Abstract
Zinc is essential for life, but toxic in excess. Thus all cells must control their internal zinc concentration. We used a systems approach, alternating rounds of experiments and models, to further elucidate the zinc control systems in Escherichia coli. We measured the response to zinc of the main specific zinc import and export systems in the wild-type, and a series of deletion mutant strains. We interpreted these data with a detailed mathematical model and Bayesian model fitting routines. There are three key findings: first, that alternate, non-inducible importers and exporters are important. Second, that an internal zinc reservoir is essential for maintaining the internal zinc concentration. Third, our data fitting led us to propose that the cells mount a heterogeneous response to zinc: some respond effectively, while others die or stop growing. In a further round of experiments, we demonstrated lower viable cell counts in the mutant strain tested exposed to excess zinc, consistent with this hypothesis. A stochastic model simulation demonstrated considerable fluctuations in the cellular levels of the ZntA exporter protein, reinforcing this proposal. We hypothesize that maintaining population heterogeneity could be a bet-hedging response allowing a population of cells to survive in varied and fluctuating environments.
Keywords: zinc homeostasis, Escherichia coli, mathematical model, bet hedging, statistical inference, stress response
1. Introduction
Zinc is an essential micronutrient for all forms of life and acts as a cofactor for all six Enzyme Commission classes [1–3]. However, at high levels, zinc is toxic to cells [4]. Thus the concentration of internal free zinc must be controlled.
In Escherichia coli, zinc can be imported by the high-affinity ABC-type zinc uptake system ZnuABC [5,6] which consists of three components: a periplasmic-binding protein, ZnuA; a membrane-spanning protein, ZnuB; and an ATPase, ZnuC [7]. The expression of znuABC is repressed by the zinc uptake regulator, Zur, which acts as a dimer, containing four zinc ions in its active repressor form [5,6,8]. In the presence of zinc, the active Zur dimer binds DNA at the znuABC promoter, competes with RNA polymerase for promoter occupancy, and consequently acts as a repressor.
Zinc can be exported by ZntA, a P-type ATPase [9]. Expression of zntA is activated by ZntR, a member of the MerR family of regulators [10,11]. In the absence of zinc, ZntR binds to DNA at the zntA promoter but does not activate expression of zntA. In the presence of zinc, ZntR is converted into a transcriptional activator, changing the DNA conformation of the zntA promoter leading to enhanced binding of RNA polymerase and transcriptional activation [12].
In addition to the high-affinity zinc uptake and export transporters ZnuABC and ZntA, E. coli possesses subsidiary zinc importers and exporters that exhibit lower affinities for zinc. ZupT, a member of the ZIP family of transporters [13] is a constitutively expressed importer that facilitates the uptake of a broad-range of metal ions with a slight preference for Zn2+ [14,15]. There are two other transporters that may participate in low-specificity zinc uptake: PitA, an inorganic phosphate transporter and MntH, an Mn2+/Fe2+ transporter of the Nramp superfamily [16,17]. Zinc export is also provided by the cation diffusion facilitator (CDF) ZitB. Transcription of zitB is directly inducible by zinc [18]. A further CDF transporter, YiiP has been implicated in zinc export, although its main substrate in vivo is Fe2+ [19,20].
Both Zur and ZntR manifest femtomolar sensitivity to zinc in vitro [8]. Therefore, it has been postulated that the concentration of internal free zinc is approximately femtomolar [21]. Subsequent measurements in vivo showed mean internal zinc concentrations mostly between 10 and 30 pM [22], although with considerable variability outside that range. In contrast, the total zinc quota in the cell has been reported by both groups as approximately 0.2 mM [8,22], approximately 2000 times higher than zinc concentration in low zinc media, and indicates efficient uptake and storage of zinc [8,22]. Much of the stored zinc is believed to be in ribosomes, in particular the ribosomal L31 protein [23–26]. Low molecular weight thiols also act as a zinc reservoir in Bacillus subtilis [27]. Therefore, we would expect that a zinc reservoir would play an important role in zinc dynamics and homeostasis.
To date, one mathematical model has been developed for the zinc regulatory system in E. coli [28]. The model was constructed to describe results from in vitro experiments analysing interactions between Zur and ZntR and DNA, and the induction of the znuABC and zntA promoters [8], and was successful in explaining these experimental data. However, this in vitro model does not consider zinc homeostasis in live cells, and so does not include in vivo processes, e.g. import and export of zinc through alternative transporters, or the binding of zinc to other proteins in the cell, accounting for the overwhelming majority of cytoplasmic zinc [8].
2. Aims of study
This study aims to improve our understanding of in vivo zinc homeostasis gene regulation, using an iterative ‘systems biology’ approach, consisting of alternating rounds of experimental and theoretical work. Our first aim was to identify the transcriptional responses of the main zinc transporters, ZnuABC and ZntA, to both genetic and chemical perturbations. Specifically, we have generated experimental data for the in vivo transcriptional activity of the znuC and zntA promoters in six strains: wild-type, ΔznuCB, ΔzntA, Δzur, ΔzntR and ΔznuCBΔzntA. Furthermore, we have quantified the responses of each of these promoters in each of the strains in detailed in vivo time course experiments following zinc stress.
Our second aim was to determine whether our existing knowledge of transcriptional regulation could explain the experimental data. We developed a new mathematical model for in vivo regulation of internal zinc levels by E. coli, using a set of ordinary differential equations (ODEs). We have integrated the model both with literature data and our newly derived experimental data using a Monte Carlo Markov chain approach [29,30]. This allowed us to evaluate model fits to the data and establish plausible ranges of parameter values, and so to evaluate the importance of alternative zinc transporter proteins and a zinc reservoir in explaining the available data on zinc regulation.
The process of fitting the mathematical model to the experimental data led to new questions that we had not anticipated. Specifically, we were forced to hypothesize that the higher levels of zinc stress that we used experimentally were partially toxic to the cells. This hypothesis was tested in a further round of experiments looking at the toxicity of zinc to E. coli cells at different zinc concentrations. This combination of model-driven hypothesis generation and experimental confirmation led to a further hypothesis that ZntA would exhibit heterogeneous expression. This was tested using a stochastic model of the system [31,32] that we simulated with the parameters obtained from the model fits to our experiments.
3. Results
3.1. Experimental characterization of transcription responses of zinc import and export proteins
Activities of the zinc-regulated znuC and zntA promoters were measured in the six strains studied: wild-type, ΔznuCB, ΔzntA, Δzur, ΔzntR and ΔznuCBΔzntA using a Lux reporter system (see Material and methods). The reporter was also tested on the hns promoter as zinc-independent control (electronic supplementary material, figure S1). Zinc concentration in the batch of LB used for these experiments was measured by ICP-MS as 12.2 μM. Excess zinc conditions include the addition of either 12.5 μM or 100 μM zinc to LB, giving total zinc concentrations of 24.7 μM and 112.2 μM, and are referred to as LB12.5 and LB100, respectively.
3.2. In vivo data in LB conditions
Altered promoter activity was observed in the strains studied under LB conditions (figure 1). The highest activity of the znuCB promoter (PznuCB) was seen in the Δzur strain, concordant with Zur being a repressor of znuABC expression [5]. Induction was also seen in the ΔznuCB strain, also expected, as less zinc import should lead to derepression of PznuC by Zur; a similar argument holds for the ΔznuCBΔzntA strain.
Figure 1.
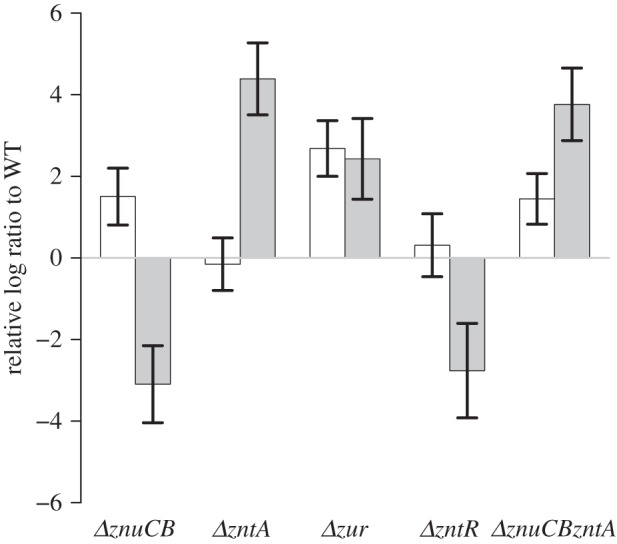
Induction of the PznuCB and PzntA in the five mutant strains relative to wild-type (log to base 2) in LB conditions. White and grey bars correspond to PznuCB and PzntA, respectively. Error bars indicate s.d.
The zntA promoter (PzntA) was strongly induced in the Δzur and ΔzntA strains. This is to be expected, as we would anticipate a rise in internal zinc concentrations as either zinc import by ZnuABC was derepressed or zinc export by ZntA was abolished. Expression of PzntA was greatly reduced in the ΔzntR strain, also expected as ZntR is the activator for zntA [10]. There is a similar reduction of zntA expression in the ΔznuCB strain, also expected, as there would be less zinc in the cell. The ΔznuCBΔzntA double mutant gave surprising results. A priori there are two possible outcomes: either the internal zinc concentration might decrease, as in the ΔznuCB strain, in which case we would expect PznuCB activity to increase and PzntA activity to decrease; or the internal zinc concentration might increase, as in the ΔzntA strain, in which case we would expect little change in PznuCB activity and an increase in PzntA activity. Instead, we observe increased activity of both promoters which appears to be incompatible with either scenario. The model provides an explanation for this phenomenon, as described below.
3.3. Time course of in vivo promoter activity following addition of zinc
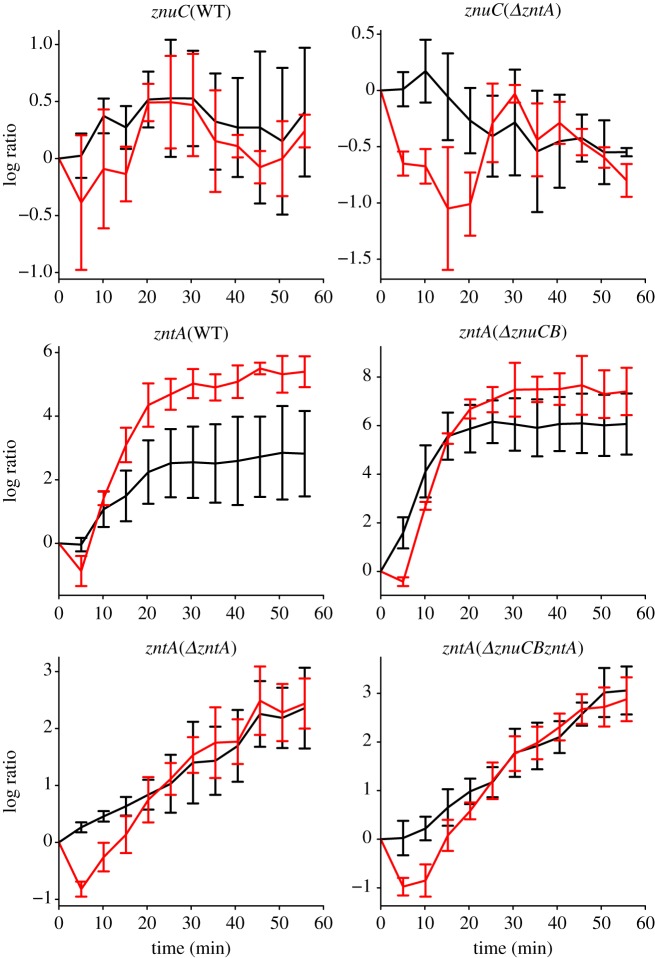
Figure 2 shows detailed time courses describing the activities of PznuCB and PzntA in the WT strain along with the most prominent responses in the mutant strains in LB12.5 and LB100; the less prominent responses are shown in electronic supplementary material, figure S2. In the WT, PznuCB shows little change in either condition, whereas PzntA shows marked increases in expression in both LB12.5 and LB100. A common feature of these and other responses is an initial decrease in induction in LB100. The induction of PzntA in LB100 (WT) and both LB12.5 and LB100 (ΔznuCB strain) were much larger (more than 4 [log2 ratio]) and faster (less than 30 min) than in the ΔzntA and ΔznuCBΔzntA strains in both LB12.5 and LB100. Although the induction of PzntA in the WT and ΔznuCB strains plateaued after 30 min following addition of zinc, the induction of PzntA in the ΔzntA and ΔznuCBΔzntA strains continued after addition of zinc until 50 min. Furthermore, in the ΔzntA strain, PznuCB showed a small decrease in induction. These observations suggest that there may be mechanisms to import external zinc into cells without ZnuABC and that ZntA is important to maintain the steady level of free zinc in E. coli cells. Interestingly, while the WT strain shows a clear difference in induction under the two concentrations of added zinc, in the mutant strains, the level of induction is similar in LB12.5 and LB100 (apart from the initial dip in promoter activity under the higher zinc concentration). The responses of the induced promoters are also considerably stronger than those observed in the hns promoter under LB100 conditions (electronic supplementary material, figure S1b) and so can be attributed to zinc-associated transcriptional change.
Figure 2.
Time series of induction of the znuC and zntA promoters following addition of 12.5 μM Zn2+ (black lines) and 100 μM Zn2+ (red lines), log to base 2 ratios normalized to the value at t = 0. Plots show the wild-type responses and selected responses from other strains that show the most marked changes in expression (the remaining responses are plotted in electronic supplementary material, figure S1). Note that in all of the plots, there is an initial decrease in the reporter expression under 100 μM zinc conditions: we propose that this results from the partial lethality of these conditions. Note also that the level of induction of PzntA in the WT strain is different under the two different conditions, whereas in the other strains, the level of induction reaches a similar level once the initial dip is reversed. Error bars indicate s.d.
3.4. A new mathematical model for in vivo zinc transport and homeostasis
We developed a mathematical model that describes the molecular processes of zinc homeostasis, in order to explain these experimental data along with literature data (figure 3). There are six variables: the concentration of ZnuABC (P1), the concentration of ZntA (P2), the concentration of active (zinc-bound) Zur (X), the concentration of active (zinc-bound) ZntR (Y), the concentration of zinc bound to ‘reservoir’ molecules, i.e. any other zinc-binding molecules in the cell (R) and the concentration of free (ionic) zinc in the cytoplasm (z). The equations are
| 3.1 |
| 3.2 |
| 3.3 |
| 3.4 |
| 3.5 |
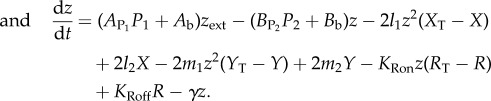 |
3.6 |
Figure 3.
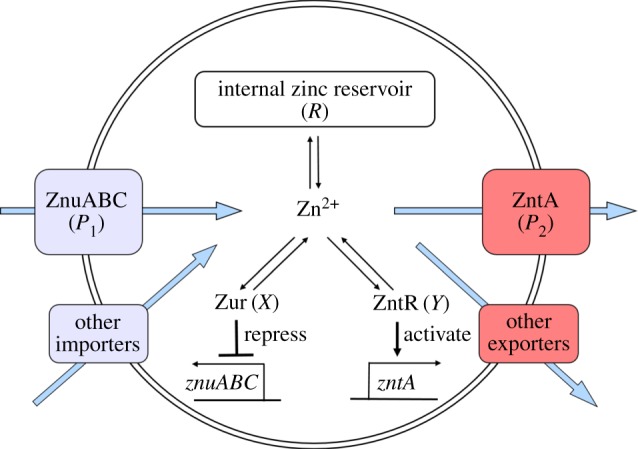
Cartoon of the mathematical model for the zinc regulation system in E. coli. Zinc import is both through the Zur-repressed ZnuABC system and through other importers. Zinc export is both through the ZntR-activated ZntA system and through other exporters. Ionic zinc can be bound and be released from the internal zinc reservoir; it also can bind to the Zur and ZntR proteins leading to their active forms as transcription factors.
ZnuABC is produced at maximal rate β1 and is repressed by active Zur with Michaelis constant K1 (equation (3.1)). ZnuABC and all other modelled components are diluted due to cell growth at rate γ; the six strains examined could potentially grow at different rates so the value of γ is strain-dependent. ZntA is produced at a basal rate r2 and is activated by active ZntR with a constant of proportionality β2 (equation (3.2)). In the equation for active Zur, XT represents the total amount of Zur in the system (assumed to be constant), and so inactive Zur is given by XT − X. The binding of two zinc ions is required to convert inactive Zur to active Zur [6], at rate l1. The active form can also revert to the inactive form at rate l2 (equation (3.3)). Similarly, the binding of two zinc ions is required to convert inactive ZntR to active ZntR [10,12,33] at rate m1 and the reversion of active ZntR to its inactive form happens at rate m2 (equation (3.4)). In the equation for the zinc reservoir (equation (3.5)), the total reservoir size is RT and so RT − R represents the number of available zinc-binding sites. Zinc binds to free reservoir binding sites with mass action kinetics with rate KRon and dissociates with rate KRoff. The reservoir molecules are replenished to balance dilution due to cell growth so that the overall concentration of reservoir molecules remains constant. Import of ionic zinc is proportional to the concentration of external zinc (equation (3.6)), with basal rate Ab and linear dependence on ZnuABC with parameter AP1. Export is proportional to internal free zinc, with basal rate Bb and linear dependence on ZntA with parameter BP2. The remaining terms in equation (3.6), for the interactions of zinc ions with Zur, ZntR and the reservoir, have already been described.
3.5. Model fitting to data
Central to this work is the fitting of the mathematical model to the experimental data. This has been accomplished using the Metropolis–Hastings algorithm for parameter inference (see Material and methods). Convergence plots from the simulations and posterior distributions for all parameters are shown in electronic supplementary material, figure S3. Point estimates and ranges for all parameter values are shown in electronic supplementary material, table S1.
3.6. The model fits published in vitro data and data under LB conditions
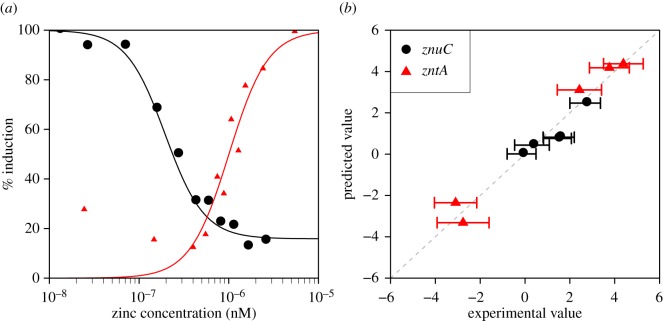
The model fit to the published zinc induction data [8] is good (figure 4a) and comparable with the fit of the previously published zinc model [28]. The model fit to our data under LB conditions is also excellent (figure 4b). In accordance with the experimental data, the model predicts that the highest induction of PznuCB was seen in the Δzur strain. Induction was also seen in the ΔznuCB and ΔznuCBΔzntA strains. Similarly, there is condordance between the experimental data and the model for PzntA, with strong induction in the ΔzntA, Δzur and ΔznuCBΔzntA strains, and strong repression in the ΔznuCB and ΔzntR strains.
Figure 4.
(a) Model fit to promoter induction data from [8] for the znuC and zntA promoters. The fit to data is extremely close and comparable to fits from previously published work [28]. (b) Model fit to the differential gene expression of the two promoters in each of the five mutant strains (log to base 2) that was reported in figure 1. There is an extremely strong concordance between the experimental data and the model values.
Interestingly, the model predicts a reasonable fit to the increased induction of both PznuCB and PzntA in the double mutant: the experimentally observed fold differences relative to the WT are 2.7 and 13.6 for the two promoters, respectively, while the model fitted fold differences are 1.7 and 18.1. The fit is achieved because the growth rate of the double mutant is predicted to be lower than that of the WT (doubling times of 110 and 64 min, respectively) leading to an apparent increase in protein expression (and by association Lux protein expression). If instead one compares only the protein synthesis terms of equations (3.1) and (3.2), it is found that PznuCB has almost the same induction (0.99-fold increased), while PzntA is 10.6-fold increased. Thus the double mutant is behaving similarly to the zntA mutant, with little change in Zur repression and substantial increase in ZntR activation.
The steady-state concentration of free zinc of WT cells in LB predicted by the model is 2.9 pM (calculated from equation (3.6) with parameters from electronic supplementary material, table S1); this is in closer accordance with the measurements of Wang et al. [22] than the predictions of Outten & O'Halloran [8]. This concentration is a dynamic equilibrium, with high predicted flux of 44 nMs−1 through the free zinc compartment. Most of this flux is a balance between zinc ions imported into the cells and absorption of those ions into the zinc reservoir.
3.7. The model can fit LB12.5 zinc induction in vivo time course data
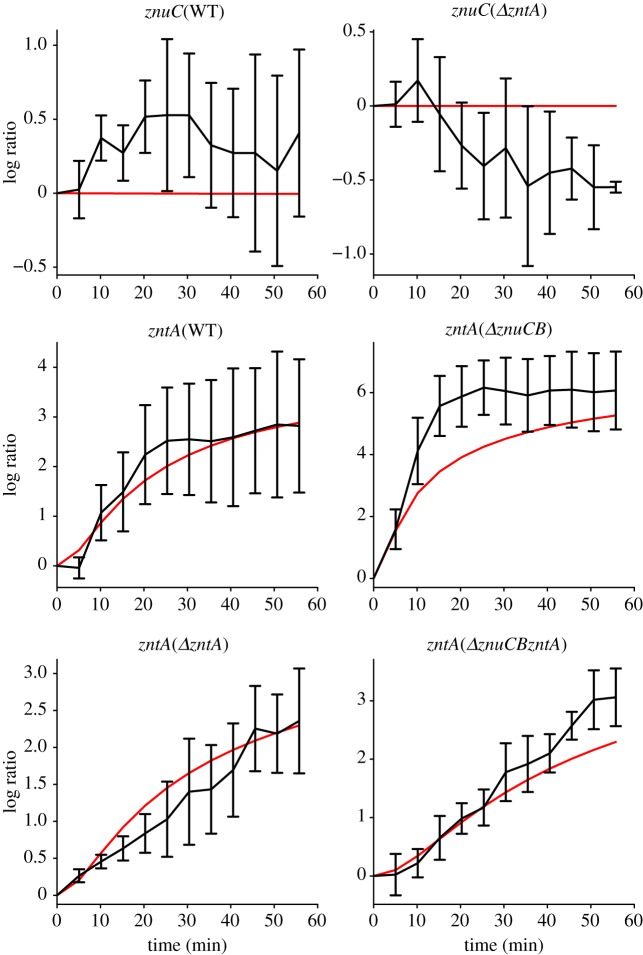
The model is able to fit all of the in vivo time course data for LB12.5 (figure 5 and electronic supplementary material, figure S4). In almost all cases, the model faithfully reproduces the experimental results, mostly fitting within the error bars (1 s.d.) of the data. The least good fit is to PznuC in the ΔzntA strain, where the model predicts little change, and experimentally there is a small decrease in expression (0.7-fold over the time course). The other interesting case is PzntA in the ΔznuCB strain, which shows a similar level of induction after 60 min, but on a slower timescale in the model compared with the experimental data. The goodness of fit can be quantified using R2 values associated with the LB12.5 time course data as a measure of the percentage of variability explained: these model fits have an R2 value of 92%. Overall, these results give considerable confidence in the model processes and parameter estimations.
Figure 5.
Log (to base 2) of expression relative to time t = 0 of induction data following addition of 12.5 μM Zn2+ for the same strain/promoter combinations as figure 1 (the remaining model fits are shown in electronic supplementary material, figure S4). Black line is experimental data; red line is model fit using best fit parameters from electronic supplementary material, table S1. The model fits every time series, matching both the timescale and degree of differential expression.
3.8. Importance of basal import, basal export and reservoir to model fitting
Our model differs from that proposed by Cui et al. [28] in that it includes, most importantly: (i) the basal import of zinc, (ii) the basal export of zinc, and (iii) the zinc reservoir. The parameters associated with these processes have all been found to be important in explaining the data. For the basal import rate Ab, the median value was 1.86 × 10−3 s−1; for the basal export rate Bb, the median value was 2.63 × 102 s−1; for the reservoir size RT, the median value was 1.97 × 105 nM; and for the on-rate to the reservoir KRon, the median value was 1.25 × 102 nMs−1. The ranges of all four parameter values do not include zero (electronic supplementary material, table S1). Graphs for all four posterior distributions are shown in electronic supplementary material, figure S2. If basal import and export are excluded from the model, then the model cannot fit the data, with R2 values reduced from 92 to 9.6% (simulation results not shown).
The importance of basal import and export of zinc can also be highlighted by considering the proportion of zinc imported and exported by the basal and zinc-induced systems in LB and LB12.5 conditions (table 1). Using the median estimated parameters from the model, we predict that, in LB, 59% of external zinc is imported by basal importers and 25% of internal zinc is exported by basal exporters. In LB12.5, the proportion of import by basal exporters remains approximately the same, while the increase in ZntA expression leads to a reduction of the proportion of zinc exported by basal exporters to just 4.8%. The importance of the reservoir can be further highlighted by the calculation that the overwhelming majority of the internal zinc is predicted to be in the zinc reservoir (the ratio of the concentrations of free zinc to reservoir zinc is approx. 3.9 × 10−8), in agreement with published data [8].
Table 1.
Percentage of zinc trafficked by basal systems. The proportion of zinc imported and exported through the basal systems as opposed to the zinc-induced systems has been calculated for the WT strains under LB and 12.5 μM added zinc conditions. The majority of zinc is predicted to be trafficked through the zinc-dependent ZnuABC and ZntA proteins, but a sizeable proportion is predicted to be trafficked through the basal (non-specific) systems. In the zinc added system, there is very little predicted change in ZnuABC expression, and so the proportion of zinc basally imported is hardly changed. In contrast, there is a considerable increase in ZntA expression, and so the proportion of zinc exported through ZntA increases from 75 to over 95%.
| condition | Ab | 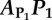 |
% zinc basal import | Bb | 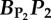 |
% zinc basal export |
|---|---|---|---|---|---|---|
| LB | 1.86 × 10−3 | 1.28 × 10−3 | 59 | 2.63 × 102 | 7.72 × 102 | 25 |
| +12.5 µM Zn2+ | 1.86 × 10−3 | 1.27 × 10−3 | 59 | 2.63 × 102 | 5.17 × 103 | 4.8 |
3.9. Impossibility of fitting model to in vivo data in LB100 conditions
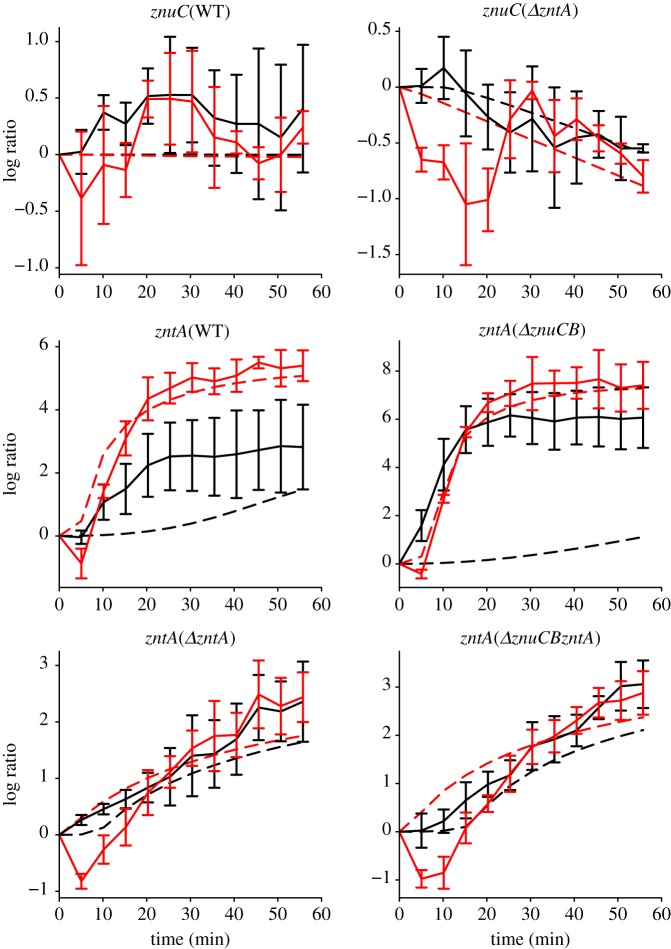
We were unable to fit the model to all of the data when we included time-series data for LB100 (figure 6 and electronic supplementary material, figure S5). While much of the data can be fitted, the model cannot fit the data in the two cases that demonstrate the highest levels of induction: PzntA in the WT and ΔznuCB strains. In both cases, the model can fit the LB100 conditions at the expense of the LB12.5 conditions, where the model predicts very little induction. As a consequence, the R2 value is reduced from 92 to 30%. The poor model fit when the LB100 data are included is not confined to the particular form of the ODE model presented in this manuscript: a wide range of different models were trialled, including models with saturating terms for zinc import, and they all displayed similar or worse patterns of behaviour (data not shown).
Figure 6.
Log (to base 2) of expression relative to time t = 0 of induction data following addition of both 12.5 μM Zn2+ and 100 μM Zn2+ for the same strain/promoter combinations as figure 1 (the remaining model fits are shown in electronic supplementary material, figure S5). Some of the model fits are very poor, especially for PzntA in the WT and ΔznuCB strains. In comparing the goodness of fits, it is important to note that the scales of the y-axes are very different in different plots, so that the worst fit (PzntA in ΔznuCB) is associated with the data showing the greatest level of promoter induction. Black and red dashed lines correspond to model fits using best fit parameters about 12.5 μM Zn2+ and 100 μM Zn2+, respectively.
In order to explain the lack of model fit, we hypothesized that the behaviour of the cells in LB100 is different from their behaviour in LB or in LB12.5. Specifically, our hypothesis is that in LB100, the population response is heterogeneous, with some cells killed by the zinc stress, and only those cells with a strong zinc export phenotype surviving. In this case, ODE models, that describe the average behaviour of a homogeneous population, could never fit these data. This hypothesis is consistent with the dip in light output seen in the majority of cases in LB100 (figure 2 and electronic supplementary material, figure S1), as it is plausible that that dip could be associated with cell killing. (The luminescence assays measure light output from the whole population, so a reduction in light output is consistent either with a lower overall level of light output in a homogeneous population, or with a heterogeneous population in which a proportion is no longer producing light.)
This leads to two testable predictions. First, we expect a degree of cell death in LB100 that would not be seen in LB or LB12.5. Second, we expect that a stochastic version of the model, which is able to describe the random variability in individual cells, would display a high level of variability in ZntA expression. When 100 μM of zinc is added, those cells expressing high levels of ZntA might survive, whereas those cells expressing low levels of ZntA might be killed. Sections 3.10 and 3.11 describe results associated with testing these hypotheses.
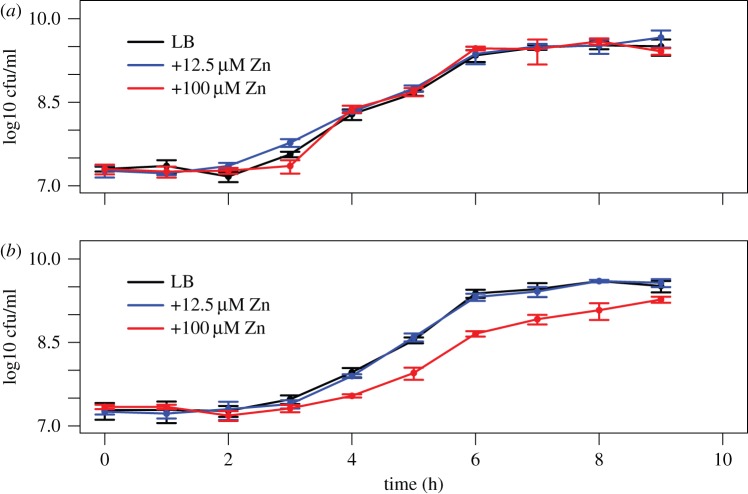
3.10. Lower viable cell counts under LB100 conditions
The WT and double mutant strains were grown in LB, LB12.5 and LB100, and the viable cell count assessed as a function of time (figure 7). The WT strain shows little difference under the three conditions studied. In contrast, the double mutant shows no difference between LB and LB12.5, but a considerably decreased viable cell count in LB100. The greatest difference, a reduction of over 80%, is seen at 6 h, with 2.4 × 109 cfu ml−1 in LB but only 4.53 × 108 cfu ml−1 in LB100. This result is consistent with the population heterogeneity hypothesis, of some cells growing, and other cells either dying or entering a non-growing state.
Figure 7.
Viable cell counts for (a) WT and (b) double mutant strains grown in either LB, LB + 12.5 μM zinc or LB + 100 μM zinc. Error bars are 1 s.d. from triplicate experiments. In all conditions, a classical growth curve is seen, displaying lag, exponential and stationary phases. The WT strain shows little difference between the three conditions. The double mutant shows little difference between LB and 12.5 μM added zinc, but the viable cell counts are considerably reduced under 100 μM added zinc conditions. This result is consistent with the hypothesis that under strong zinc stress, there is a heterogeneous response in the population, with some cells growing, and others either dying or entering a non-growing state. Thus these results are experimental confirmation of the hypothesis put forward as a consequence of the mathematical model.
3.11. Prediction of stochasticity of ZntA protein levels in single cells from a stochastic model
In order to explore the ZnuABC and ZntA protein levels in single cells, a stochastic model was developed. This model contains exactly the same processes as the ODE model described above, but uses a discrete chemical reaction scheme to describe them [31], and thus captures intrinsic variability due to molecular events [34]. The complete chemical reaction scheme is given in the supporting SBML file (zinc.xml).
Outputs from an example simulation are shown in figure 8. The ZnuABC and ZntA protein levels display very different behaviours. ZnuABC rises to a steady state of approximately 1943 molecules per cell, and then shows fluctuations around this steady state with a standard deviation of approximately 434 (coefficient of variability 22%), which is greater than Poisson noise, but consistent with negative regulation [35]. In contrast, the ZntA curve shows much greater variability, with a mean of 268 molecules per cell and s.d. of 324 (coefficient of variability 121%), and bursty expression leading to sharp peaks followed by dilution to low numbers of proteins per cell. This pattern occurs on a timescale considerably slower than the cell cycle; thus individual cells in a population would contain different levels of ZntA, with some cells having high expression and other cells with low expression. This confirms our second prediction.
Figure 8.
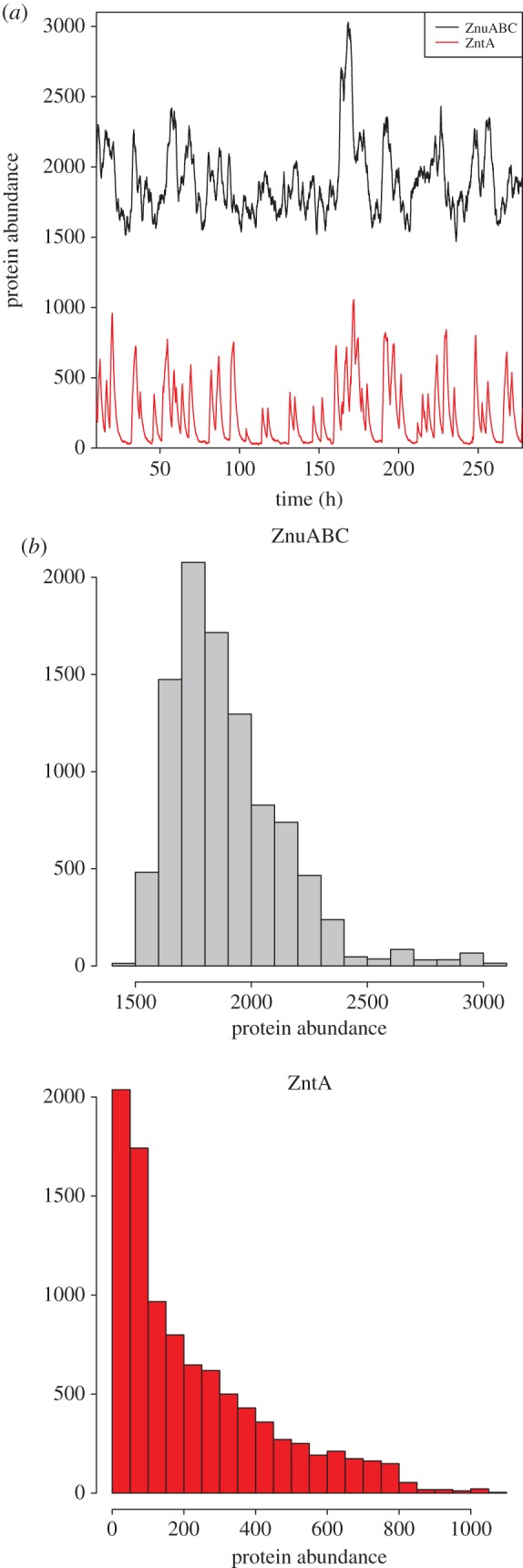
(a) Realization of a stochastic simulation showing ZnuC and ZntA protein abundance. There are small fluctuations in ZnuC abundance around a mean consistent with Poisson noise. In contrast, there are very large fluctuations in ZntA abundance, consistent with bursty production of protein. The timescale of the simulation is considerably longer than a cell cycle so the fluctuations are representative of the variability that would be seen in a population of cells. (b) Corresponding histograms of protein expression of ZnuC and (c) ZntA showing that ZnuC has a relatively tight distribution (mean 1943, s.d. 434, coefficient of variability 22%), while ZntA has a very broad distribution (mean 268, s.d. 324, coefficient of variability 121%).
The difference observed in the model in the variabilities of ZnuABC and ZntA arises from the actions of their regulators. In LB, the majority of Zur molecules are bound by zinc, while the majority of ZntR molecules are unbound by zinc. Zur is a repressor in this form, and PznuCB is usually bound by the Zur–zinc complex. The fluctuations in ZnuABC production arise due to the occasional unbinding of Zur followed by binding of another Zur dimer. These events are relatively common and are largely independent of internal free zinc. In contrast, ZntR is an activator when bound by zinc. PzntA is usually not bound by ZntR, and ZntA is only produced when a ZntR–zinc complex is formed and binds to PzntA. Because of the scarcity of free (ionic) zinc, these events are much rarer, and when they do occur, a large burst of protein is produced. ZntA production is also sensitive to stochasticity in free zinc abundance.
4. Discussion
The aim of this work is to develop a detailed understanding of the responses of the zinc homeostasis system in E. coli K-12 to added zinc, with a specific focus on the Zur-regulated importer system ZnuABC and the ZntR-regulated exporter ZntA. We have generated novel experimental data on six strains, with different key zinc-regulated genes deleted, including time course transcriptional responses of zinc uptake and efflux genes to added zinc. These data, along with data derived by previous groups, have been integrated with a detailed, mechanistic, mathematical model, using a Monte Carlo approach to fit this model to the data. As part of the model fitting, we could only fit data with the smaller concentration of added zinc (12.5 μM) and we could not use the data from the higher concentration of added zinc (100 μM). This led us to the hypothesis that under higher zinc concentrations, the cells demonstrate a heterogeneous response to zinc stress: some cells either do not grow or die, whereas others mount a successful response.
We tested this hypothesis both experimentally, by measuring viable cell counts, and theoretically, by constructing a stochastic model for the system in order to assess the level of heterogeneity in the cell responses. Both these approaches confirmed our hypothesis: in experiments, the viable cell counts were lower with 100 μM added zinc for the double mutant strain; and theoretically we have shown that the cells display considerable heterogeneity of expression of the ZntA exporter even under ‘normal’ conditions. While the lower viable cell counts could potentially be attributed to slower growth of a homogeneous population, such an interpretation is incompatible both with the analysis of the time course data and with the output of the stochastic model.
These results highlight the value of experimental evaluation of the heterogeneity of the zinc response in a population of cells, for example by using single cell assays with fluorescent reporters and flow cytometry. Stochastic models describing population heterogeneity could be calibrated against such data. These experiments would overcome the potential ambiguity of the viable cell counts. Moreover, although we have reported stochastic fluctuations of the ZntA protein, we propose that the double mutant, which cannot produce ZntA, itself has a heterogeneous population response. Therefore, we anticipate that other relevant proteins are expressed heterogeneously, possibly including the non-specific exporters. Future stochastic models could investigate such heterogeneity and could be meaningfully constructed once detailed population level data from single cell assays are available.
There are three main conclusions to be drawn from this research. First, the model shows that zinc import and export is not just mediated by the specific inducible or repressible Zur/ZnuABC and ZntR/ZntA systems, but also by non-specific transporters, in concordance with previously published experimental work. These include the alternative lower specificity Zn2+ importers ZupT, PitA and MntH [14,16,17] and alternative lower specificity zinc exporters ZitB [18] and YiiP [19,20]. This is likely to result from the fundamental chemistry of transition metal ions: biological import and export systems appear to lack recognition specificity between metals such as Zn, Cd and Pb. Thus, many transition metal import and export systems are likely to be cross-reactive, e.g. ZntA has been reported to be able to export Cd2+ and Pb2+ under certain conditions as well as Zn2+ [36]. Thus the net phenotype to ensure uptake of essential metals but removal of toxins will depend on the totality of import and export systems in a cell.
Second, the model shows the importance of the zinc reservoir, that contains the vast majority of zinc in the cell [8,22]. Previous mathematical models have not included this, but the reservoir is necessary for the cell for two reasons: (i) for the functional mobilization of zinc by turnover of L31, liberating zinc for other essential uses in times of zinc depletion, and replacement of L31 by the paralog YkgM protein which lacks zinc; and (ii) the reservoir acts as a buffer to protect against zinc toxicity from free zinc in the cell.
Third is the proposed heterogenous expression of ZntA and response of the population to zinc stress. Heterogeneous gene expression has been suggested as a mechanism for ‘bet-hedging’: greater overall clonal fitness because a sub-population is primed to respond to potential lethal stress [37,38]. Our results suggest that this might be the case for the zinc export system in E. coli. Given the cross-reactivity of metals, we speculate that it could be the case for other metal export systems too, leading to a population of cells with sub-populations capable of responding to a wide range of environmental stresses. Such speculation would require further experimental work [39].
The model also makes predictions relevant to the concentration of internal free zinc. Previous studies have estimated this to be in the femtomolar range [8], whereas other studies have measured it in the picomolar range [22]. It has been argued [22] that the discepency between the femtomolar sensitivity of Zur and ZntR and the picomolar internal zinc pool may arise because the zinc occupancy of these transcription factors does not rapidly equilibriate [22]. Our model estimates picomolar internal free zinc concentration, which is more consistent with the measurements made by Wang et al. [22]. Moreover, these zinc concentration measurements show considerable variability, which is consistent with our model, both in terms of the very high flux through the free zinc compartment, and the predicted stochasticity of zinc-regulated exporter protein expression.
Although the model is able to fit the experimental data very well, two features of the model could be improved. First are the model structure and parameters for the zinc reservoir. We assume a fixed potential reservoir size, and the optimal parameters suggest that this size matches the reported concentration of zinc in the cells [40]. Moreover, the on- and off-rates for such zinc binding are poorly estimated, despite the use of informative priors. It is likely that the potential reservoir size is able to respond to the levels of zinc and that the overall size may exceed the zinc content. We have not included this in our model because of a lack of relevant experimental data. Second, we assume that the changes in growth rate between the different strains do not affect the rate of production of zinc-associated or Lux proteins. This may or may not be reasonable, and further experimentation would be appropriate.
The experimental measurements have used a Lux assay [41] to assess promoter activity. There are two areas for consideration. First, the Lux assay is an indirect measure of promoter expression and protein activity. The measured light output arises from a set of coupled chemical reactions that produce the light and recycle the substrates needed for light production [42]. Thus, although we have assumed a linear relationship between light output and protein synthesis, it is likely that this relationship is nonlinear. That said, the same plasmid lux constructs have been used previously to assess responses of several E. coli promoters to acid stress [43]. In that work, the lux reporters were compared with independent qPCR experiments and there was high concordance between the two reporter technologies. This gives us confidence in the reliability of the reporter results.
The second consideration is that the response of the lux promoter itself can be sensitive to metabolic change, and especially cell death. Indeed, Lux reporters can be used as a signal for toxicity [44]. However, such use is effective only at much higher zinc concentrations than in our study, typically greater than 100 μM in E. coli cells [44]. This is compatible with our own data, where no cytotoxicity was observed at 12.5 μM added zinc (figure 7a,b), some cytotoxicity at 100 μM added zinc (figure 7b) and more marked signal reduction at 200 μM added zinc (electronic supplementary material, figure S1c). Thus we are confident of our use of the lux reporter at lower concentrations of added zinc. As discussed above, a mixed cellular response at higher concentrations of zinc would suggest that future experimental and modelling approaches, that address the responses of a heterogeneous population, could be appropriate.
5. Material and methods
5.1. Bacterial strains and plasmids
Bacterial strains were constructed by the One-step inactivation method [45] and P1 transduction. PCR products were amplified with the primers: TOP631 and TOP632 (zur deletion); TOP1464 and TOP1465 (zntR deletion); TOP1468 and 1469 (znuCB deletion); and TOP1470 and 1471 (zntA deletion) (electronic supplementary material, table S2). The template plasmids: pKD13 (zur deletion); pKD4 (znuCB deletion) and pKD3 (zntA and zntR deletions) were electroporated into E. coli K-12 BW25113 cells containing pKD46. Kanamycin or chloramphenicol resistant clones were selected. Chromosomal gene disruption was confirmed by PCR using the specific primer for each gene (electronic supplementary material, table S2) and the common primer, k1 or c1 (described in [45]). The Δzur::Km, ΔznuCB::Km, ΔzntA::Cm and zntR::Cm mutations were transferred from E. coli K-12 BW25113 into E. coli K-12 MG1655 CGSC 7740 by P1 transduction (electronic supplementary material, table S3). Kanamycin resistance was cured from MG1655 Δzur::Km and MG1655 ΔznuCB::Km for the Lux assays, to enable the strains to be subsequently transformed with the Lux reporter plasmids, which carried the kanamycin resistance marker. This was done using FLP expressed from pCP20 [45]. The double knockout mutant of znuCB and zntA (MG1655 ΔzntA::Cm ΔznuCB) was constructed by P1 transduction of the zntA::Cm allele into MG1655 ΔznuCB cells. Luciferase reporter plasmids were constructed based on the pLUX plasmid which is a low-copy plasmid carrying the Photorhabdus luminescens lux operon [46]. DNA fragments (approx. 200 bp) containing promoters PznuC and PzntA were amplified with the primers, TOP1494 and 1495 (PznuC), TOPN01 and TOPN02 (PzntA), and TOP1499 and TOP1500 (Phns) (electronic supplementary material, table S2) and cloned into pLUX as XhoI/BamHI fragments. Escherichia coli K-12 DH5α cells were transformed with the plasmids, and transformants were selected on the basis of kanamycin resistance. pLUX clones containing PznuC, PzntA and Phns were validated by DNA sequence analysis. These plasmid constructs were subsequently introduced into the strains, MG1655, MG1655 ΔzntA::Cm, MG1655 zntR::Cm, MG1655 Δzur, MG1655 ΔznuCB and MG1655 ΔzntA::Cm ΔznuCB.
5.2. Lux assay
The bioluminescence reaction in bacteria involves the oxidation of reduced riboflavin phosphate (FMNH2), oxygen and a long chain fatty aldehyde. The Photorhabdus luminescens lux operon consists of luxCDE, encoding a fatty acid reductase complex enzyme for the synthesis of the fatty aldehyde substrate and luxAB, encoding the luciferase for the bioluminescent reaction [42]. Because FMNH2 and oxygen are present in E. coli cells, we could detect the bioluminescence in cells expressing the lux operon from zinc activated or repressed promoter/lux fusions without the addition of extra substrates. Pre-cultures of the various reporter plasmid carrying strains were grown overnight at 37°C, and used to inoculate fresh LB/kanamycin (at 1/500 v v−1) and grown for 3 h under aerobic conditions at 37°C. Two hundred microlitre aliquots of these cultures were transferred into 96-well microtitre plates (Porvair, UK). These cultures were incubated at 37°C for 10 min without shaking. Following incubation, bioluminescence was immediately measured using a GENios Pro plate luminometer (Tecan) for the static experiment. For the time course experiment, after the 3 h incubation, different amounts of ZnSO4 were added before the measurement of light output from the culture were taken. Twelve light output measurements were taken over 1 h at 5-min intervals. All experiments were performed using three biological replicates. For the LB experiments, we performed four replicate bioluminescence measurements for each biological replicate. To approximate the bioluminescent activity per unit cell mass, we divided the luminescence activity by the absorbance (OD595) of the cell culture. Thus, for all experiments, both luminescence and absorbance measurements were taken and data were collected and processed as follows:
5.3. Bacterial growth measurements
All strains were grown in Luria Bertani broth containing varying levels of zinc sulfate as described above. Cultures of mutant strains were also supplemented with the appropriate antibiotics in order to maintain selection for the mutant. Optical density measurements were taken from each strain type in each media type in triplicate using an Ultrospec 2000 spectrophotometer (Pharmacia Biotech). Readings were taken using a 10-fold dilution and subsequent correction to maintain linearity between the estimation of cell number and observed optical density. Viable counts were taken using a variation of the Miles and Misra method [47]. Tenfold serial dilutions were made from individual cultures and triplicate 5 μl spots were placed onto prewarmed LB-agar plates. These were incubated for 12–16 h at 37°C, and colony numbers were recorded using dilutions that gave between 20 and 100 individually discernable colonies. Viable counts were calculated on the basis of the appropriate corrections for dilution factor and 5 μl spot size.
5.4. Zinc concentration in LB
The zinc concentration of the LB media used in these experiments was analysed using Thermo-Fisher Scientific X-Series II inductively coupled mass spectrometry (ICP-MS). Samples were applied via an autosampler (Cetac ASX-520) through a concentric glass nebulizer (Thermo-Fisher Scientific) at a rate of 1 ml min−1. Interfering ions were removed by the hexapole collision cell (7% hydrogen in helium) and data analysed using Plasmalab software (v. 2.5.4; Thermo-Fisher Scientific).
5.5. Monte Carlo simulations
Parameter estimations were carried out using the Metropolis–Hastings algorithm [29,30]. For the majority of parameters, uninformative priors have been used. Informative priors have been used for the parameters l1, m1, KRon, KRoff and the six γ parameters (electronic supplementary material, table S4). For the growth rate parameters, prior distributions were based on the growth rates of strains grown in LB. For each growth curve, the Richards model [47] was fitted to estimate the growth rate. The mean rate for each strain was computed, along with the mean standard deviation of all strains, to provide a Gaussian prior for each strain. As proposal distributions, a lognormal distribution was used and the variances of the distributions were empirically chosen in order to ensure acceptance probabilities close to 0.234 [48]. The parameters were updated separately in each step, and 4 000 000 iterations were carried out. To calculate the steady states of the system, Newton algorithm of GSL library [49] encoded in C++ was applied. ODE calculations were performed by the cvode solver with Newton iterations provided by the Sundials library [50].
5.6. Calculations of dynamical and stochastic simulations
For model simulations with a specific parameter value set, ODE calculations were performed by the deSolve package [51] in the statistical environment R. Stochastic simulations based on the Gibson–Bruck algorithm [31] were performed until 1 × 106 s, i.e. 277 h, using Dizzy (v. 1.11.3) [52] using 29 chemical reactions including 14 species (the reaction scheme is given in the supporting SBML file zinc.xml).
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dorota Herman for mathematical modelling advice; Anna Page for initial viable cell count experiments; Scott Young and Darren Hepworth for assistance with the ICP-MS; Ishan Ajmera for the Biomodels submission; and Diane Levine for help with the media summary. The super-computing resource was provided by National Institute of Genetics, Research Organization of Information and Systems, Japan.
Data accesability
Experimental data are available on Figshare (http://dx.doi.org/10.6084/m9.figshare.1311513). Mathematical models are available on Biomodels (MODEL1502180000).
Funding statement
This research was funded by the Biology and Biotechnology Research Council Japan Partnering Award BB/H531586/1 and Research Grant BB/I001875/1, and the Japan Science and Technology Strategic Japanese-UK Cooperative Program on Systems Biology.
References
- 1.Berg JM, Shi Y. 1996. The galvanization of biology: a growing appreciation for the roles of zinc. Science 271, 1081–1085. ( 10.1126/science.271.5252.1081) [DOI] [PubMed] [Google Scholar]
- 2.Coleman JE. 1998. Zinc enzymes. Curr. Opin. Chem. Biol. 2, 222–234. ( 10.1016/S1367-5931(98)80064-1) [DOI] [PubMed] [Google Scholar]
- 3.Clayton SR, Heurlier K, Oshima T, Hobman JL. 2011. Copper and zinc stress in bacteria. In Stress response in pathogenic bacteria (Advances in Molecular and Cellular Microbiology) (ed. Kidd SP.), 19th edn, pp. 245–265. Wallingford, UK: CABI. [Google Scholar]
- 4.Nies DH. 1999. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 51, 730–750. ( 10.1007/s002530051457) [DOI] [PubMed] [Google Scholar]
- 5.Patzer SI, Hantke K. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28, 1199–1210. ( 10.1046/j.1365-2958.1998.00883.x) [DOI] [PubMed] [Google Scholar]
- 6.Patzer SI, Hantke K. 2000. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J. Biol. Chem. 275, 24 321–24 332. ( 10.1074/jbc.M001775200) [DOI] [PubMed] [Google Scholar]
- 7.Yatsunyk LA, et al. 2008. Structure and metal binding properties of ZnuA, a periplasmic zinc transporter from Escherichia coli. J. Biol. Inorg. Chem. 13, 271–288. ( 10.1007/s00775-007-0320-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outten CE, O'Halloran TV. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488–2492. ( 10.1126/science.1060331) [DOI] [PubMed] [Google Scholar]
- 9.Rensing C, Mitra B, Rosen BP. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl Acad. Sci. USA 94, 14 326–14 331. ( 10.1073/pnas.94.26.14326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brocklehurst KR, Hobman JL, Lawley B, Blank L, Marshall SJ, Brown NL, Morby AP. 1999. ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol. Microbiol. 31, 893–902. ( 10.1046/j.1365-2958.1999.01229.x) [DOI] [PubMed] [Google Scholar]
- 11.Brown NL, Barrett SR, Camakaris J, Lee BT, Rouch DA. 1995. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol. Microbiol. 17, 1153–1166. ( 10.1111/j.1365-2958.1995.mmi_17061153.x) [DOI] [PubMed] [Google Scholar]
- 12.Outten CE, Outten FW, O'Halloran TV. 1999. DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR homologue in Escherichia coli. J. Biol. Chem. 274, 37 517–37 524. ( 10.1074/jbc.274.53.37517) [DOI] [PubMed] [Google Scholar]
- 13.Grass G, Wong MD, Rosen BP, Smith RL, Rensing C. 2002. ZupT is a Zn(II) uptake system in Escherichia coli. J. Bacteriol. 184, 864–866. ( 10.1128/JB.184.3.864-866.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taudt N, Grass G. 2010. Point mutations change specificity and kinetics of metal uptake by ZupT from Escherichia coli . Biometals 23, 643–656. ( 10.1007/s10534-010-9319-z) [DOI] [PubMed] [Google Scholar]
- 15.Grass G, Franke S, Taudte N, Nies NH, Kucharski LM, Maguire ME, Rensing C. 2005. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J. Bacteriol. 187, 1604–1611. ( 10.1128/JB.187.5.1604-1611.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beard SJ, Hashim R, Wu G, Binet MR, Hughes MN, Poole RK. 2000. Evidence for the transport of zinc(II) ions via the pit inorganic phosphate transport system in Escherichia coli. FEMS Microbiol. Lett. 184, 231–235. ( 10.1111/j.1574-6968.2000.tb09019.x) [DOI] [PubMed] [Google Scholar]
- 17.Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MFM. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 35, 1065–1078. ( 10.1046/j.1365-2958.2000.01774.x) [DOI] [PubMed] [Google Scholar]
- 18.Grass G, Fan B, Rosen BP, Franke S, Nies DH, Rensing C. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183, 4664–4667. ( 10.1128/JB.183.15.4664-4667.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grass G, Otto M, Fricke B, Haney CJ, Rensing C, Nies DH, Munkelt D. 2005b. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 183, 9–18. ( 10.1007/s00203-004-0739-4) [DOI] [PubMed] [Google Scholar]
- 20.Lu M, Fu D. 2007. Structure of the zinc transporter YiiP. Science 317, 1746–1748. ( 10.1126/science.1143748) [DOI] [PubMed] [Google Scholar]
- 21.Waldron KJ, Robinson NJ. 2009. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 7, 25–35. ( 10.1038/nrmicro2057) [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Hurst TK, Thompson RB, Fierke CA. 2011. Genetically encoded ratiometric biosensors to measure intracellular exchangeable zinc in Escherichia coli . J. Biomed. Opt. 16, 087011 ( 10.1117/1.3613926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanamiya H, et al. 2004. Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Mol. Microbiol. 52, 273–283. ( 10.1111/j.1365-2958.2003.03972.x) [DOI] [PubMed] [Google Scholar]
- 24.Akanuma G, Nanamiya H, Natori Y, Nomura N, Kawamura F. 2006. Liberation of zinc-containing L31 (RpmE) from ribosomes by its paralogous gene product YtiA, in Bacillus subtilis. J. Bacteriol. 188, 2715–2720. ( 10.1128/JB.188.7.2715-2720.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hensley MP, Tierney DL, Crowder MW. 2011. Zn(II) Binding to Escherichia coli 70S ribosomes. Biochemistry 50, 9937–9939. ( 10.1021/bi200619w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hensley MP, Gunasekera TS, Easton JA, Sigdel TK, Sugarbaker SA, Klingbeil L, Breece RM, Tierney DL, Crowder MW. 2012. Characterization of Zn(II)-responsive ribosomal proteins YkgM and L31 in E. coli. J. Inorg. Biochem. 111, 164–172. ( 10.1016/j.jinorgbio.2011.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Z, Chandrangsu P, Helmann TC, Romsang A, Gaballa A, Helmann JD. 2014. Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Mol. Microbiol. 94, 756–70. ( 10.1111/mmi.12794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui J, Kaandorp JA, Lloyd CM. 2008. Simulating in vitro transcriptional response of zinc homeostasis system in Escherichia coli. BMC Syst. Biol. 2, 89 ( 10.1186/1752-0509-2-89) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hastings WK. 1970. Monte Carlo sampling methods using Markov chains and their applications. Biometrika 57, 97–109. ( 10.1093/biomet/57.1.97) [DOI] [Google Scholar]
- 30.Herman D, Thomas CM, Stekel DJ. 2011. Global transcription regulation of RK2 plasmids: a case study in the combined use of dynamical mathematical models and statistical inference for integration of experimental data and hypothesis exploration. BMC Syst. Biol. 5, 119 ( 10.1186/1752-0509-5-119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson MA, Bruck J. 2000. Efficient exact stochastic simulation of chemical systems with many species and many channels. J. Phys. Chem. A 104, 1876–1889. ( 10.1021/jp993732q) [DOI] [Google Scholar]
- 32.Wilkinson DJ. 2009. Stochastic modelling for quantitative description of heterogeneous biological systems. Nat. Rev. Genet. 10, 122–133. ( 10.1038/nrg2509) [DOI] [PubMed] [Google Scholar]
- 33.Changela A, Chen K, Xue Y, Holschen J, Outten CE, O'Halloran TV, Mondragon A. 2003. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301, 1383–1387. ( 10.1126/science.1085950) [DOI] [PubMed] [Google Scholar]
- 34.Swain PS, Elowitz MB, Siggia ED. 2002. Intrinsic and extrinsic contributions to stochasticity in gene expression. Proc. Natl Acad. Sci. USA 99, 12 795–12 800. ( 10.1073/pnas.162041399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stekel DJ, Jenkins DJ. 2008. Strong negative self regulation of prokaryotic transcription factors increases the intrinsic noise of protein expression. BMC Syst. Biol. 2, 6 ( 10.1186/1752-0509-2-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma R, Rensing C, Rosen BP, Mitra B. 2000. The ATP hydrolytic activity of purified ZntA, a Pb(II)/Cd(II)/Zn(II)-translocating ATPase from Escherichia coli. J. Biol. Chem. 275, 3873–3878. ( 10.1074/jbc.275.6.3873) [DOI] [PubMed] [Google Scholar]
- 37.Levy SF, Ziv N, Siegal ML. 2012. Bet hedging in yeast by heterogeneous, age-correlated expression of a stress protectant. PLoS Biol. 10, e1001325 ( 10.1371/journal.pbio.1001325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller J, Hense BA, Fuchs TM, Utz M, Pötsche CH. 2013. Bet-hedging in stochastically switching environments. J. Theor. Biol. 336, 144–157. ( 10.1016/j.jtbi.2013.07.017) [DOI] [PubMed] [Google Scholar]
- 39.Delvigne F, Goffin P. 2014. Microbial heterogeneity affects bioprocess robustness: dynamic single-cell analysis contributes to understanding of microbial populations. Biotechnol. J. 9, 61–72. ( 10.1002/biot.201300119) [DOI] [PubMed] [Google Scholar]
- 40.Ishihama Y, Schmidt T, Rappsilber J, Mann M, Hartl FU, Kerner MJ, Frishman D. 2008. Protein abundance profiling of the Escherichia coli cytosol. BMC Genomics 9, 102 ( 10.1186/1471-2164-9-102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szittner R, Meighen E. 1990. Nucleotide sequence, expression, and properties of luciferase coded by lux genes from a terrestrial bacterium. J. Biol. Chem. 265, 16 581–16 587. [PubMed] [Google Scholar]
- 42.Welham PA, Stekel DJ. 2009. Mathematical model of the Lux luminescence system in the terrestrial bacterium Photorhabdus luminescens. Mol. BioSyst. 5, 68–76. ( 10.1039/B812094C) [DOI] [PubMed] [Google Scholar]
- 43.Burton NA, Johnson MD, Antczak P, Robinson A, Lund PA. 2010. Novel aspects of the acid response network of E. coli K-12 are revealed by a study of transcriptional dynamics. J. Mol. Biol. 401, 726–742. ( 10.1016/j.jmb.2010.06.054) [DOI] [PubMed] [Google Scholar]
- 44.Ivask A, Rolova T, Kahru A. 2009. A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing. BMC Biotechnol. 9, 41 ( 10.1186/1472-6750-9-41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645. ( 10.1073/pnas.120163297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miles AA, Misra SS, Irwin JO. 1938. An estimation of the bactericidal power of the blood. J. Hyg. (Lond.) 38, 732–749. ( 10.1017/S002217240001158X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards JF. 1969. A flexible growth function for empirical use. J. Exp. Bot. 10, 290–301. ( 10.1093/jxb/10.2.290) [DOI] [Google Scholar]
- 48.Roberts GO, Gelman A, Gilks WR. 1997. Weak convergence and optimal scaling of random walk metropolis algorithm. Ann. Appl. Probab. 7, 110–120. ( 10.1214/aoap/1034625254) [DOI] [Google Scholar]
- 49.Galassi M, Davies J, Theiler J, Gough B, Jungman G, Alken P, Booth M, Rossi F. 2009. GNU scientific library reference manual, 3rd edn Godalming, UK: Network Theory Ltd. [Google Scholar]
- 50.Cohen SD, Hindmarsh AC. 1996. CVODE, a stiff/nonstiff ODE solver in C. Compute. Phys. 10, 138–143. ( 10.1063/1.4822377) [DOI] [Google Scholar]
- 51.Soetaert K, Petzoldt T, Setzer WR. 2010. Solving differential equations in R: package deSolve. J. Stat. Softw. 33, 1–25.20808728 [Google Scholar]
- 52.Ramsey S, Orrell D, Bolouri H. 2005. Dizzy: stochastic simulation of large-scale genetic regulatory networks. J. Bioinform. Comput. Biol. 3, 415–436. ( 10.1142/S0219720005001132) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.