mentored program for “late bloomers” (ie, MDs without PhDs) that would be the equivalent of a K12 (DL2) plus K08 or K23. As part of the curriculum, specific training in bioinformatics, phenotyping, and other research tools for advanced clinical research capability could be incorporated into the curriculum.
This same multi-institute-sponsored program is also intended to support intense and focused training for postdoctoral fellows with MD or MD/PhD degrees who are earlier in their research career development and who wish to augment specialized laboratory skill sets that may not be available in their local academic environment. On traveling to one of the designated specialized laboratories, these individuals would be mentored by a senior scientist with the requisite laboratory and educational expertise. It is hoped that such experiences will create new avenues for hematologic research collaboration for these individuals and facilitate their abilities to apply for and acquire sustainable research funding during this critical early career stage.
Modeling workforce and research funding.
Drs Richard Larson and Navid Ghaffarzadegan and colleagues at the Engineering Systems Division, Virginia Tech, have performed pioneering work in modeling the PhD biomedical workforce in the United States.9,10 During the second workshop, Dr Ghaffarzadegan presented data on how these models may help predict workforce needs for hematology in the United States. He pointed out the reproductive nature of the scientific workforce and how a high-quality science workforce engenders (ie, educates and mentors) the next-generation science workforce.
This phenomenon can be modeled using a demographically derived R0 = 1 replacement function, whereby, in a steady state, each mentor enables his own scientific successor. When R0 > 1, the scientific workforce grows exponentially. When complexities confound the simple replacement of generational workforces, these can be mathematically attributed within the model accordingly to create the best fit within the model.
A number of questions arose as to how such a model might be best used to predict future societal needs for hematologists. Dr Ghaffarzadegan pointed out that the purpose of the model is to provide policy insights rather than implement a means to stabilize a workforce. An example of why this may be salient for hematology is that downstream workforce needs may be complicated by the need for mentoring in new scientific expertise outside the traditional training purview of the hematologist. To include these disciplines in such a model requires collaboration between individuals with expertise in mathematical modeling and the hematology mentoring community. Included in such collaborations would be consideration of influencing economic forces within the medical system in general and hematology specifically.
Well-designed data collection and tracking are essential for workforce assessment and planning. Hence, a session in the second workshop was dedicated to a discussion of what types of data might be critical to collect and analyze retrospectively and prospectively to define more precisely the status of the workforce in nonmalignant hematology. Relevant questions that cannot be adequately answered without cross-sectional or longitudinal data collection include (1) What influences trainees to pursue careers in hematology? (2) How many hematologists will be needed in the United States in 5, 10, and 20 years and how many are entering and leaving the field (the R0)? and (3) What will be the research needs of the United States in this field in subsequent decades and what human resources will be required to insure the ongoing scientific innovation that will lead to advances in the field? The data must be built on a cross-sectional understanding of the size and distribution of the hematologic workforce presently, the assessed capacity of the specialty to adapt to current and future health care system needs, and the integrated role that hematologic researchers need to play in future team-science direction and implementation and in training future physician-scientists in hematology. For these reasons, we cannot estimate with acceptable confidence intervals how many hematologists will be needed in the United States in 5, 10, or 20 years. However, by implementing strategies for data collection, advancing the next-generation mathematical models, and assessing the overlap of research needs with other complementary medical subspecialties and their research workforce requirements, national needs might be better estimated in the future and thus allow optimal resource allocations and better resource planning.
Protecting and enhancing the current and future hematology workforce
A particular concern expressed by participants in these workshops was that, given the difficulty experienced by mentors in securing and maintaining research funding support (NIH and otherwise), mentees will wonder about their own potential for a long and successful career in research. In addition to some of the strategies for improving funding for Early Stage Investigators (discussed earlier), other strategies being explored within the NIH and more broadly include funding investigators rather than research projects. This approach would potentially provide funding security for longer periods, reducing the daunting reapplication focus that exacerbates these career concerns. Other ideas that were brought forward include government–private sector partnerships with the pharmaceutical industry: United States–centric companies need a secure academic workforce because they are dependent on the same developmental pipeline for research hematologists as are government and academia.
The need to develop exit pathways for trainees was mentioned earlier. A commitment to fundamental medical research requires passion and commitment by the trainee over many years, oftentimes with associated financial sacrifice. If, along the way, he or she loses this requisite passion for a research career for any reason, prior integration of clinical and research training would alleviate some of the time penalty for abandoning the research path. Creating such paths will require broad cooperation among responsible stakeholders.
Some novel programs have recently been created to begin to address some of these challenges. ASH has recently established a Bridge Grant Program to provide interim research funding for investigators who wrote meritorious grants for funding from the NIH. This allows them to keep key staff engaged while they reapply for longer-term funding. The criteria for awarding bridge monies include the importance of the individual to his or her local academic hematology community, as well as the excellence of the science. In addition, the NHLBI and NIDDK have prioritized their R56 bridge funding mechanism, which is similar to the creative one established by ASH, and targets grants that fell just short of the funding cutoff.
It is apparent that no single strategy will enhance the hematologic research workforce. However, by undertaking some of the strategies described herein and connecting them to other, yet to be proposed creative recruitment and retention strategies,10 there is a foundation for creating an integrated and collaborative pathway to enhancing the nonmalignant hematologic research workforce in the United States. In Table 2, several possible work groups to address these strategic goals are proposed.
Table 2.
Possible work groups to address strategic goals for enhancing the specialty of hematology in the United States
| Clinical |
| • Exploring hospitalist and other career enhancement opportunities through engagement of hospitals |
| • Engagement with and assessing the impact of regulatory entities such as the ABIM, ABP, American Board of Legal Medicine, Accreditation Council for Graduate Medical Education, The Joint Commission, and others |
| • Impact of the Patient Protection and Affordable Care Act and the Patient-Centered Outcomes Research Institute |
| • Enhancing mentorship of the clinical hematologist |
| Training |
| • Research training: exploring opportunities for redesigning the pathway to research independence |
| • Enhancing flexibility in training along clinical/translational/basic pathways with requisite “entry and exit ramps” to protect the trainee against time penalties |
| • Exploring opportunities for special trainee pathways (eg, medicine/pediatrics; critical care across the age spectrum; chronic disease management across the age spectrum) |
| • Fostering/expanding/supporting mentorship for research and career development |
| Research |
| • Enhancement of basic and clinical research mentorship |
| • Centers of Excellence creation/expansion for specialized training and broadened mentorship |
| • Establishment of an integrated longitudinal pathway for researcher development |
| • Exploration of public-private partnership opportunities for research support |
| • Creation of funding mechanisms that encourage established scientists to take risks and that support innovation |
| • Development of funding mechanisms to bridge established investigators when needed to maintain a science workforce |
| • Consideration of other ways to make the pursuit of translational or basic research a securer career option |
| Data collection and analyses |
| • Database creation and tracking of trainees in hematology |
| • Modeling of societal needs and trends |
| • Assessment of who should be partnering with whom and what data are critical |
| • Education of the public about how hematology care and research impact their health and well-being |
Conclusion
Research by investigators in hematology has been highly impactful for at least the last 35 years and shows evidence of being evermore impactful at the highest level over this same period. Despite this extraordinary record of sustained productivity, the number of NIH applications and awards in this discipline has declined by ∼70% over the last 13 years. To optimize hematologic research and training capabilities in the United States, the specialty needs to (1) broaden training capacities to incorporate new scientific expertise at the molecular, cellular, and computational level; (2) expand partnerships among government, academia, regulatory organizations, and professional organizations, most notably ASH, to create new flexibilities for certification that do not increase the length of training; (3) build the discipline’s strength in comparative effectiveness assessments; and (4) broaden its scope to include not only the traditional capabilities such as coagulation, diagnostics, and transfusion therapies but also new ones at the interface with other medical disciplines, such as critical care medicine, geriatrics, and obstetrics. These efforts need to be coupled with secure methods to support and retain productive hematology researchers in academic medical schools and at other research sites.
Acknowledgments
The authors thank NIH Division Director Michael S. Lauer for his portfolio analysis and Terrie Squadere of the NIH who provided assistance with manuscript preparation.
The opinions expressed in this manuscript are those of the authors and workshop participants. They should not be interpreted to represent the position of the NIH, the US Department of Health and Human Services, the federal government, or ASH. A list of workshop participants can be found in the Appendix.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.K.H. and D.M.D. organized and cochaired the workshops, analyzed the portfolio data, and wrote the manuscript; B.S.C. cochaired the workshops and provided input into the manuscript development, including writing and editing; and J.L.A. helped lead a workshop, wrote the manuscript, and provided ongoing editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: W. Keith Hoots, Division of Blood Diseases and Resources, National Heart, Lung, and Blood Institute, National Institutes of Health, 6701 Rockledge Drive, Room 9136, Bethesda, MD 20892-7950; e-mail: hootswk@nhlbi.nih.gov.
Appendix
The following is a list of workshop participants:
Charles S. Abrams; Hussein Ahmad; Gowthami Arepally; Janis L. Abkowitz; Thomas Abshire; James P. AuBuchon, FCAP; Mila N. Becker, Esq; Edward J. Benz; Nancy Berliner; R. Lorraine Brown, CPHP; Michael P. Busch; Andrew P. Cap, FACP; James F. Casella; Henry Chang; Alan R. Cohen; Barry S. Coller; Donna DiMichele; David Garcia; David Ginsburg; Navid Ghaffarzadegan; Simone Glynn; Jonathan Goldsmith; W. Keith Hoots; Armand Keating; Craig Kessler; Christian J. Ketchum; Andrei Kindzelski; Barbara Konkle; Andrew Leavitt; Stephanie Lee; Norma Lerner; Martha Liggett, Esq; Rebecca Link; Naomi L. C. Luban; Harvey Luksenburg; Steven E. McKenzie; Kathryn McLaughlin; Jeffery L. Miller; Phyllis Mitchell; Gisele Muller-Parker; Diane Nugent; Eugene P. Orringer; Mary-Elizabeth Percival; Charles Parker; Lisa C. Richardson; Rebekah S. Rasooly; Rita Sarkar; David T. Scadden; Alan N. Schechter; Gary Schiller; Susan B. Shurin; Gerald A. Soff; Mike Soucie; John Tisdale; Alexis A. Thompson; Clare J. Twist; Matthew Ulrickson; Gregory M. Vercellotti, FACP; Paul Wallace; Ronald Warren; Ellen Werner; Gilbert C. White II; David A. Williams; Ted Wun, FACP, FRCPA; Daniel G. Wright; Neal S. Young, MACP; and Shimian Zou.
References
- 1.Parker C, Hoots K, Keating A. If it’s a benign disease, why is the patient so sick? The Hematologist. 2012;9(5):2,7.
- 2.National Institutes of Health. Physician -Scientist Workforce Working Group Report. http://acd.od.nih.gov/reports/PSW_Report_ACD_06042014.pdf. Accessed June 2014. [Google Scholar]
- 3.American Academy of Arts and Sciences Committee on New Models for U.S. Science & Technology Policy. Restoring the Foundation: the Vital Role of Research in Preserving the American Dream. Available at https://www.amacad.org/restoringthefoundation. Accessed Fall 2014. [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, et al. PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camicia G, Pozner R, de Larrañaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42(4):286–294. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 6.Coller BS. The Hematologist discoverer. The Hematologist. 2013;10(6):7. [Google Scholar]
- 7.Coller BS. Translational research and the physician-scientist. In: Schafer AI, ed. The Vanishing Physician-Scientist? Ithaca, NY: Cornell University Press; 2009:67-83. [Google Scholar]
- 8.Goodman DC, Robertson RG. Accelerating physician workforce transformation through competitive graduate medical education funding. Health Aff (Millwood) 2013;32(11):1887–1892. doi: 10.1377/hlthaff.2013.0451. [DOI] [PubMed] [Google Scholar]
- 9.Larson RC, Ghaffarzadegan N, Xue Y. Too many PhD graduates or too few academic openings: the basic reproductive number R0 in academia. Syst Res Behav Sci. 2014;31(6):745-750. [DOI] [PMC free article] [PubMed]
- 10.Larson RC, Ghaffarzadegan N, Diaz MG. Magnified effects of changes in NIH research funding levels. Serv Sci. 2012;4(4):382–395. doi: 10.1287/serv.1120.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]