Abstract
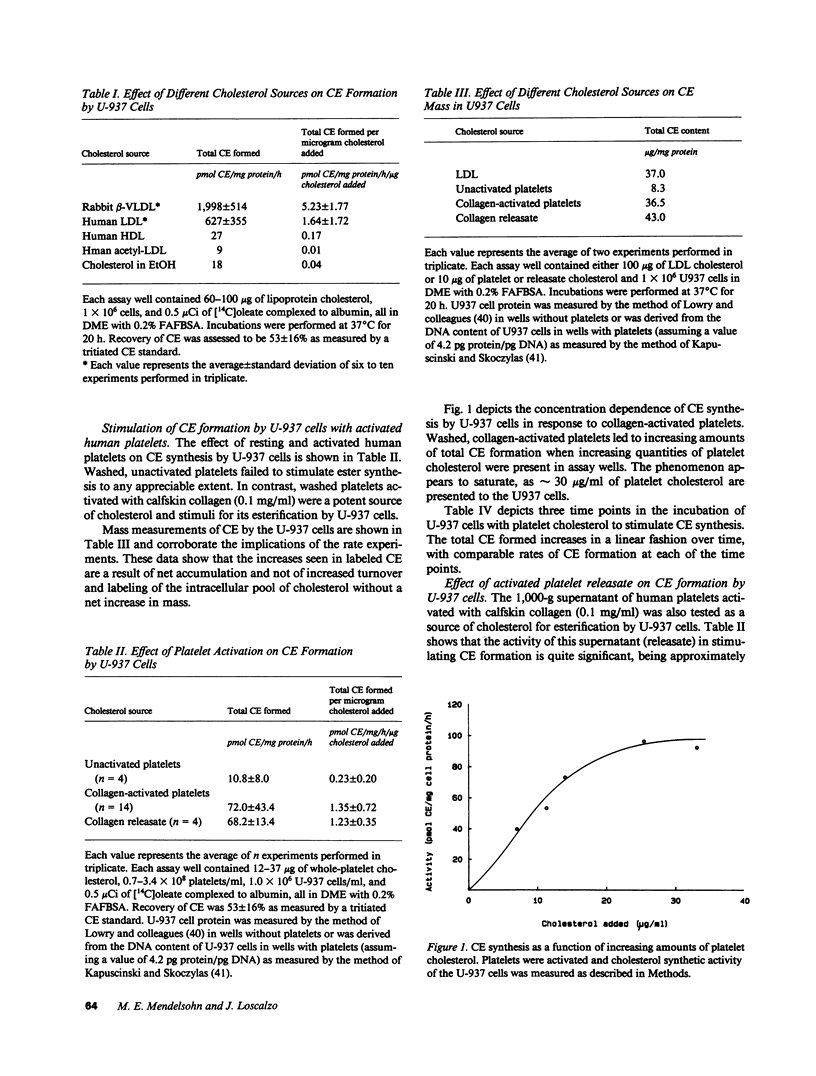
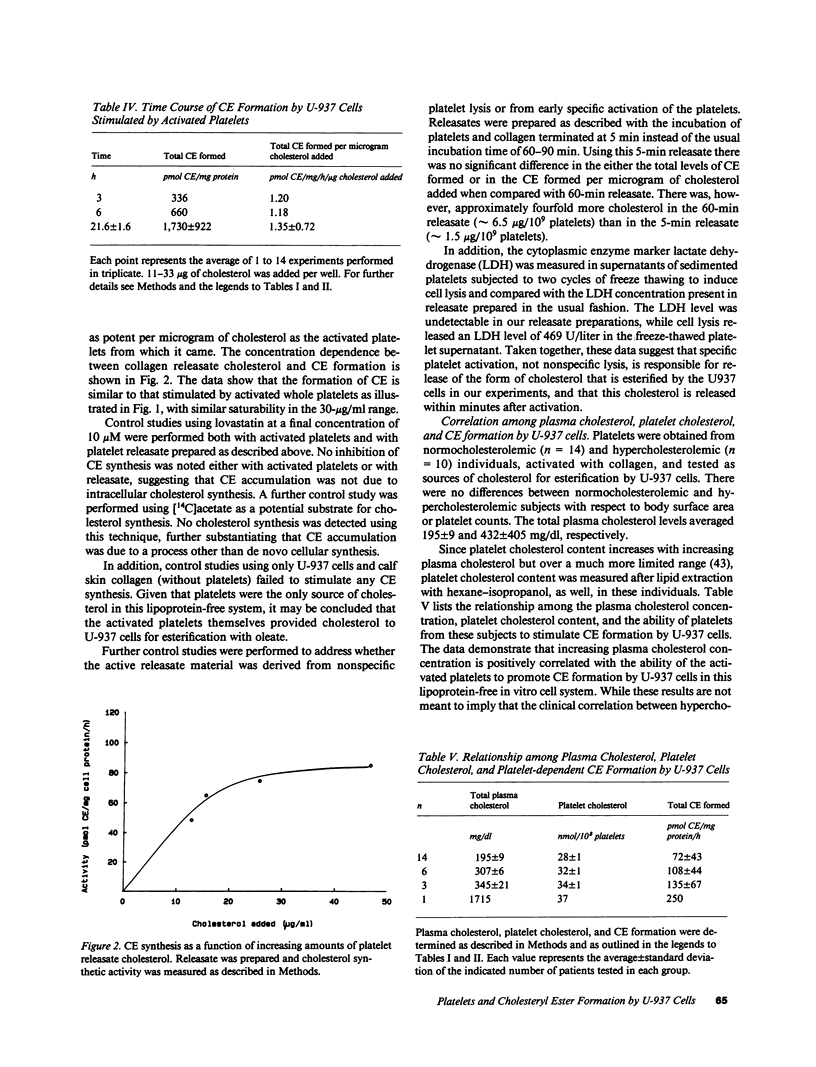
The conversion of tissue macrophages into cholesteryl ester-rich foam cells is a crucial early event in atherogenesis. We studied the platelet as a potential source of cholesterol for esterification by macrophages because (a) platelets are rich in free cholesterol, (b) they adhere to macrophages early in atherogenesis, and (c) vascular injury can induce foam cell formation in the absence of hyperlipoproteinemia. We found that washed, activated human platelets from normocholesterolemic donors stimulated cholesteryl ester formation by the human monocyte-derived cell, U-937. Platelet cholesterol, released from platelets activated with calf skin collagen, was approximately equipotent at donating cholesterol to U-937 cells for esterification as normal human low density lipoprotein cholesterol. The stimulation of cholesteryl ester formation by activated human platelets demonstrated both concentration and time dependence. When hypercholesterolemic donors were studied, it was found that increasing plasma levels of cholesterol correlated directly with the ability of these hypercholesterolemic platelets to support cholesteryl ester synthesis by U-937 cells. Cholesterol-donating activity was also found in a 1,000-g supernatants of activated platelets. These observations point to a new and potentially important role for platelets in atherogenesis and suggest a mechanism for foam cell formation in the absence of marked hypercholesterolemia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviram M., Brook J. G., Lees A. M., Lees R. S. Low density lipoprotein binding to human platelets: role of charge and of specific amino acids. Biochem Biophys Res Commun. 1981 Mar 16;99(1):308–318. doi: 10.1016/0006-291x(81)91746-0. [DOI] [PubMed] [Google Scholar]
- Aviram M., Brook J. G. Platelet interaction with high and low density lipoproteins. Atherosclerosis. 1983 Mar;46(3):259–268. doi: 10.1016/0021-9150(83)90176-4. [DOI] [PubMed] [Google Scholar]
- Björkerud S., Bondjers G. Arterial repair and atherosclerosis after mechanical injury. 5. Tissue response after induction of a large superficial transverse injury. Atherosclerosis. 1973 Sep-Oct;18(2):235–255. doi: 10.1016/0021-9150(73)90103-2. [DOI] [PubMed] [Google Scholar]
- Boers G. H., Smals A. G., Trijbels F. J., Fowler B., Bakkeren J. A., Schoonderwaldt H. C., Kleijer W. J., Kloppenborg P. W. Heterozygosity for homocystinuria in premature peripheral and cerebral occlusive arterial disease. N Engl J Med. 1985 Sep 19;313(12):709–715. doi: 10.1056/NEJM198509193131201. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Krieger M., Ho Y. K., Anderson R. G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979 Sep;82(3):597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss L. K., Plow E. F. Interaction of plasma lipoproteins with human platelets. Blood. 1984 Aug;64(2):365–374. [PubMed] [Google Scholar]
- Derksen A., Cohen P. Extensive incorporation of (2-14C) mevalonic acid into cholesterol precursors by human platelets in vitro. J Biol Chem. 1973 Nov 10;248(21):7396–7403. [PubMed] [Google Scholar]
- Derksen A., Meguid M. M., Cohen P. Non-human primate platelets and arterial tissue cannot convert preformed [14C]lanosterol into[14C]cholesterol in vivo. Biochem J. 1976 Jul 15;158(1):157–159. doi: 10.1042/bj1580157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deykin D., Desser R. K. The incorporation of acetate and palmitate into lipids by human platelets. J Clin Invest. 1968 Jul;47(7):1590–1602. doi: 10.1172/JCI105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth J. L., Kraemer F. B., Cooper A. D. Transport of beta-very low density lipoproteins and chylomicron remnants by macrophages is mediated by the low density lipoprotein receptor pathway. J Biol Chem. 1987 Feb 15;262(5):2316–2325. [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Falcone D. J., Mated N., Shio H., Minick C. R., Fowler S. D. Lipoprotein-heparin-fibronectin-denatured collagen complexes enhance cholesteryl ester accumulation in macrophages. J Cell Biol. 1984 Oct;99(4 Pt 1):1266–1274. doi: 10.1083/jcb.99.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. J., Moore S., Singal D. P. Repeated endothelial injury and induction of atherosclerosis in normolipemic rabbits by human serum. Lab Invest. 1975 Mar;32(3):404–415. [PubMed] [Google Scholar]
- Friedman R. J., Stemerman M. B., Wenz B., Moore S., Gauldie J., Gent M., Tiell M. L., Spaet H. The effect of thrombocytopenia on experimental arteriosclerotic lesion formation in rabbits. Smooth muscle cell proliferation and re-endothelialization. J Clin Invest. 1977 Nov;60(5):1191–1201. doi: 10.1172/JCI108872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brown M. S. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A., Radin N. S. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978 Oct 1;90(1):420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Ross R., Slichter S. J., Scott C. R. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976 Sep;58(3):731–741. doi: 10.1172/JCI108520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider J. G., Boyett R. L. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978 May;19(4):514–518. [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Knight B. L., Soutar A. K. Degradation by cultured fibroblasts and macrophages of unmodified and 1,2-cyclohexanedione-modified low-density lipoprotein from normal and homozygous familial hypercholesterolaemic subjects. Biochem J. 1982 Jan 15;202(1):145–152. doi: 10.1042/bj2020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller E., Koller F., Doleschel W. Specific binding sites on human blood platelets for plasma lipoproteins. Hoppe Seylers Z Physiol Chem. 1982 Apr;363(4):395–405. doi: 10.1515/bchm2.1982.363.1.395. [DOI] [PubMed] [Google Scholar]
- Koo C., Wernette-Hammond M. E., Innerarity T. L. Uptake of canine beta-very low density lipoproteins by mouse peritoneal macrophages is mediated by a low density lipoprotein receptor. J Biol Chem. 1986 Aug 25;261(24):11194–11201. [PubMed] [Google Scholar]
- Kruth H. S. Platelet-mediated cholesterol accumulation in cultured aortic smooth muscle cells. Science. 1985 Mar 8;227(4691):1243–1245. doi: 10.1126/science.3975612. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. B., Oh S. Y. Altered metabolism (in vivo and in vitro) of plasma lipoproteins after selective chemical modification of lysine residues of the apoproteins. J Clin Invest. 1979 Sep;64(3):743–750. doi: 10.1172/JCI109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. Ultrastructural findings on platelet depositions in initial atherogenesis. Wien Klin Wochenschr. 1986 Apr 4;98(7):212–214. [PubMed] [Google Scholar]
- Mazurov A. V., Preobrazhensky S. N., Leytin V. L., Repin V. S., Smirnov V. N. Study of low density lipoprotein interaction with platelets by flow cytofluorimetry. FEBS Lett. 1982 Jan 25;137(2):319–322. doi: 10.1016/0014-5793(82)80375-x. [DOI] [PubMed] [Google Scholar]
- McCully K. S., Wilson R. B. Homocysteine theory of arteriosclerosis. Atherosclerosis. 1975 Sep-Oct;22(2):215–227. doi: 10.1016/0021-9150(75)90004-0. [DOI] [PubMed] [Google Scholar]
- Moore S., Ihnatowycz T. O. Vessel injury and atherosclerosis. Adv Exp Med Biol. 1978;102:145–161. doi: 10.1007/978-1-4757-1217-9_9. [DOI] [PubMed] [Google Scholar]
- Moore S. Thromboatherosclerosis in normolipemic rabbits. A result of continued endothelial damage. Lab Invest. 1973 Nov;29(5):478–487. [PubMed] [Google Scholar]
- Murphy-Chutorian D. R., Wexman M. P., Grieco A. J., Heininger J. A., Glassman E., Gaull G. E., Ng S. K., Feit F., Wexman K., Fox A. C. Methionine intolerance: a possible risk factor for coronary artery disease. J Am Coll Cardiol. 1985 Oct;6(4):725–730. doi: 10.1016/s0735-1097(85)80473-3. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Printz D. J., Boyd D., Joy L., Steinberg D. Macrophage oxidation of low density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis. 1986 Sep-Oct;6(5):505–510. doi: 10.1161/01.atv.6.5.505. [DOI] [PubMed] [Google Scholar]
- Raymond T. L., Reynolds S. A., Swanson J. A. Lipoproteins of the extravascular space: enhanced macrophage degradation of low density lipoproteins from interstitial inflammatory fluid. J Lipid Res. 1985 Nov;26(11):1356–1362. [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Sandberg H., Andersson L. O., Höglund S. Isolation and characterization of lipid-protein particles containing platelet factor 3 released from human platelets. Biochem J. 1982 Apr 1;203(1):303–311. doi: 10.1042/bj2030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick B. P., Schick P. K., Chase P. R. Lipid composition of guinea pig platelets and megakaryocytes. The megakaryocyte as a probable source of platelet lipids. Biochim Biophys Acta. 1981 Jan 26;663(1):239–248. doi: 10.1016/0005-2760(81)90210-1. [DOI] [PubMed] [Google Scholar]
- Schick B. P., Schick P. K. Cholesterol exchange in platelets, erythrocytes and megakaryocytes. Biochim Biophys Acta. 1985 Feb 8;833(2):281–290. doi: 10.1016/0005-2760(85)90200-0. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Anaya-Galindo R., Bennett J., Colman R. W., Cooper R. A. Platelet hypersensitivity induced by cholesterol incorporation. J Clin Invest. 1975 Mar;55(3):636–643. doi: 10.1172/JCI107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Bennett J. S., Colman R. W., Cooper R. A. Abnormalities of cholesterol-phospholipid composition in platelets and low-density lipoproteins of human hyperbetalipoproteinemia. J Lab Clin Med. 1977 Feb;89(2):341–353. [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tabas I., Weiland D. A., Tall A. R. Unmodified low density lipoprotein causes cholesteryl ester accumulation in J774 macrophages. Proc Natl Acad Sci U S A. 1985 Jan;82(2):416–420. doi: 10.1073/pnas.82.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber M. G., Kayden H. J. Low density lipoprotein receptor activity in human monocyte-derived macrophages and its relation to atheromatous lesions. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5466–5470. doi: 10.1073/pnas.77.9.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Via D. P., Plant A. L., Craig I. F., Gotto A. M., Jr, Smith L. C. Metabolism of normal and modified low-density lipoproteins by macrophage cell lines of murine and human origin. Biochim Biophys Acta. 1985 Mar 6;833(3):417–428. doi: 10.1016/0005-2760(85)90099-2. [DOI] [PubMed] [Google Scholar]
- Wang-Iverson P., Ginsberg H. N., Peteanu L. A., Le N. A., Brown W. V. Apo E-mediated uptake and degradation of normal very low density lipoproteins by human monocyte/macrophages: a saturable pathway distinct from the LDL receptor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):578–586. doi: 10.1016/0006-291x(85)90645-x. [DOI] [PubMed] [Google Scholar]


