Abstract
Compared to humans, non-human primates have very little control over their vocal production. Nonetheless, some primates produce various call combinations, which may partially offset their lack of acoustic flexibility. A relevant example is male Campbell's monkeys (Cercopithecus campbelli), which give one call type (‘Krak’) to leopards, while the suffixed version of the same call stem (‘Krak-oo’) is given to unspecific danger. To test whether recipients attend to this suffixation pattern, we carried out a playback experiment in which we broadcast naturally and artificially modified suffixed and unsuffixed ‘Krak’ calls of male Campbell's monkeys to 42 wild groups of Diana monkeys (Cercopithecus diana diana). The two species form mixed-species groups and respond to each other's vocalizations. We analysed the vocal response of male and female Diana monkeys and overall found significantly stronger vocal responses to unsuffixed (leopard) than suffixed (unspecific danger) calls. Although the acoustic structure of the ‘Krak’ stem of the calls has some additional effects, subject responses were mainly determined by the presence or the absence of the suffix. This study indicates that suffixation is an evolved function in primate communication in contexts where adaptive responses are particularly important.
Keywords: alarm calls, syntax, field experiment, guenon
1. Introduction
Research on primate vocal behaviour continues to show surprising levels of complexity, both at the production and comprehension levels [1]. The predation context has been a particularly rewarding source for new findings, probably because individuals are under strong selective pressure to use communication signals efficiently to protect genetic relatives and other valuable group members [2–4]. In some species, natural selection has favoured the evolution of acoustically distinct alarm calls with call variants related to the type of predator, the degree of threat or the appropriate anti-predator behaviour. Evidence is not restricted to primates but also includes a range of other taxa, including birds [5–7], non-primate mammals (prairie dogs (Cynomys gunnisoni) [8], suricates [9]) and non-human primates (lemurs (Lemur catta) [10], Old World monkeys (Cercopithecoidae) [11–14], New World monkeys (Platyrrhini) [15–18], apes (Hominoidea) [19]). Although these findings have been interpreted in terms of potential parallels to human language, animal alarm call systems usually lack flexibility, arbitrariness in acoustic structure and generativity, indicating profound differences between animal communication and human language [20–22]. Instead, animal communication tends to be very limited in the amount of acoustic variation available to the signaller to interact with others.
However, recent research has shown that there is another level of complexity in animal communication, in that some species combine basic acoustic units into more complex vocal structures. Such combinatorial abilities may have evolved in some species to partially offset their lack of flexibility in generating acoustic variation. Many bird and some mammal species have been observed to combine vocal units to produce more complex sequences [23–25], which in primates has been associated with differences in ‘meanings’ [26–30]. A particularly interesting example is the Campbell's monkeys' (Cercopithecus campbelli) alarm call system. Here, adult males have a repertoire of three basic alarm calls (‘Krak’, ‘Hok’, ‘Wak’), which have been termed ‘call stems’, each of which can occur with an acoustically invariable ‘suffix’ (‘oo’) [31]. Here, we use the term ‘suffixation’ to refer to this phenomenon: the act of adding an acoustically invariable component to different call stems. In previous research, we have found that suffixation appears to broaden the call's ‘meaning’ by, for example, transforming highly specific alarm calls (‘Krak’), mainly given to leopards, to general alert calls (‘Krak-oo’), given to a wide range of events, including falling branches, interactions with neighbouring groups and other general disturbances [14,29,32].
The goal of this study is to test the ‘suffixation’ hypothesis experimentally, by testing whether the presence or the absence of the suffix ‘oo’ in Campbell's monkey calls causes relevant differences in behavioural responses. To this end, we focused on the recipients by carrying out playback experiments with Diana monkeys (Cercopithecus diana diana). Diana and Campbell's monkeys regularly form mixed-species associations [33], coordinate their travel directions and attend to each other's alarm calls [12,34–36]. Although testing other Campbell's monkey groups would have been the obvious choice, we opted for testing Diana monkeys, mainly to avoid confounding effects of territorial behaviour. For example, it is likely that playing back Campbell's monkey calls triggered hostile responses towards the presumed intruder rather than quantifiable responses to the subtle acoustic differences generated by suffixation [14,33].
We created playback stimuli that consisted of natural ‘Krak’ and ‘Krak-oo’ calls and the corresponding artificially altered calls, i.e. natural ‘Krak-oo’ calls with the ‘oo’ suffix deleted (artificial ‘Krak’ calls) and natural ‘Krak’ calls with an ‘oo’ suffix added (artificial ‘Krak-oo’ calls). We chose this design to rule out the possibility that there are subtle acoustic variations within the ‘Krak’ stem, depending on whether it was produced on its own or as part of a ‘Krak-oo’. All calls were recorded from local male Campbell's monkeys. We predicted that if suffixation is communicatively relevant, then other monkeys should react according to the presence or the absence of the suffix, regardless of the origin of the call stem. In particular, we predicted that the animals would give more alarm calls and fewer affiliative calls to playbacks of natural and artificially edited ‘Krak’ calls than to playbacks of natural and artificially edited ‘Krak-oo’ calls.
2. Results
(a). Call rates
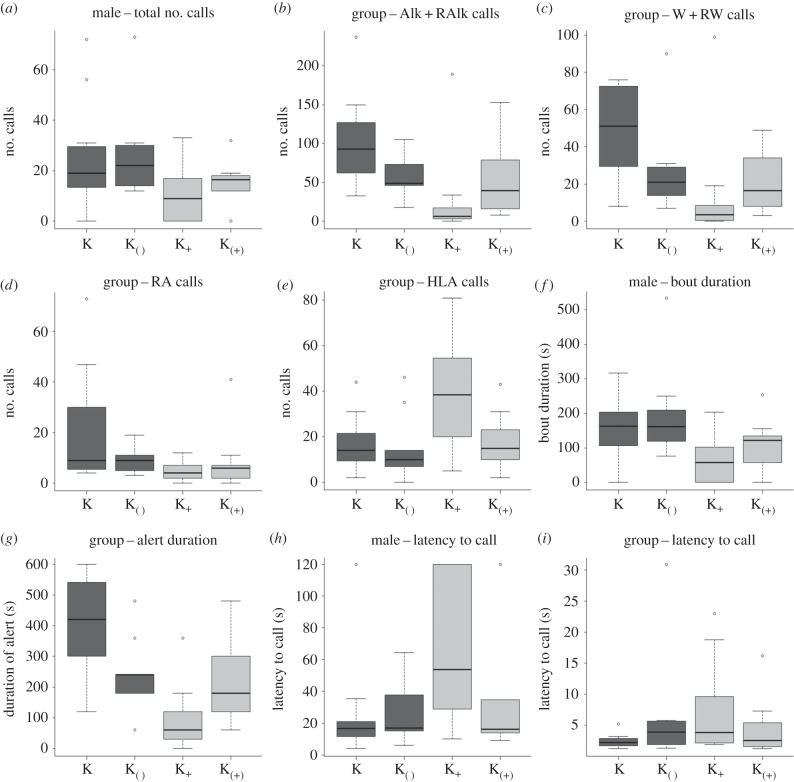
We tested 42 different groups of Diana monkeys with the four different playback conditions, i.e. natural ‘Krak’ (N = 11), natural ‘Krak-oo’ (N = 12), artificial ‘Krak’ (N = 9) and artificial ‘Krak-oo’ (N = 10). We analysed the number of calls given by Diana monkeys after each playback using a Generalised Linear Mixed Model (GLMM, model 1). As predicted, male Diana monkeys gave significantly more alarm calls after hearing ‘Krak’ calls (natural or artificial) than ‘Krak-oo’ calls (natural or artificial; figure 1), while the acoustic structure of the ‘Krak’ stem had no significant impact (table 1). Diana monkey females gave more alarm calls and fewer social calls after hearing ‘Krak’ than ‘Krak-oo’ calls (natural or artificial; figure 1), but we also found that the acoustic structure of the ‘Krak’ stem had an additional impact. We thus carried out two more GLMMs (models 2 and 3) and compared the corrected Akaike Information Criterion (AICc) values obtained for the two models. The difference between AICc values was greater than 2 for all variables, and the lower AICc value was obtained systematically if the model included ‘suffix’ as the only fixed factor (table 1). This indicates that the presence of the suffix was the main factor to explain female call rates (see §4e for more details).
Figure 1.
Median and inter-quartile range in the four experimental conditions natural ‘Krak’ (K, N = 11), artificial ‘Krak’ (K( ), N = 9), natural ‘Krak-oo’ (K+, N = 12) and artificial ‘Krak-oo’ (K(+), N = 10) for each variable studied. (a–e) The number of calls given, respectively, by the male (a) and by the group with (b) ‘Alk’ alarm call units, given alone and combined with an R unit, (c) ‘W’ alarm call units, given alone and combined with an R unit, (d) number of ‘RA’ alert calls given (combination of ‘R’ and ‘A’ call units) and (e) sum of three positive social call units and combinations between them (i.e. H, L, A call units and HA and LA calls). Plots (f) and (g) show the duration of alarm, respectively, for the male (f) and the group (g). Finally, (h) and (i) show latency to give first call, respectively, for the male (h) and the group (i).
Table 1.
Results of the statistical analysis. (a) Results of the GLMM and of the Δ(AICc) analysis for each number of calls given by the subjects. (b) Results of the LMM and of the Δ(AICc) analysis for males' bout duration, groups' alert duration and for males' and groups' latency to give first call. Tables show χ²- and p-values from the first model (i.e. GLMM-1 or LMM-1) for each of the two fixed factors included in the model (i.e. origin of the ‘Krak’ stem and presence of an ‘oo’ suffix). Significant p-values (under 0.05) are in bold. Tables also show the AICc values of the second and third models and the absolute value of the subtraction between these two AICcs: |Δ(AICc)|. The lower AICc value, which corresponds to the main parameter explaining the results, is in bold.
| ‘Krak’ stem |
suffixation |
|||||||
|---|---|---|---|---|---|---|---|---|
| (a) emitter | call type | χ² | p-value | χ² | p-value | AICc ‘stem’ | AICc ‘suffix’ | |Δ(AICc)| |
| males | alarm | 2.87 | >0.05 | 82.85 | <0.0001 | — | — | — |
| females | Alk + RAlk | 219.09 | <0.0001 | 312.25 | <0.0001 | 1817.49 | 1718.86 | 98.63 |
| W + RW | 71.53 | <0.0001 | 167.03 | <0.0001 | 1114.24 | 1008.95 | 105.29 | |
| RA | 50.87 | <0.0001 | 66.61 | <0.0001 | 614.80 | 596.98 | 17.82 | |
| HLA | 44.00 | <0.0001 | 52.87 | <0.0001 | 736.08 | 722.16 | 13.92 | |
| ‘Krak’ stem |
suffixation |
|||||||
|---|---|---|---|---|---|---|---|---|
| (b) emitter | variable | χ² | p-value | χ² | p-value | AICc ‘stem’ | AICc ‘suffix’ | |Δ(AICc)| |
| males | bout duration | 0.028 | >0.05 | 10.13 | <0.01 | — | — | — |
| latency to call | 1.86 | >0.05 | 6.45 | <0.05 | — | — | — | |
| females | ‘alert’ duration | 12.04 | <0.001 | 21.32 | <0.0001 | 549.84 | 543.26 | 6.58 |
| latency to call | 3.32 | >0.05 | 0.49 | >0.05 | — | — | — | |
(b). Calling durations
We compared the duration of the males' alarm calling and the rest of the groups' alert calling across conditions using Linear Mixed Models (LMMs). As predicted, playbacks of ‘Krak’ calls elicited longer responses in both measures than ‘Krak-oo’ calls, regardless of whether they were natural or artificial (figure 1). In our models, male alarm call duration was significantly explained by the presence of the suffix alone, while the groups' alert call duration was explained by both suffixation and the structure of the ‘Krak’ stem (table 1). As before, we compared two more LMM models (models 2 and 3). Again, the difference between their AICcs was more than 2, which showed that the model with the lower AICc—corresponding to the third model (with suffixation only)—contained the factor having the main impact on the monkeys' behaviour. This hence indicated that the presence of suffix was the main factor to drive alert duration (table 1).
(c). Latencies to first calls
Finally, we analysed the males' and the groups' latencies to give first calls (figure 1h,i). Here again, suffixation was the only significant factor to explain the males' latency to call, but for the groups' latencies to call, we found no significant effects (table 1).
3. Discussion
With this study, we demonstrated experimentally that suffixation is a salient acoustic feature in Campbell's monkey vocal communication. As predicted, Diana monkeys reacted more strongly to ‘Krak’ calls (usually indicating leopard presence) than to ‘Krak-oo’ calls (indicating a general threat). Diana monkeys consistently produced more alarm and fewer social calls, gave their first call earlier, called and remained vigilant for longer after hearing unsuffixed -‘Krak’- calls (natural or artificial) than suffixed -‘Krak-oo’- calls (natural or artificial, figure 1). Overall, the presence or the absence of the suffix was the only parameter that had a systematic and sustained effect on Diana monkey responses, suggesting that the ‘oo’ suffix is communicatively relevant in that ‘Krak-oo’ calls are a combination of a ‘Krak’ stem with an ‘oo’ suffix.
These findings are novel because previous animal communication studies have only reported combinatorial abilities at the sequence level. Although there are a few examples of combinatorial phenomena at the call unit level [37,38] we are not aware of any study that has investigated experimentally whether this is communicatively relevant to recipients [39]. The only comparable studies with non-human primates have focused on discrimination and categorization abilities of grammatical rules in human speech or artificial grammars [40–42], but never as part of the animals' own natural communication systems. Our study thus demonstrates experimentally that suffixation can be communicatively relevant in the natural vocal communication of free-ranging, untrained animals in biologically relevant contexts.
Reactions to natural and artificial ‘Krak’ calls were more similar to each other than reactions to natural and artificial ‘Krak-oo’ calls, perhaps because artificially adding ‘oo’ parts to existing ‘Krak’ calls was technically more challenging than deleting the ‘oo’ from ‘Krak-oo’ calls. This may have led to less naturally sounding stimuli for artificial ‘Krak-oo’ than ‘Krak’ calls, a difference that may have been perceived by the Diana monkeys. Although suffixation had the strongest effect on the monkeys' behaviour, the acoustic structure of the ‘Krak’ stem (i.e. whether playback stimuli were created from natural ‘Kraks’ or natural ‘Krak-oo’ calls) also had a significant impact on some female response variables (table 1). It is also clear that the presence of a leopard (a reliable trigger of male ‘Krak’ calls) represents a different psychological experience from hearing the sounds of a falling tree (a reliable trigger of male ‘Krak-oo’ calls). These differences in perceived danger and urgency appear to have left acoustic traces in the calls' structure, a mechanism suggested by several authors [31,43,44]. Our results demonstrate that Diana monkeys perceived these subtle acoustic differences in the ‘Krak’ stem, although they relied more on the presence or the absence of the suffix in their responses (figure 1).
How exactly such findings should be interpreted, especially what types of internal states are involved in callers and recipients, is the topic of an ongoing debate [45–51]. Some authors prefer to invoke notions related to human-like emotions, while others offer more cognitive interpretations. For example, one prominent theory proposes that the calls' acoustic structure directly affects recipient arousal, without much intervening processing [52]. Another view is that monkeys form associations between acoustic structures and the corresponding external events that trigger them, to the effect that acoustic structures become carriers of meaning [53]. A third view is that animals interpret acoustic information in relation to the current context, which is based on evidence that the same calls can trigger different reactions depending on the current context [54,55].
We are not able to contribute much to this discussion with our current data. On the one hand, previous studies with Campbell's monkeys have shown a direct correlation between acoustic structure and the external events that triggered them, as well as adequate recipient responses to experimentally presented exemplars of calls [14,31,32,56] in line with a ‘semantic’ interpretation. On the other hand, some of the Campbell's monkey calls may contain specific acoustic features that have a direct impact on the recipients' nervous systems, as proposed by Owren & Rendall [52]. For instance, sharp onsets in alarm calls may enhance levels of internal arousal and thus trigger movement. In our case, this is a less likely explanation because although both ‘Krak’ and ‘Krak-oo’ calls share the sharp onset, only ‘Krak’ calls elicited strong behavioural reactions. In another study, ‘boom’ calls (a natural indicator of non-predatory contexts) were artificially added to Campbell's monkey alarm calls, which also had a significant effect on behavioural responses [14,26]. Nevertheless, what internal states, if any, are causally responsible for mediating between calls and reactions will need to be investigated by other, more targeted research.
This experiment also provides further evidence for complex interspecific communication, with Diana monkeys demonstrating surprising discriminative skills when exposed to the calls of another species. We consider it likely that similar interspecific communicative abilities are also present in other species, in line with the idea that polyspecific primate groups are more than mere assemblies of different groups to avoid predators but instead form supra-social organizations with animals interacting with each other on a daily basis as individuals [33,57]. So far, interspecific communication has been largely found in the predation context, in some cases between predator and prey. For example, Diana monkeys also distinguish between some of the calls of one of their predators, the chimpanzees [58], between the different alarm call types produced by sympatric putty-nosed monkeys [36] or between the alarm calls of different species of guinea fowl [54]. These perception abilities are most probably a consequence of the frequent associations of Diana monkeys with other primate species and observing predator–prey interactions in other species, suggesting that similar abilities exist in other primates.
Finally, the suffixation mechanism described here is unlikely to be an isolated phenomenon in primate communication. Related work on female Diana monkeys' vocal communication has shown that the contact calls of adult females also consist of acoustically distinct elements that are combined in structured ways with probable effects on the information they may convey [37,59]. In other work, female Campbell's monkeys were found to combine two social call units to convey information associated with arousal [44] and social bonds (affiliated females produce a second unit with similar frequency modulation shapes) [60]. In red-capped mangabeys (Cercocebus torquatus), both sexes produce context-specific combinations of call units in sex-specific ways, while contextually similar call types are produced in sequences, with length and complexity depending on the vocal activity of other group members [61]. Although these phenomena require more rigorous experimental testing, they suggest that affixation is a widely present feature of non-human primates' communication. The more general hypothesis is that vocal complexity (as seen in combinatorial systems) is the evolutionary outcome of social complexity [61–65], suggesting that similar phenomena should be found in other species with complex social demands, notably some of the great ape species.
Further research is needed to get a deeper understanding of these combinatorial mechanisms within different primate calls. For Campbell's monkeys, the observed vocal combinations effectively enlarge their vocal repertoire, despite these animals' limited articulatory control. Future research will have to focus on the differences in perceived meaning of the other combinations that have been found in natural communication, notably between ‘Hok’ and ‘Hok-oo’ and between ‘Wak’ and ‘Wak-oo’ calls, to determine whether suffixation consistently changes relatively specific messages to more general ones, as suggested by Ouattara et al. [13]. Findings will be of interest because they suggest that basic features of human speech, such as duality of patterning [66], can evolve independently in species that are not so closely related to humans.
4. Material and methods
(a). Study site and subjects
Field experiments were conducted between May and July 2013 in Taï National Park, Ivory Coast, the largest preserved tropical rainforest in West Africa. The experimenter (C. Coye) and her field assistant conducted playback tests on unhabituated free-ranging groups of Diana monkeys, living in a roughly 50 km² area surrounding the C.R.E station (Centre de recherche en écologie, 5°50′ N, 7°21′ W). Diana and Campbell's monkeys are arboreal forest primates that live in small groups of one adult male and several adult females (Diana: 7–13, Campbell: 4–7) with their offspring. The density is about 2.5 groups per km²; with home ranges of about 56.0 ha around the research station [33]. Although illegal, hunting has drastically decimated the population in other areas of the park. Diana and Campbell's monkeys form polyspecific associations on a daily basis, also with other sympatric primates [33]. Both male and female vocal repertoires are well described for both species [13,14,37,64,67].
(b). Playback stimuli
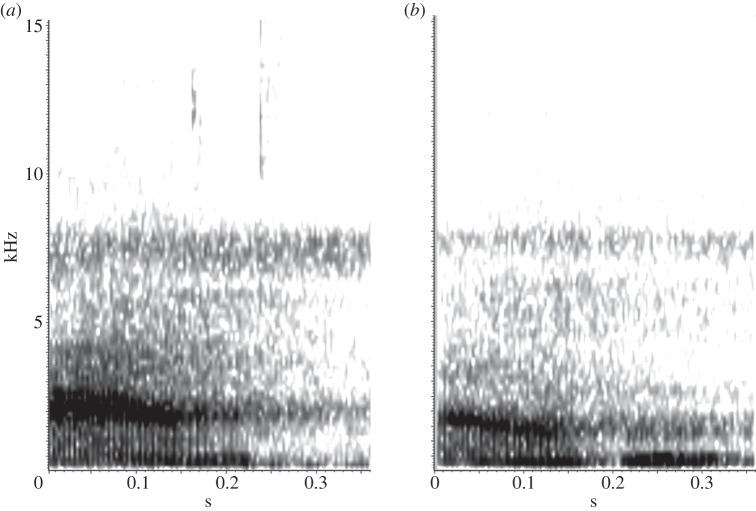
Structure of alarm calls may vary depending on the origin and identity of the caller [55,68,69] so we only used recordings from identified male Campbell's monkey from the general study area. Playback stimuli were edited from recordings made by K. Ouattara from two free-ranging Campbell's males in Taï National Park, using Raven Pro 1.5, and were selected on the basis of recording quality, from a dataset classified by acoustic analysis for a previous study [32]. Playback stimuli consisted of vocal sequences of 1 min (58.8 ± 0.95 s; mean ± s.e.) with inter-call durations of 3 s, reflecting the natural structure of vocal sequences in this species [29,69]. Each male contributed with one sequence per playback category, resulting in a total of eight sequences two natural ‘Krak’ call sequences, two natural ‘Krak-oo’ call sequences, two artificial ‘Krak’ call sequences (natural ‘Krak-oo’ from which the ‘oo’ suffix was deleted) and two artificial ‘Krak-oo’ call sequences (natural ‘Krak’ calls with an ‘oo’ suffix each added; figure 2). To ensure that subjects’ reactions are due to the presence or the absence of the ‘oo’ suffix, we created sequences by adding (artificial ‘Krak-oo’ sequences) or deleting (artificial ‘Krak’ sequences) ‘oo’ parts to the calls used to create the sequences of natural stimuli. All ‘oo’ suffixes added came from natural ‘Krak-oo’ calls from the same males. The calls were processed with a low-pass filter to remove high-frequency background noise (above 16 kHz, above the frequency range of the male calls, figure 2). Calls were amplified to obtain a naturalistic intensity of around 90 db at 1 m from the speaker.
Figure 2.
Spectrographic representation of (a) ‘Krak’ and (b) ‘Krak-oo’ calls. Black circle: ‘oo’ suffix.
(c). Experimental protocol
Thirteen trials were conducted in a random order for each stimulus category, with never more than four trials per day. None of the Diana monkey groups studied were habituated to human presence and the exact locations of their home ranges were unknown. To avoid retesting the same groups twice in short succession, the GPS position was recorded using a Garmin map-62 after each trial, and we subsequently did not test any Diana monkey group in an area of 1 km² (twice the average home range size) around the location of the experiment for at least one month. Each stimulus category was never played more than once at the same location.
For each trial, the experimenters searched for a Diana monkey group by listening for their contact calls. The playback and recording equipment were then silently positioned at 1.7 m above ground, 25–50 m away from the group, ensuring that the monkeys remained unaware of the experimenters' presence. Unhabituated Diana monkeys produce alarm calls to humans and sometimes approach and stare at observers, so detection is easily recognized. Playback stimuli were broadcast with a Philips GoGear Vibe player connected to a Nagra DSM speaker/amplifier and a Bose 151 Environmental speaker. Recording equipment consisted of a Sennheiser K6/ME66 directional microphone and a Marantz PMD660 solid-state recorder (sampling rate 44.1 kHz, resolution 16 bits, WAV sound format). Before each stimulus presentation, the experimenters waited at least 15 min to ensure that the male had not produced any loud calls and that the group had not noticed our presence, otherwise the trial was discarded.
(d). Dependent variables
The vocal response of the study group was recorded and analysed for both the adult male and the females with their offspring. Diana monkeys show strong sexual dimorphism in vocal behaviour; the calls of the adult males are very different from calls given by the females and immature group members [67]. Hence, we analysed separately male alarm calls—taking into account the total call bout given—and the group's call rates. The latter were analysed for 5 min following the start of each playback since previous work has shown that, after this time, individuals have usually returned to their baseline call rates, regardless of stimulus type [34].
We counted the total number of alarm calls given by the adult male, and the total number of calls given by the group, classified as four ‘social’ call units (H, L, R, A) and two ‘alarm’ call units (Alk, W) [37] (see the figure showing vocal repertoire of female Diana monkeys in the electronic supplementary material). Female alarm call units are given only to disturbances but never in peaceful contexts (C. Coye 2013, unpublished data). The six basic call units can be combined into five combined call types (HA and LA social positive calls, RA alert calls, RAlk and RW alarm calls) [37]. ‘Social’ call units are part of calls given in affiliative and peaceful situations (H, L, A). To obtain reasonable sample sizes while respecting biological saliency, we discriminated the following call types and units: Alk call units combined or not to an R call (hence forming the ‘Alk + RAlk’ alarm group), W call units combined or not to an R call (‘W + RW’ alarm group), R and RA alert calls (lumped together under the name RA in this analysis) and lumped all social calls (H, L, A and combinations between them) into one group, which led to the following sample sizes: NAlk+RALK = 2488, NW+RW = 1136, NRA = 458, NHLA = 973. For each trial, we also recorded the group's latency to give their first call. All groups responded with calls to the playback stimuli. Finally, we measured the time spent in ‘alert’ by the group, defined as when more than five alarm units or calls (Alk, W, RAlk, RW or RA) were produced over 30 s.
For the males, we measured the total duration of each call bout (time between the first and last calls); when a male did not call, a call bout duration equal to zero was attributed. Finally, we measured the latency to give the first call. In some trials (N = 7), the male did not call, in which case we assigned a dummy latency of 128.8 s, corresponding to twice the maximum observed latency to call for all males.
(e). Statistical analysis
We considered each playback as an independent event. Among the 52 playback trials performed, 10 were excluded owing to equipment failure or because of early detection of the experimenters or the equipment, which generated a final sample size of N = 11 natural ‘Krak’ [K], N = 12 natural ‘Krak-oo’ [K+], N = 9 artificial ‘Krak’ [K( )] and N = 10 artificial ‘Krak-oo’ [K(+)].
We tested the impact of both the origin of the ‘Krak’ part of calls (taken either from a ‘Krak’ or from a ‘Krak-oo’ call) and the presence of an ‘oo’ suffix in the calls, for each variable described. To this end, we used a generalised linear mixed model (GLMM) with a Poisson distribution and a log link or a linear mixed model (LMM) with a Gaussian distribution and an identity link, using the glmer( ) and the lmer( ) functions from the ‘lme4’ R package, respectively. We systematically used GLMMs to analyse the number of calls produced, and LMMs to analyse the duration of calling and alert as well as the latency to give the first call (separately for the adult male and the rest of the group).
For both GLMM and LMM, we included the origin of the ‘Krak’ stem (i.e. taken from a natural ‘Krak’ or from a ‘Krak-oo’ call) and the presence of an ‘oo’ suffix as crossed fixed factors. The identity of the Campbell's monkey call producer was entered as a random factor (two males). Then, we performed an analysis of variance (ANOVA), using the ANOVA( ) function from the ‘car’ R package, running type II Wald χ²-tests to study the effect of the fixed factors.
In some analyses, both the origin of the ‘Krak’ stem and the presence of the suffix had a significant impact. To compare the relative influence of these two factors, we carried out two additional GLMMs (distribution: Poisson, link: log) and LMMs (distribution: Gaussian, link: identity), using the glmer( ) and lmer( ) functions of the ‘lme4’ R package. All models included caller identity as a random factor but only one of the two possible fixed factors: either the origin of ‘Krak’ stem or the presence of suffix. We then compared the respective corrected AICc for both models and considered the one with the lower AICc to be significantly more accurate, provided the absolute value of the difference between the two AICc (i.e. |Δ(AICc)|) was greater than two [70,71]. All statistical tests were computed with R v. 3.0.2.
Supplementary Material
Acknowledgements
In Côte d'Ivoire, we thank the Minister of Scientific Research and the ‘Office Ivoirien des Parcs et Réserves' (OIPR) for permission to conduct research in Taï National Park. We thank A. Bitty and the Centre Suisse de Recherches Scientifiques for logistic support, our field assistants B. Diero and F. Gnepa for their invaluable help with data collection, the Taï Chimpanzee Project (TCP) and the ‘Centre de Recherche en Ecologie' (CRE) for their support in the field. We are very grateful for comments and fruitful discussions from P. Le Gouar, M. Hervé, C. Rochais, H. Thielges and D. Kremers.
Ethics statement
The study has been conducted in accordance with the current laws in France, in Scotland and in Ivory Coast and has been approved by the University of St Andrews (School of Psychology) ethics committee and by the Ivorian Office of Parks and Reserves.
Data accessibility
The dataset supporting this article can be downloaded from the University of Rennes 1, Home document repository: https://ecm.univ-rennes1.fr/nuxeo/nxdoc/default/1b1b9b09-5dea-48d4-a071-ec236297db27/view_documents. All statistical tests were computed using the R software which can be downloaded at http://cran.r-project.org/bin/windows/base/.
Funding statement
Research has been funded by the French Ministry of Research, Institut Universitaire de France, ANR ‘Orilang’ and the European Union's Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement no. 283871.
Authors' contributions
All authors contributed to this work equally: A.L. and K.Z. gave financial support to this project; all authors developed the concept and designed experiments. K.Z., K.O. and C.C. organized the field mission to Ivory Coast, C.C. and K.O. performed the experiment and collected the data and stimuli. All authors worked on data analysis and prepared the manuscript.
References
- 1.Lemasson A, Ouattara K, Zuberbühler K. 2013. Exploring the gaps between primate calls and human language. In The evolutionary emergence of language: evidence and inference (eds Botha Rudolf, Everaert Martin.), pp. 181–203. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Macedonia JM, Evans CS. 1993. Essay on contemporary issues in ethology: variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 93, 177–197. ( 10.1111/j.1439-0310.1993.tb00988.x) [DOI] [Google Scholar]
- 3.Zuberbühler K, Jenny D. 2002. Leopard predation and primate evolution. J. Hum. Evol. 43, 873–886. ( 10.1006/jhev.2002.0605) [DOI] [PubMed] [Google Scholar]
- 4.Stephan C, Zuberbühler K. 2008. Predation increases acoustic complexity in primate alarm calls. Biol. Lett. 4, 641–644. ( 10.1098/rsbl.2008.0488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Templeton CN, Greene E, Davis K. 2005. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937. ( 10.1126/science.1108841) [DOI] [PubMed] [Google Scholar]
- 6.Suzuki TN. 2014. Communication about predator type by a bird using discrete, graded and combinatorial variation in alarm calls. Anim. Behav. 87, 59–65. ( 10.1016/j.anbehav.2013.10.009) [DOI] [Google Scholar]
- 7.Courter JR, Ritchison G. 2010. Alarm calls of tufted titmice convey information about predator size and threat. Behav. Ecol. 21, 936–942. ( 10.1093/beheco/arq086) [DOI] [Google Scholar]
- 8.Slobodchikoff CN, Kiriazis J, Fischer C, Creef E. 1991. Semantic information distinguishing individual predators in the alarm calls of Gunnison's prairie dogs. Anim. Behav. 42, 713–719. ( 10.1016/S0003-3472(05)80117-4) [DOI] [Google Scholar]
- 9.Manser MB. 2001. The acoustic structure of suricates' alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. Lond. B 268, 2315–2324. ( 10.1098/rspb.2001.1773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira ME, Macedonia JM. 1991. Ringtailed lemur anti-predator calls denote predator class, not response urgency. Anim. Behav. 41, 543–544. ( 10.1016/S0003-3472(05)80861-9) [DOI] [Google Scholar]
- 11.Seyfarth RM, Cheney DL, Marler P. 1980. Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim. Behav. 28, 1070–1094. ( 10.1016/S0003-3472(80)80097-2) [DOI] [Google Scholar]
- 12.Zuberbühler K. 2000. Referential labelling in Diana monkeys. Anim. Behav. 59, 917–927. ( 10.1006/anbe.1999.1317) [DOI] [PubMed] [Google Scholar]
- 13.Ouattara K, Zuberbühler K, N'goran EK, Gombert J-E, Lemasson A. 2009. The alarm call system of female Campbell's monkeys. Anim. Behav. 78, 35–44. ( 10.1016/j.anbehav.2009.03.014) [DOI] [Google Scholar]
- 14.Ouattara K, Lemasson A, Zuberbühler K. 2009. Campbell's monkeys concatenate vocalizations into context-specific call sequences. Proc. Natl Acad. Sci. USA 106, 22 026–22 031. ( 10.1073/pnas.0908118106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirchhof J, Hammerschmidt K. 2006. Functionally referential alarm calls in tamarins (Saguinus fuscicollis and Saguinus mystax)—evidence from playback experiments. Ethology 112, 346–354. ( 10.1111/j.1439-0310.2006.01165.x) [DOI] [Google Scholar]
- 16.Wheeler BC. 2010. Production and perception of situationally variable alarm calls in wild tufted capuchin monkeys (Cebus apella nigritus). Behav. Ecol. Sociobiol. 64, 989–1000. ( 10.1007/s00265-010-0914-3) [DOI] [Google Scholar]
- 17.Caesar C, Zuberbühler K. 2012. Referential alarm calling behaviour in New World primates. Curr. Zool. 58, 680–697. [Google Scholar]
- 18.Cäsar C, Byrne R, Young RJ, Zuberbühler K. 2012. The alarm call system of wild black-fronted titi monkeys, Callicebus nigrifrons. Behav. Ecol. Sociobiol. 66, 653–667. ( 10.1007/s00265-011-1313-0) [DOI] [Google Scholar]
- 19.Slocombe KE, Zuberbühler K. 2005. Functionally referential communication in a chimpanzee. Curr. Biol. 15, 1779–1784. ( 10.1016/j.cub.2005.08.068) [DOI] [PubMed] [Google Scholar]
- 20.Hauser MD, Chomsky N, Fitch WT. 2002. The faculty of language: what is it, who has it, and how did it evolve? Science 298, 1569–1579. ( 10.1126/science.298.5598.1569) [DOI] [PubMed] [Google Scholar]
- 21.Hammerschmidt K, Fischer J. 2008. Constraints in primate vocal production. In Evolution of communicative flexibility: complexity, creativity, and adaptability in human and animal communication (eds Oller DK, Griebel U.), pp. 93–119. Cambridge, MA: MIT. [Google Scholar]
- 22.Corballis MC. 2003. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behav. Brain Sci. 26, 199–208. ( 10.1017/S0140525X03000062) [DOI] [PubMed] [Google Scholar]
- 23.Kanwal JS, Matsumura S, Ohlemiller K, Suga N. 1994. Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J. Acoust. Soc. Am. 96, 1229–1254. ( 10.1121/1.410273) [DOI] [PubMed] [Google Scholar]
- 24.Clucas BA, Freeberg TM, Lucas JR. 2004. Chick-a-dee call syntax, social context, and season affect vocal responses of Carolina chickadees (Poecile carolinensis). Behav. Ecol. Sociobiol. 57, 187–196. ( 10.1007/s00265-004-0847-9) [DOI] [Google Scholar]
- 25.Riesch R, Ford JKB, Thomsen F. 2008. Whistle sequences in wild killer whales (Orcinus orca). J. Acoust. Soc. Am. 124, 1822–1829. ( 10.1121/1.2956467) [DOI] [PubMed] [Google Scholar]
- 26.Zuberbühler K. 2002. A syntactic rule in forest monkey communication. Anim. Behav. 63, 293–299. ( 10.1006/anbe.2001.1914) [DOI] [Google Scholar]
- 27.Arnold K, Zuberbühler K. 2006. The alarm-calling system of adult male putty-nosed monkeys, Cercopithecus nictitans martini. Anim. Behav. 72, 643–653. ( 10.1016/j.anbehav.2005.11.017) [DOI] [Google Scholar]
- 28.Clarke E, Reichard UH, Zuberbühler K. 2006. The syntax and meaning of wild gibbon songs. PLoS ONE 1, e73 ( 10.1371/journal.pone.0000073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouattara K, Lemasson A, Zuberbühler K. 2009. Anti-predator strategies of free-ranging Campbell's monkeys. Behaviour 146, 1687–1708. ( 10.1163/000579509X12469533725585) [DOI] [Google Scholar]
- 30.Clay Z, Zuberbühler K. 2011. Bonobos extract meaning from call sequences. PLoS ONE 6, e18786 ( 10.1371/journal.pone.0018786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keenan S, Lemasson A, Zuberbühler K. 2013. Graded or discrete? A quantitative analysis of Campbell's monkey alarm calls. Anim. Behav. 85, 109–118. ( 10.1016/j.anbehav.2012.10.014) [DOI] [Google Scholar]
- 32.Ouattara K, Lemasson A, Zuberbühler K. 2009. Campbell's monkeys use affixation to alter call meaning. PLoS ONE 4, e7808 ( 10.1371/journal.pone.0007808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGraw WS, Zuberbühler K, Noë R. 2007. Monkeys of the Tai forest: an African primate community. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Zuberbühler K. 2000. Interspecies semantic communication in two forest primates. Proc. R. Soc. Lond. B 267, 713–718. ( 10.1098/rspb.2000.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolters S, Zuberbühler K. 2003. Mixed-species associations of Diana and Campbell's monkeys: the costs and benefits of a forest phenomenon. Behaviour 140, 371–385. ( 10.1163/156853903321826684) [DOI] [Google Scholar]
- 36.Eckardt W, Zuberbühler K. 2004. Cooperation and competition in two forest monkeys. Behav. Ecol. 15, 400–411. ( 10.1093/beheco/arh032) [DOI] [Google Scholar]
- 37.Candiotti A, Zuberbühler K, Lemasson A. 2012. Context-related call combinations in female Diana monkeys. Anim. Cogn. 15, 327–339. ( 10.1007/s10071-011-0456-8) [DOI] [PubMed] [Google Scholar]
- 38.Bouchet H, Laporte M, Candiotti A, Lemasson A. 2014. Flexibilité vocale sous influences sociales chez les primates non-humains. Rev. Primatol. 5|2013, document 53. ( 10.4000/primatologie.1794) [DOI] [Google Scholar]
- 39.Zuberbühler K, Lemasson A. 2014. Primate communication: meaning from strings of calls. In Language and recursion (eds Lowenthal F, Lefebvre L.), pp. 115–125. New York, NY: Springer. [Google Scholar]
- 40.Fitch WT, Hauser MD. 2004. Computational constraints on syntactic processing in a nonhuman primate. Science 303, 377–380. ( 10.1126/science.1089401) [DOI] [PubMed] [Google Scholar]
- 41.Saffran J, Hauser M, Seibel R, Kapfhamer J, Tsao F, Cushman F. 2008. Grammatical pattern learning by human infants and cotton-top tamarin monkeys. Cognition 107, 479–500. ( 10.1016/j.cognition.2007.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endress AD, Cahill D, Block S, Watumull J, Hauser MD. 2009. Evidence of an evolutionary precursor to human language affixation in a non-human primate. Biol. Lett. 5, 749–751. ( 10.1098/rsbl.2009.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schehka S, Zimmermann E. 2009. Acoustic features to arousal and identity in disturbance calls of tree shrews (Tupaia belangeri). Behav. Brain Res. 203, 223–231. ( 10.1016/j.bbr.2009.05.007) [DOI] [PubMed] [Google Scholar]
- 44.Lemasson A, Remeuf K, Rossard A, Zimmermann E. 2012. Cross-taxa similarities in affect-induced changes of vocal behavior and voice in arboreal monkeys. PLoS ONE 7, e45106 ( 10.1371/journal.pone.0045106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hauser MD. 1996. The evolution of communication. Cambridge, MA: MIT Press. [Google Scholar]
- 46.Simões CS, et al. 2010. Activation of frontal neocortical areas by vocal production in marmosets. Front. Integr. Neurosci. 4, 123 ( 10.3389/fnint.2010.00123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coudé G, Ferrari PF, Rodà F, Maranesi M, Borelli E, Veroni V, Monti F, Rozzi S, Fogassi L. 2011. Neurons controlling voluntary vocalization in the macaque ventral premotor cortex. PLoS ONE 6, e26822 ( 10.1371/journal.pone.0026822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owren MJ, Amoss RT, Rendall D. 2011. Two organizing principles of vocal production: implications for nonhuman and human primates. Am. J. Primatol. 73, 530–544. ( 10.1002/ajp.20913) [DOI] [PubMed] [Google Scholar]
- 49.Wheeler BC, Fischer J. 2012. Functionally referential signals: a promising paradigm whose time has passed. Evol. Anthropol. Issues News Rev. 21, 195–205. ( 10.1002/evan.21319) [DOI] [PubMed] [Google Scholar]
- 50.Collier K, Bickel B, van Schaik CP, Manser MB, Townsend SW. 2014. Language evolution: syntax before phonology? Proc. R. Soc. B 281, 20140263 ( 10.1098/rspb.2014.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarantino A, Clay Z. 2015. Contextually variable signals can be functionally referential. Anim. Behav. 100, e1–e8. ( 10.1016/j.anbehav.2014.08.017) [DOI] [Google Scholar]
- 52.Owren MJ, Rendall D. 2001. Sound on the rebound: bringing form and function back to the forefront in understanding nonhuman primate vocal signaling. Evol. Anthropol. Issues News Rev. 10, 58–71. ( 10.1002/evan.1014) [DOI] [Google Scholar]
- 53.Seyfarth RM, Cheney DL. 2010. Production, usage, and comprehension in animal vocalizations. Brain Lang. 115, 92–100. ( 10.1016/j.bandl.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 54.Zuberbühler K. 2000. Causal cognition in a non-human primate: field playback experiments with Diana monkeys. Cognition 76, 195–207. ( 10.1016/S0010-0277(00)00079-2) [DOI] [PubMed] [Google Scholar]
- 55.Arnold K, Zuberbühler K. 2013. Female putty-nosed monkeys use experimentally altered contextual information to disambiguate the cause of male alarm calls. PLoS ONE 8, e65660 ( 10.1371/journal.pone.0065660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuberbühler K. 2001. Predator-specific alarm calls in Campbell's monkeys, Cercopithecus campbelli. Behav. Ecol. Sociobiol. 50, 414–422. ( 10.1007/s002650100383) [DOI] [Google Scholar]
- 57.Gautier J-P, Gautier-Hion A. 1983. Comportement vocal des males adultes et organisation supraspecifique dans les troupes polyspecifiques de cercopitheques. Folia Primatol. (Basel) 40, 161–174. ( 10.1159/000156097) [DOI] [PubMed] [Google Scholar]
- 58.Zuberbühler K. 2000. Causal knowledge of predators' behaviour in wild Diana monkeys. Anim. Behav. 59, 209–220. ( 10.1006/anbe.1999.1296) [DOI] [PubMed] [Google Scholar]
- 59.Candiotti A, Zuberbühler K, Lemasson A. 2012. Convergence and divergence in Diana monkey vocalizations. Biol. Lett. 8, 382–385. ( 10.1098/rsbl.2011.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lemasson A, Hausberger M. 2004. Patterns of vocal sharing and social dynamics in a captive group of Campbell's monkeys (Cercopithecus campbelli campbelli). J. Comp. Psychol. 118, 347–359. ( 10.1037/0735-7036.118.3.347) [DOI] [PubMed] [Google Scholar]
- 61.Bouchet H, Blois-Heulin C, Lemasson A. 2013. Social complexity parallels vocal complexity: a comparison of three non-human primate species. Front. Psychol. 4, 390 ( 10.3389/fpsyg.2013.00390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blumstein DP. 2003. Social complexity but not the acoustic environment is responsible for the evolution of complex alarm communication. In Adaptive strategies and diversity in marmots, pp 31–38. Lyon, France: International Network on Marmots. [Google Scholar]
- 63.Freeberg TM. 2006. Social complexity can drive vocal complexity group size influences vocal information in Carolina chickadees. Psychol. Sci. 17, 557–561. ( 10.1111/j.1467-9280.2006.01743.x) [DOI] [PubMed] [Google Scholar]
- 64.Lemasson A, Hausberger M. 2011. Acoustic variability and social significance of calls in female Campbell's monkeys (Cercopithecus campbelli campbelli). J. Acoust. Soc. Am. 129, 3341–3352. ( 10.1121/1.3569704) [DOI] [PubMed] [Google Scholar]
- 65.McComb K, Semple S. 2005. Coevolution of vocal communication and sociality in primates. Biol. Lett. 1, 381–385. ( 10.1098/rsbl.2005.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hockett C. 1960. The origin of speech. Sci. Am. 203, 88–111. ( 10.1038/scientificamerican0960-88) [DOI] [PubMed] [Google Scholar]
- 67.Zuberbühler K, Noë R, Seyfarth RM. 1997. Diana monkey long-distance calls: messages for conspecifics and predators. Anim. Behav. 53, 589–604. ( 10.1006/anbe.1996.0334) [DOI] [Google Scholar]
- 68.Schlenker P, Chemla E, Arnold K, Lemasson A, Ouattara K, Sumir K, Stephan C, Ryder R, Zuberbühler K. 2013. Monkey semantics: two ‘dialects' of Campbell's monkey alarm calls. Linguist. Philos. 37, 439–501. ( 10.1007/s10988-014-9155-7) [DOI] [Google Scholar]
- 69.Lemasson A, Ouattara K, Bouchet H, Zuberbühler K. 2010. Speed of call delivery is related to context and caller identity in Campbell's monkey males. Naturwissenschaften 97, 1023–1027. ( 10.1007/s00114-010-0715-6) [DOI] [PubMed] [Google Scholar]
- 70.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. Berlin, Germany: Springer. [Google Scholar]
- 71.Mazerolle MJ. 2004. Mouvements et reproduction des amphibiens en tourbières perturbées. PhD Thesis Laval, Quebec: Université de Laval. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article can be downloaded from the University of Rennes 1, Home document repository: https://ecm.univ-rennes1.fr/nuxeo/nxdoc/default/1b1b9b09-5dea-48d4-a071-ec236297db27/view_documents. All statistical tests were computed using the R software which can be downloaded at http://cran.r-project.org/bin/windows/base/.