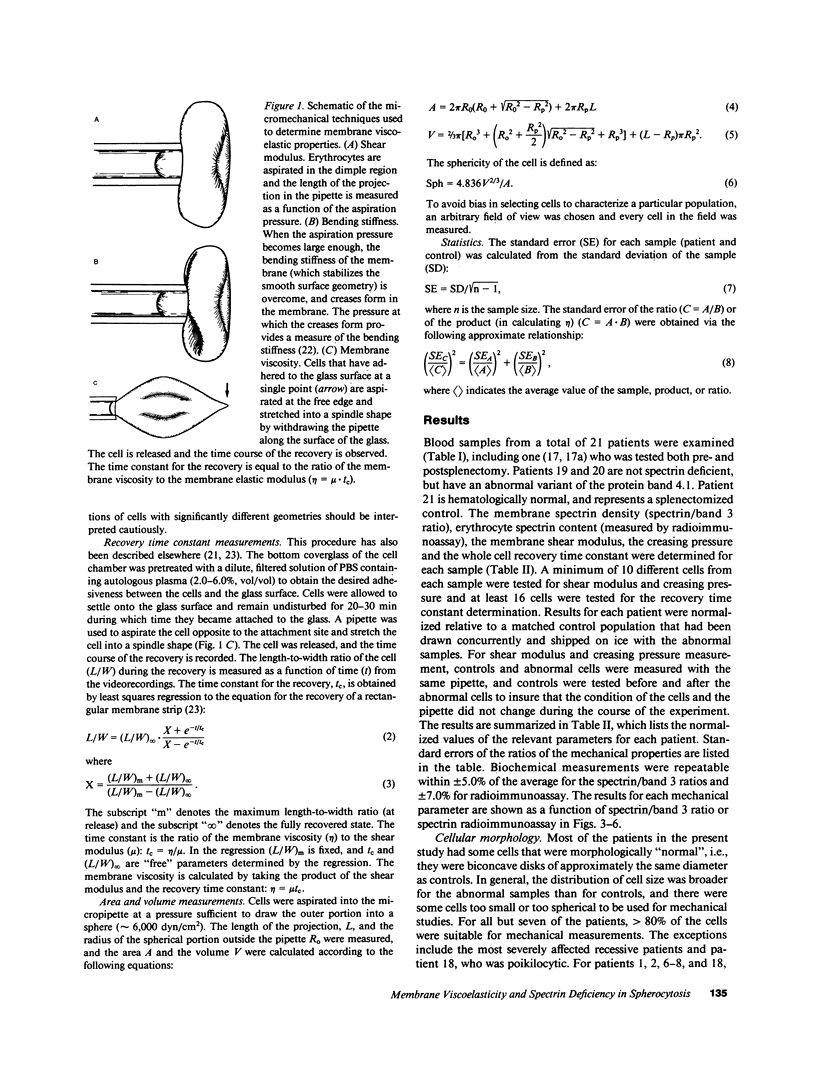
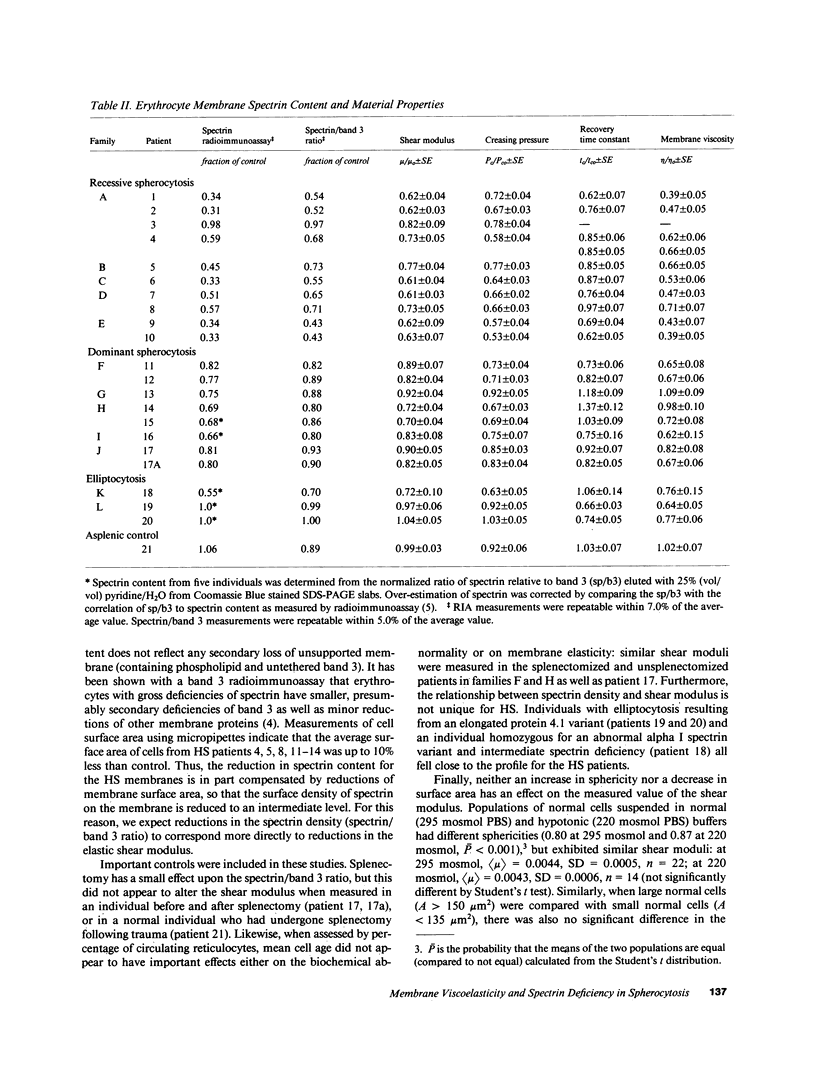
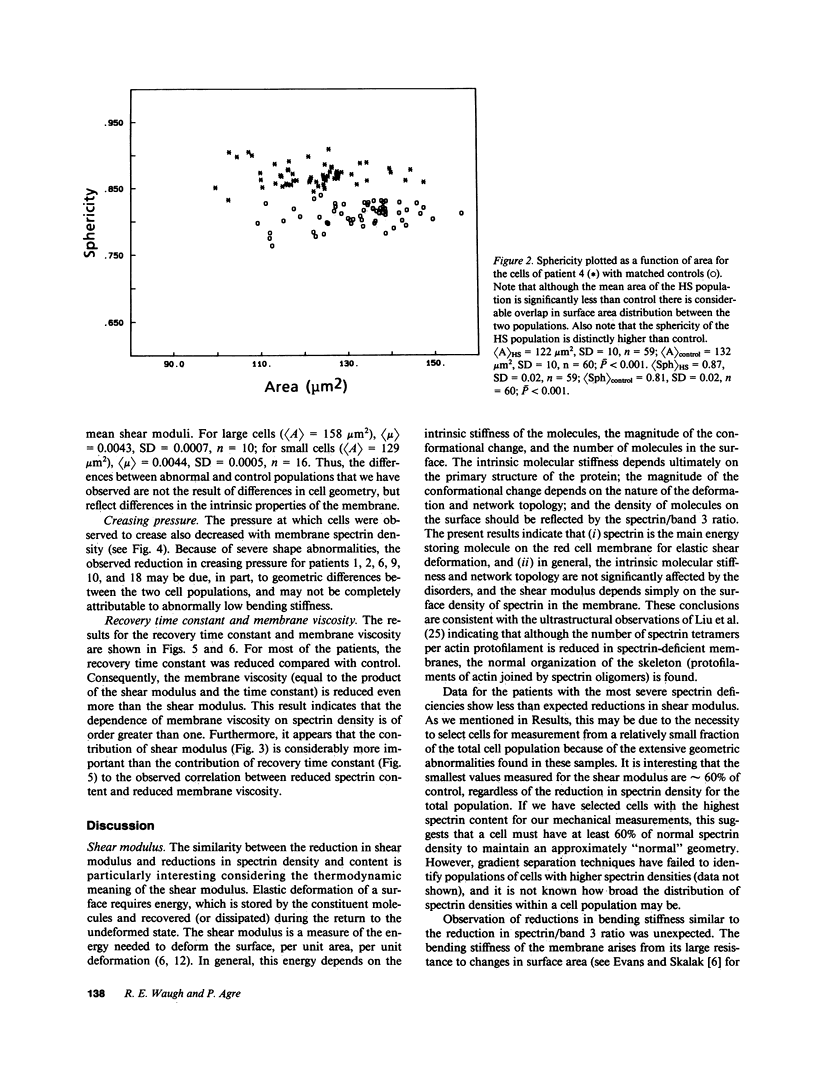
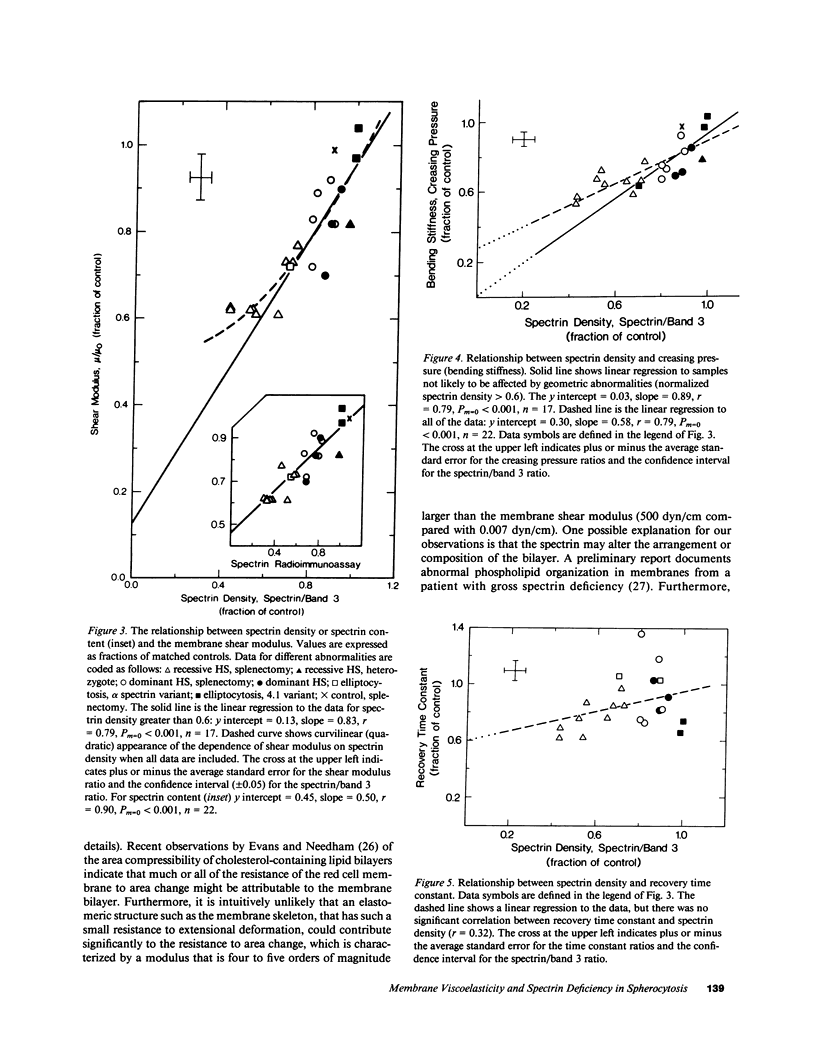
Abstract
Hereditary spherocytosis is a common hemolytic anemia associated with deficiencies in spectrin, the principal structural protein of the erythrocyte membrane-skeleton. We have examined 20 different individuals from 10 spherocytosis kindreds and 2 elliptocytosis kindreds to determine the effects of different levels of spectrin deficiency on the viscoelastic properties of the erythrocyte membrane. Micropipettes were used to perform single-cell micromechanical measurements of approximately 1,000 individual cells to determine the membrane elastic shear modulus, the apparent membrane bending stiffness, and whole cell recovery time constant for the different cell populations. The membrane viscosity was calculated by the product of the shear modulus and the recovery time constant. Results show correlation between the fractional reduction in shear modulus and the fractional reduction in spectrin content (determined by spectrin radioimmunoassay) and spectrin density (determined by the ratios of spectrin to band 3 on electrophoresis gels) suggesting that membrane shear elasticity is directly proportional to the surface density of spectrin on the membrane (P less than 0.001). The apparent membrane bending stiffness is also reduced in proportion to the density of spectrin (P less than 0.001). The membrane viscosity is reduced relative to control (P less than 0.001), but the nature of the relationship between spectrin density and membrane viscosity is less clearly defined. These studies document striking relationships between partial deficiencies of erythrocyte spectrin and specific viscoelastic properties of the mutant membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Asimos A., Casella J. F., McMillan C. Inheritance pattern and clinical response to splenectomy as a reflection of erythrocyte spectrin deficiency in hereditary spherocytosis. N Engl J Med. 1986 Dec 18;315(25):1579–1583. doi: 10.1056/NEJM198612183152504. [DOI] [PubMed] [Google Scholar]
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Lux S. E. Hereditary spherocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):15–43. [PubMed] [Google Scholar]
- Bennett V. Isolation of an ankyrin-band 3 oligomer from human erythrocyte membranes. Biochim Biophys Acta. 1982 Aug 12;689(3):475–484. doi: 10.1016/0005-2736(82)90305-4. [DOI] [PubMed] [Google Scholar]
- Bennett V. Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96:313–324. doi: 10.1016/s0076-6879(83)96029-9. [DOI] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Chien S., Sung K. L., Skalak R., Usami S., Tözeren A. Theoretical and experimental studies on viscoelastic properties of erythrocyte membrane. Biophys J. 1978 Nov;24(2):463–487. doi: 10.1016/S0006-3495(78)85395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J. 1983 Jul;43(1):27–30. doi: 10.1016/S0006-3495(83)84319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., La Celle P. L. Intrinsic material properties of the erythrocyte membrane indicated by mechanical analysis of deformation. Blood. 1975 Jan;45(1):29–43. [PubMed] [Google Scholar]
- Evans E. A., Skalak R. Mechanics and thermodynamics of biomembranes: part 2. CRC Crit Rev Bioeng. 1979 Nov;3(4):331–418. [PubMed] [Google Scholar]
- Evans E., Mohandas N., Leung A. Static and dynamic rigidities of normal and sickle erythrocytes. Major influence of cell hemoglobin concentration. J Clin Invest. 1984 Feb;73(2):477–488. doi: 10.1172/JCI111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Buxbaum K. L., Evans E. A. Temperature dependence of the viscoelastic recovery of red cell membrane. Biophys J. 1980 Jan;29(1):177–182. doi: 10.1016/S0006-3495(80)85124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Worthy P. R., Evans E. A. Red cell extensional recovery and the determination of membrane viscosity. Biophys J. 1979 Apr;26(1):101–114. doi: 10.1016/S0006-3495(79)85238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash G. B., Meiselman H. J. Red cell and ghost viscoelasticity. Effects of hemoglobin concentration and in vivo aging. Biophys J. 1983 Jul;43(1):63–73. doi: 10.1016/S0006-3495(83)84324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash G. B., Wyard S. J. Erythrocyte membrane elasticity during in vivo ageing. Biochim Biophys Acta. 1981 May 6;643(2):269–275. doi: 10.1016/0005-2736(81)90072-9. [DOI] [PubMed] [Google Scholar]
- O'Keefe E., Vennett V. Use of immunoglobulin-loaded protein A-bearing staphylococci as a primary solid phase immunoadsorbent in radioimmunoassay. J Biol Chem. 1980 Jan 25;255(2):561–568. [PubMed] [Google Scholar]
- Takakuwa Y., Tchernia G., Rossi M., Benabadji M., Mohandas N. Restoration of normal membrane stability to unstable protein 4.1-deficient erythrocyte membranes by incorporation of purified protein 4.1. J Clin Invest. 1986 Jul;78(1):80–85. doi: 10.1172/JCI112577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözeren A., Skalak R., Fedorciw B., Sung K. L., Chien S. Constitutive equations of erythrocyte membrane incorporating evolving preferred configuration. Biophys J. 1984 Mar;45(3):541–549. doi: 10.1016/S0006-3495(84)84191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Effects of 2,3-diphosphoglycerate on the mechanical properties of erythrocyte membrane. Blood. 1986 Jul;68(1):231–238. [PubMed] [Google Scholar]
- Waugh R. E. Effects of abnormal cytoskeletal structure on erythrocyte membrane mechanical properties. Cell Motil. 1983;3(5-6):609–622. doi: 10.1002/cm.970030526. [DOI] [PubMed] [Google Scholar]
- Waugh R. E. Effects of inherited membrane abnormalities on the viscoelastic properties of erythrocyte membrane. Biophys J. 1987 Mar;51(3):363–369. doi: 10.1016/S0006-3495(87)83358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R., Evans E. A. Thermoelasticity of red blood cell membrane. Biophys J. 1979 Apr;26(1):115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I. Herediatary spherocytosis. A review. Arch Intern Med. 1975 Oct;135(10):1316–1323. doi: 10.1001/archinte.135.10.1316. [DOI] [PubMed] [Google Scholar]



