Abstract
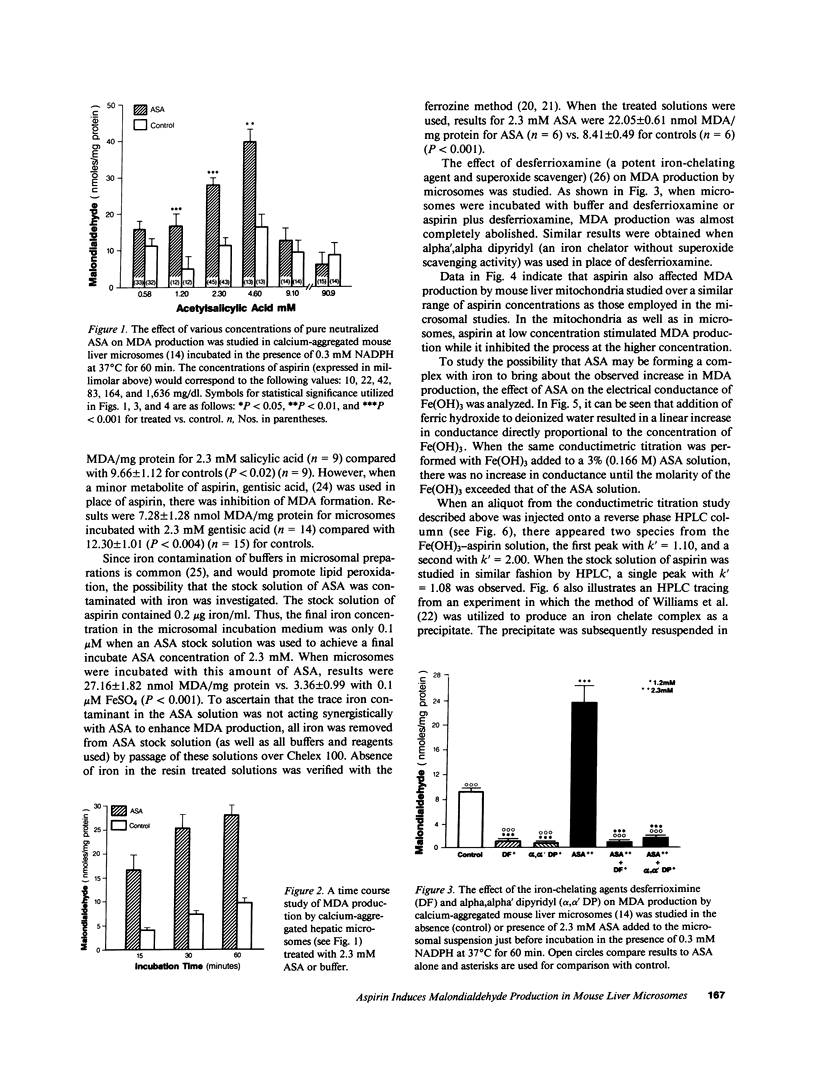
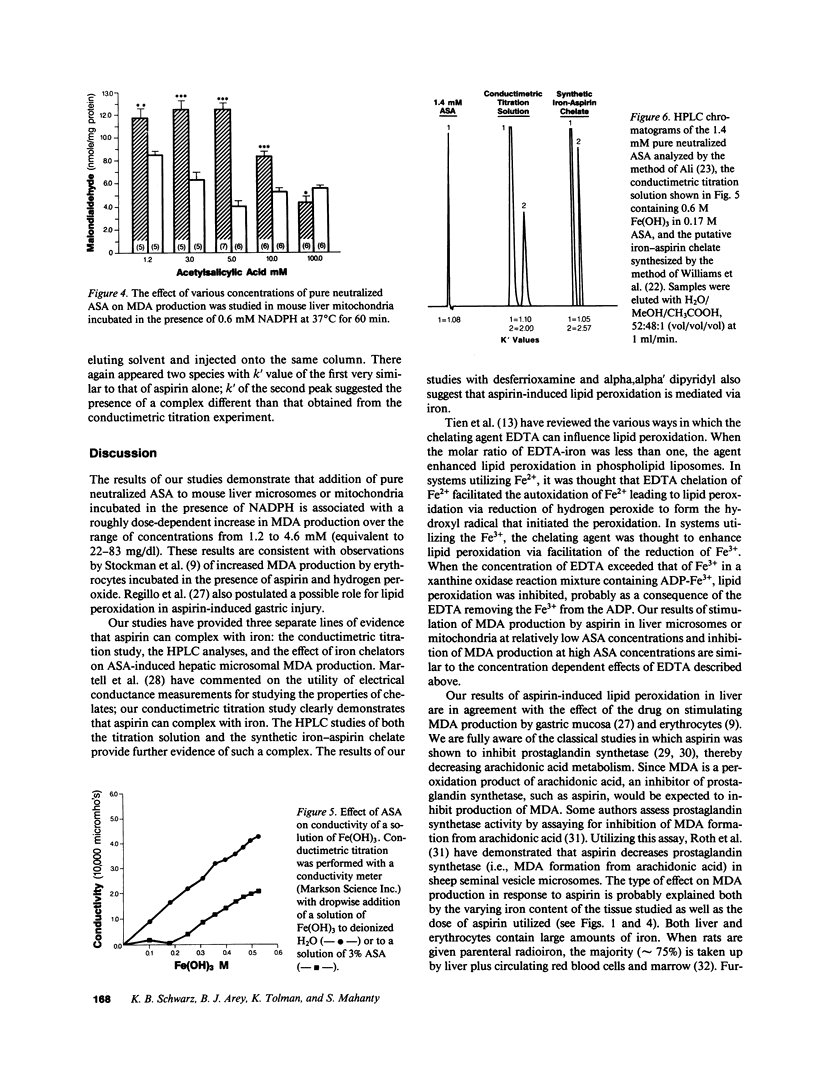
To investigate the possibility that lipid peroxidation is the mechanism responsible for aspirin-induced liver damage, pure neutralized acetylsalicylic acid (ASA), 0.6-90.9 mM, was added to calcium-aggregated mouse liver microsomes followed by incubation in NADPH buffer at 37 degrees C for 60 min and subsequent measurement of malondialdehyde (MDA). MDA production at ASA concentrations from 1.2 to 4.6 mM was greater than control (P less than 0.004). Peak MDA values were observed with 4.6 mM ASA, 39.58 +/- 6.73 nmol MDA/mg protein vs. 16.16 +/- 2.85 (P less than 0.004). Higher concentrations of ASA were inhibitory compared with the value at 4.6 mM (P less than 0.001). Aspirin had similar effects on MDA production by mouse liver mitochondria. MDA production with either ASA or buffer was completely suppressed by the potent iron-chelating agents desferrioxamine and alpha,alpha' dipyridyl when these were added to the microsomal preparations. Since MDA production in this system is known to be affected by iron-chelating agents (enhanced at low concentration, inhibited at higher concentration), the iron-chelating properties of ASA were investigated. Conductivity titration curves of Fe(OH)3 added to water or ASA suggested that the ASA was complexing with iron. The presence of an iron-ASA complex was established by high pressure liquid chromatographic analysis of the solution from this study. We conclude that aspirin enhances MDA production by hepatic microsomes and mitochondria via an aspirin-iron chelate and that this represents at least one mechanism by which aspirin may produce liver damage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. L. Application of gas-liquid chromatography and high-performance liquid chromatography to the analysis of trace amounts of salicylic acid, acetylsalicylic anhydride and acetylsalicylsalicylic acid in aspirin samples and aspirin formulations. J Chromatogr. 1976 Nov 3;126:651–663. doi: 10.1016/s0021-9673(01)84109-1. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Tavill A. S., Brittenham G. M., Park C. H., Recknagel R. O. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983 Mar;71(3):429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Carpenter S. J., Healey J. F. Iron and the liver: subcellular distribution of iron and decreased microsomal cytochrome P-450 in livers of iron-loaded rats. Arch Pathol Lab Med. 1979 Jan;103(1):21–29. [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Cheney B. A., Lothe K., Morgan E. H., Sood S. K., Finch C. A. Internal iron exchange in the rat. Am J Physiol. 1967 Feb;212(2):376–380. doi: 10.1152/ajplegacy.1967.212.2.376. [DOI] [PubMed] [Google Scholar]
- Glikman P., Gutnisky A., Gimeno M. F., Gimeno A. L. Effect of acetylsalicylic acid on iron absorption in the rat. Experientia. 1981 Jun;37(6):588–589. doi: 10.1007/BF01990066. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, GEBICKI J. M., HOFFSTEN P. E., WEINSTEIN J., SCOTT A. Swelling and lysis of rat liver mitochondria induced by ferrous ions. J Biol Chem. 1963 Feb;238:828–835. [PubMed] [Google Scholar]
- Halpin T. J., Holtzhauer F. J., Campbell R. J., Hall L. J., Correa-Villaseñor A., Lanese R., Rice J., Hurwitz E. S. Reye's syndrome and medication use. JAMA. 1982 Aug 13;248(6):687–691. [PubMed] [Google Scholar]
- Hochstein P., Nordenbrand K., Ernster L. Evidence for the involvement of iron in the ADP-activated peroxidation of lipids in microsomes and mitochondria. Biochem Biophys Res Commun. 1964;14:323–328. doi: 10.1016/s0006-291x(64)80004-8. [DOI] [PubMed] [Google Scholar]
- Hurwitz E. S., Barrett M. J., Bregman D., Gunn W. J., Pinsky P., Schonberger L. B., Drage J. S., Kaslow R. A., Burlington D. B., Quinnan G. V. Public Health Service study of Reye's syndrome and medications. Report of the main study. JAMA. 1987 Apr 10;257(14):1905–1911. [PubMed] [Google Scholar]
- Kaplowitz N., Kuhlenkamp J., Goldstein L., Reeve J. Effect of salicylates and phenobarbital on hepatic glutathione in the rat. J Pharmacol Exp Ther. 1980 Feb;212(2):240–245. [PubMed] [Google Scholar]
- Kornbrust D. J., Mavis R. D. Microsomal lipid peroxidation. I. Characterization of the role of iron and NADPH. Mol Pharmacol. 1980 May;17(3):400–407. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowrey K., Glende E. A., Jr, Recknagel R. O. Destruction of liver microsomal calcium pump activity by carbon tetrachloride and bromotrichloromethane. Biochem Pharmacol. 1981 Jan 15;30(2):135–140. doi: 10.1016/0006-2952(81)90184-2. [DOI] [PubMed] [Google Scholar]
- Maines M. D. Evidence for the catalytic activity of endogenous iron in the lipid peroxidative destruction of heme by allylisopropylacetamide in the rat liver. Int J Biochem. 1980;12(5-6):781–785. doi: 10.1016/0020-711x(80)90162-7. [DOI] [PubMed] [Google Scholar]
- Martens M. E., Lee C. P. Reye's syndrome: salicylates and mitochondrial functions. Biochem Pharmacol. 1984 Sep 15;33(18):2869–2876. doi: 10.1016/0006-2952(84)90209-0. [DOI] [PubMed] [Google Scholar]
- Montgomery M. R., Clark C., Holtzman J. L. Iron species of hepatic microsomes from control and phenobarbital-treated rats. Arch Biochem Biophys. 1974 Jan;160(1):113–118. doi: 10.1016/s0003-9861(74)80015-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Ishihara N., Shimokawa T., Kojima S. Effect of clofibrate on lipid peroxidation in rats treated with aspirin and 4-pentenoic acid. J Biochem. 1987 Jan;101(1):81–88. doi: 10.1093/oxfordjournals.jbchem.a121910. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. IV. Dependence on Fe3+. J Biol Chem. 1971 Jan 10;246(1):263–269. [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest. 1966 Jan;15(1 Pt 1):132–148. [PubMed] [Google Scholar]
- Roders M. K., Glende E. A., Jr, Recknagel R. O. NADPH dependent lipid peroxidation of calcium bound microsomes. Res Commun Chem Pathol Pharmacol. 1976 Oct;15(2):393–396. [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M., Riegelman S., Harris P. A., Sholkoff S. D., Eyring E. J. Kinetics of acetylsalicylic acid disposition in man. Nature. 1967 Jul 22;215(5099):413–414. doi: 10.1038/215413a0. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Starko K. M., Ray C. G., Dominguez L. B., Stromberg W. L., Woodall D. F. Reye's syndrome and salicylate use. Pediatrics. 1980 Dec;66(6):859–864. [PubMed] [Google Scholar]
- Stockman J. A., 3rd, Lubin B., Oski F. A. Aspirin-induced hemolysis: the role of concomitant oxidant (H2O2) challenge. Pediatr Res. 1978 Sep;12(9):927–931. doi: 10.1203/00006450-197809000-00009. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Aust S. D. Rat liver microsomal NADPH-dependent release of iron from ferritin and lipid peroxidation. J Free Radic Biol Med. 1985;1(4):293–300. doi: 10.1016/0748-5514(85)90134-5. [DOI] [PubMed] [Google Scholar]
- Tien M., Morehouse L. A., Bucher J. R., Aust S. D. The multiple effects of ethylenediaminetetraacetate in several model lipid peroxidation systems. Arch Biochem Biophys. 1982 Oct 15;218(2):450–458. doi: 10.1016/0003-9861(82)90367-8. [DOI] [PubMed] [Google Scholar]
- Tolman K. G., Peterson P., Gray P., Hammar S. P. Hepatotoxicity of salicylates in monolayer cell cultures. Gastroenterology. 1978 Feb;74(2 Pt 1):205–208. [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Waldman R. J., Hall W. N., McGee H., Van Amburg G. Aspirin as a risk factor in Reye's syndrome. JAMA. 1982 Jun 11;247(22):3089–3094. [PubMed] [Google Scholar]
- Williams D. A., Walz D. T., Foye W. O. Synthesis and biological evaluation of tetrakis(acetylsalicylato)-mu-dicopper(II). J Pharm Sci. 1976 Jan;65(1):126–128. doi: 10.1002/jps.2600650129. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman H. J. Effects of aspirin and acetaminophen on the liver. Arch Intern Med. 1981 Feb 23;141(3 Spec No):333–342. doi: 10.1001/archinte.141.3.333. [DOI] [PubMed] [Google Scholar]


