Abstract
This study related ATP levels with membrane damage, lipid abnormalities, and cell death in energy-depleted LLC-PK1 cells. Oxidative phosphorylation was inhibited by antimycin A, and glycolysis was regulated by graded glucose deprivation to achieve stepwise ATP depletion. Over a range of ATP levels down to approximately equal to 5% of normal, over 5 h, cells were altered only minimally, or injured reversibly. Such cells maintained mitochondrial potential, and retained more K+ than cells without an energy source. Over the same duration, cells without an energy source were lethally injured. Treatment with antimycin induced increments of triglycerides and decreases of phospholipids. With severe ATP depletion (approximately equal to 5-10% of normal after 5 h), decrease of phospholipids was marked. Cells in which ATP was not measurable (or was less than 5% of normal) showed comparable phospholipid declines but, in addition, showed massive and progressive increase of unesterified fatty acids. The results identified a low threshold of ATP, at best 5-10% of normal, which preserved viability in LLC-PK1 cells despite major loss of membrane phospholipids. This threshold also determined the ability of cells to maintain their normally low levels of unesterified fatty acids. Failure of energy-dependent mechanisms that normally metabolize unesterified fatty acids may be a correlate of the extent of energy depletion that determines lethal injury.
Full text
PDF

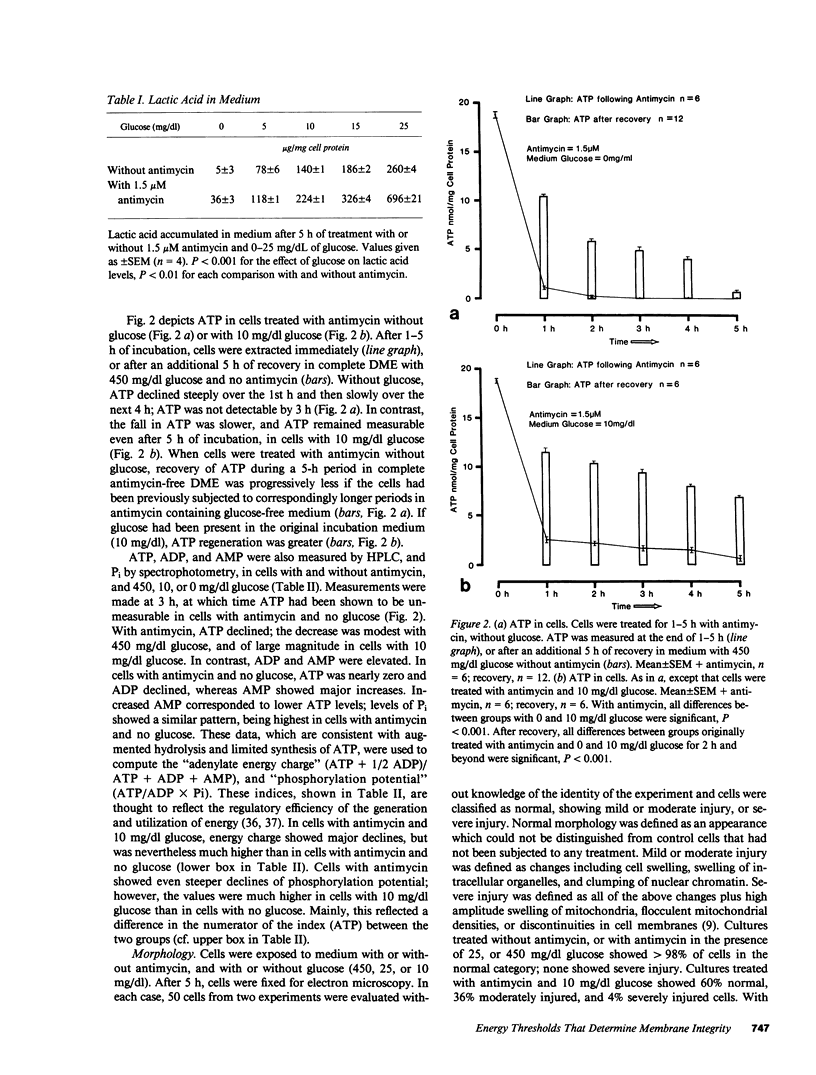
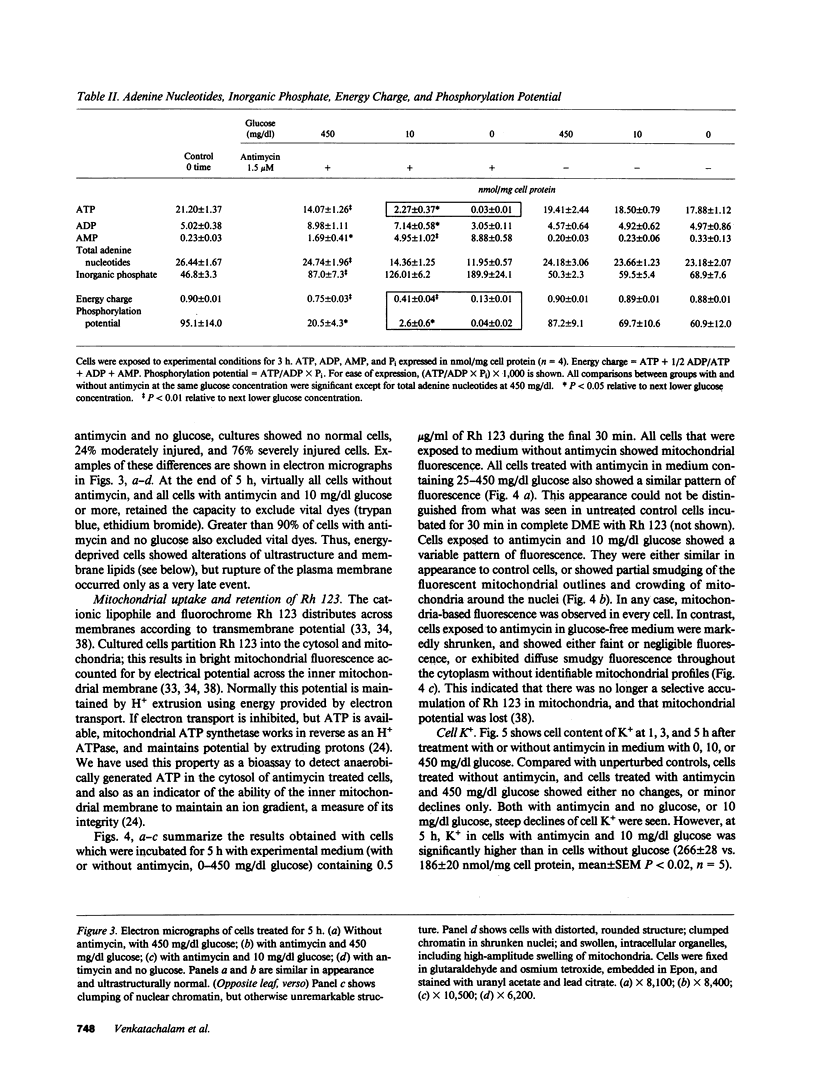
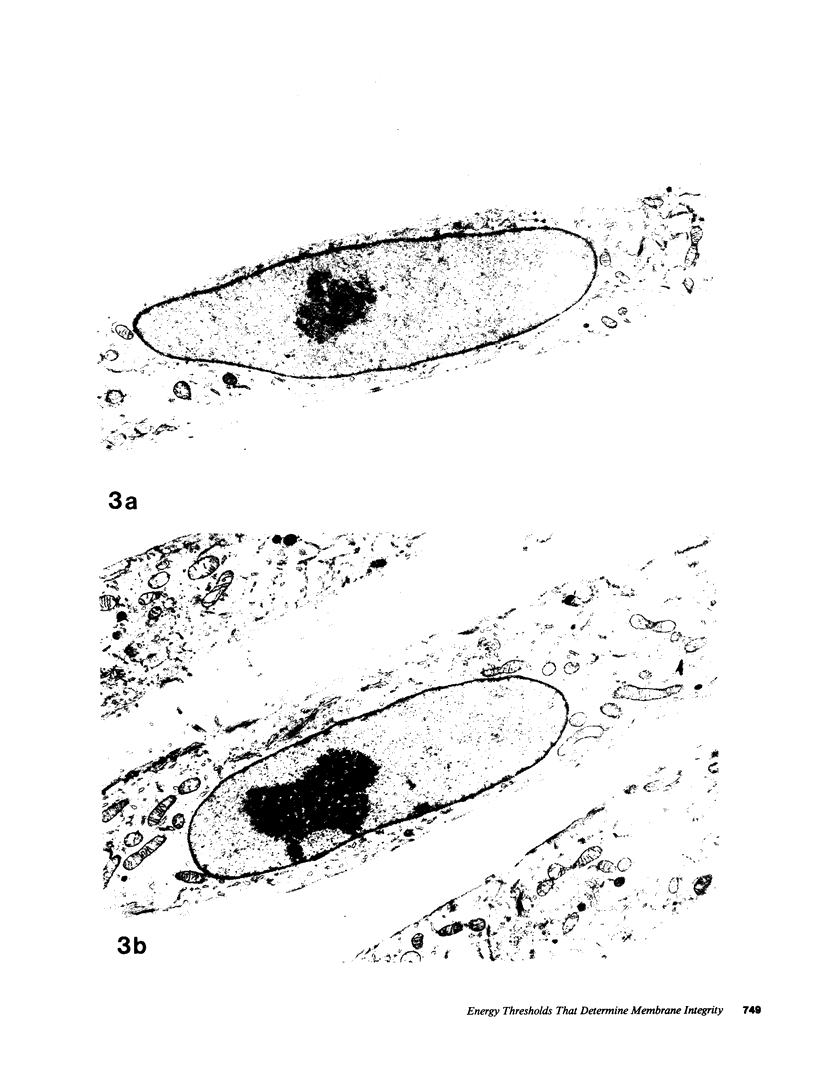
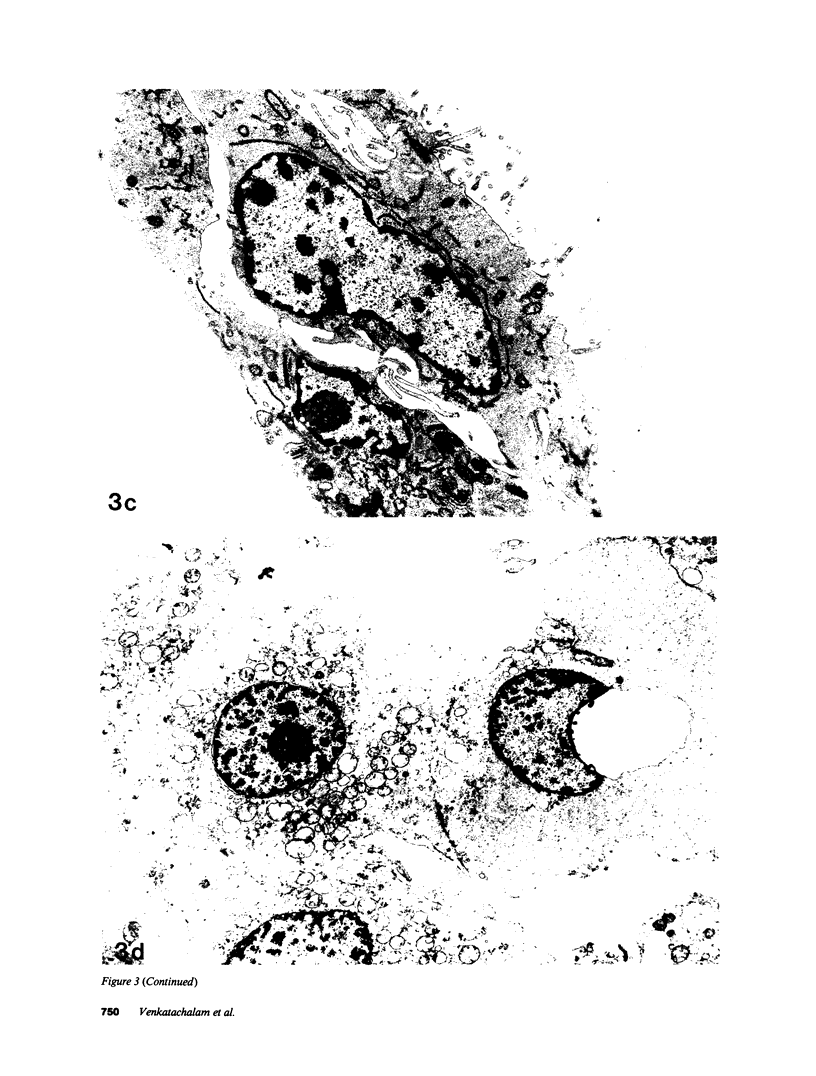




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. The fusion of erythrocytes by fatty acids, esters, retinol and alpha-tocopherol. Biochem J. 1973 Sep;136(1):147–155. doi: 10.1042/bj1360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed K., Thomas B. S. The effects of long chain fatty acids on sodium plus potassium ion-stimulated adenosine triphosphatase of rat brain. J Biol Chem. 1971 Jan 10;246(1):103–109. [PubMed] [Google Scholar]
- Allan D., Michell R. H. The relationship between Ca2+-mediated polyphosphoinositide phosphodiesterase activity, 1, 2-diacylglycerol accumulation, and microvesiculation in erythrocytes. Prog Clin Biol Res. 1979;30:523–529. [PubMed] [Google Scholar]
- Arslan P., Corps A. N., Hesketh T. R., Metcalfe J. C., Pozzan T. cis-Unsaturated fatty acids uncouple mitochondria and stimulate glycolysis in intact lymphocytes. Biochem J. 1984 Jan 15;217(2):419–425. doi: 10.1042/bj2170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Aveldaño M. I., Bazán N. G. Differential lipid deacylation during brain ischemia in a homeotherm and a poikilotherm. Content and composition of free fatty acids and triacylglycerols. Brain Res. 1975 Dec 12;100(1):99–110. doi: 10.1016/0006-8993(75)90244-9. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Bastin J., Cambon N., Thompson M., Lowry O. H., Burch H. B. Change in energy reserves in different segments of the nephron during brief ischemia. Kidney Int. 1987 Jun;31(6):1239–1247. doi: 10.1038/ki.1987.137. [DOI] [PubMed] [Google Scholar]
- Bradford B. U., Marotto M., Lemasters J. J., Thurman R. G. New, simple models to evaluate zone-specific damage due to hypoxia in the perfused rat liver: time course and effect of nutritional state. J Pharmacol Exp Ther. 1986 Jan;236(1):263–268. [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Renal ischemia: a new perspective. Kidney Int. 1984 Oct;26(4):375–383. doi: 10.1038/ki.1984.185. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Bonventre J. V., Malis C. D., Leaf A. Calcium and ischemic injury. N Engl J Med. 1986 Jun 26;314(26):1670–1676. doi: 10.1056/NEJM198606263142604. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Leaf A., Bonventre J. V. Mechanism of protection by verapamil and nifedipine from anoxic injury in isolated cardiac myocytes. Am J Physiol. 1984 Mar;246(3 Pt 1):C323–C329. doi: 10.1152/ajpcell.1984.246.3.C323. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Leaf A., Bonventre J. V. Mitochondrial function and intracellular calcium in anoxic cardiac myocytes. Am J Physiol. 1986 Jan;250(1 Pt 1):C18–C25. doi: 10.1152/ajpcell.1986.250.1.C18. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Chien K. R., Han A., Sen A., Buja L. M., Willerson J. T. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984 Mar;54(3):313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Sen A., Reynolds R., Chang A., Kim Y., Gunn M. D., Buja L. M., Willerson J. T. Release of arachidonate from membrane phospholipids in cultured neonatal rat myocardial cells during adenosine triphosphate depletion. Correlation with the progression of cell injury. J Clin Invest. 1985 Jun;75(6):1770–1780. doi: 10.1172/JCI111889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Stubbs M., Miyata Y., Ditre C. M. Regulation of cellular metabolism by intracellular phosphate. Biochim Biophys Acta. 1977 Oct 12;462(1):20–35. doi: 10.1016/0005-2728(77)90186-4. [DOI] [PubMed] [Google Scholar]
- Farber J. L. Biology of disease: membrane injury and calcium homeostasis in the pathogenesis of coagulative necrosis. Lab Invest. 1982 Aug;47(2):114–123. [PubMed] [Google Scholar]
- Gazitt Y., Loyter A., Reichler Y., Ohad I. Correlation between changes in the membrane organization and susceptibility to phospholipase C attack induced by ATP depletion of rat erythrocytes. Biochim Biophys Acta. 1976 Feb 6;419(3):479–492. doi: 10.1016/0005-2736(76)90260-1. [DOI] [PubMed] [Google Scholar]
- Goldenberg H., Fernandez A. Simplified method for the estimation of inorganic phosphorus in body fluids. Clin Chem. 1966 Dec;12(12):871–882. [PubMed] [Google Scholar]
- Gronow G. H., Cohen J. J. Substrate support for renal functions during hypoxia in the perfused rat kidney. Am J Physiol. 1984 Oct;247(4 Pt 2):F618–F631. doi: 10.1152/ajprenal.1984.247.4.F618. [DOI] [PubMed] [Google Scholar]
- Higgins T. J., Bailey P. J., Allsopp D. Interrelationship between cellular metabolic status and susceptibility of heart cells to attack by phospholipase. J Mol Cell Cardiol. 1982 Nov;14(11):645–654. doi: 10.1016/0022-2828(82)90162-6. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Steenbergen C., Jr Nucleotide metabolism and cellular damage in myocardial ischemia. Annu Rev Physiol. 1985;47:727–749. doi: 10.1146/annurev.ph.47.030185.003455. [DOI] [PubMed] [Google Scholar]
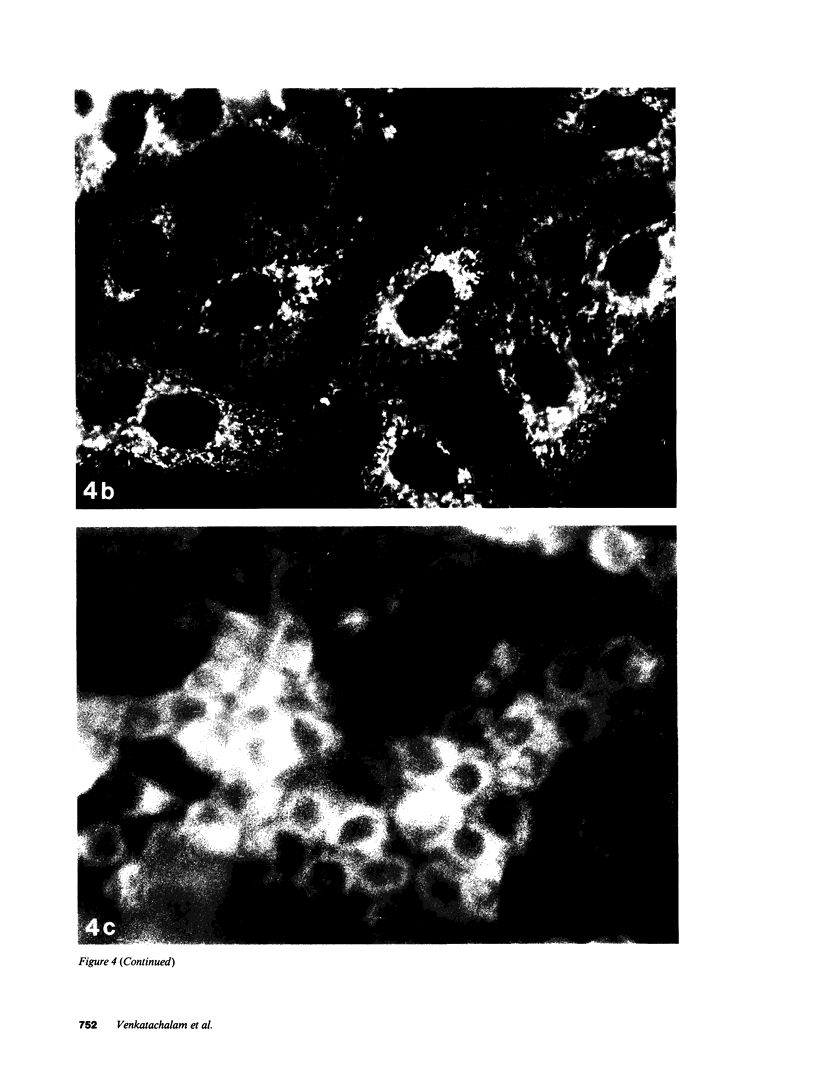
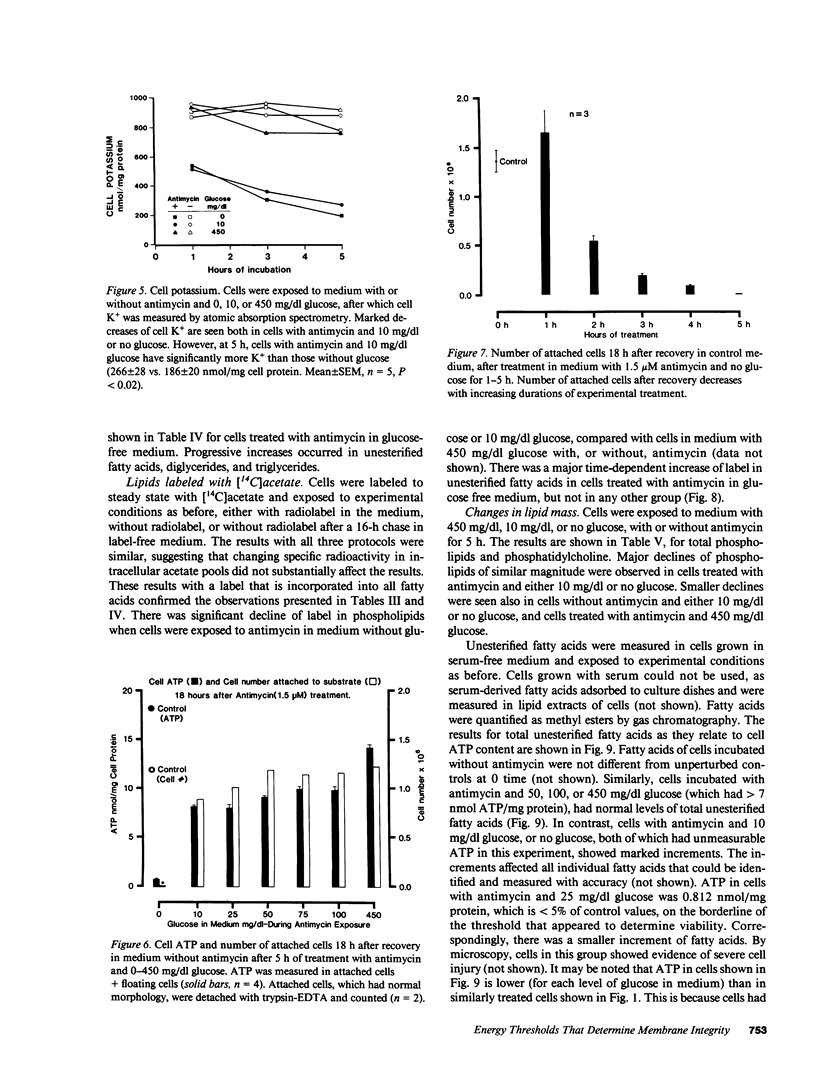
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M., Nash-Adler P., Watras J., Messineo F. C., Takenaka H., Louis C. F. Fatty acid effects on calcium influx and efflux in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. Biochim Biophys Acta. 1982 Apr 23;687(1):17–26. doi: 10.1016/0005-2736(82)90165-1. [DOI] [PubMed] [Google Scholar]
- LEAF A. On the mechanism of fluid exchange of tissues in vitro. Biochem J. 1956 Feb;62(2):241–248. doi: 10.1042/bj0620241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHNINGER A. L., REMMERT L. F. An endogenous uncoupling and swelling agent in liver mitochondria and its enzymic formation. J Biol Chem. 1959 Sep;234:2459–2464. [PubMed] [Google Scholar]
- LIEBECQ C., JACQUEMOTTE-LOUIS M. Nucléotides de l'adénine. V. Mécanisme de l'hydrolyse non enzymatique de l'adénosine triphosphate. Bull Soc Chim Biol (Paris) 1958;40(5-6):759–765. [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Martin J. K., Luthra M. G., Wells M. A., Watts R. P., Hanahan D. J. Phospholipase A2 as a probe of phospholipid distribution in erythrocyte membranes. Factors influencing the apparent specificity of the reaction. Biochemistry. 1975 Dec 16;14(25):5400–5408. doi: 10.1021/bi00696a003. [DOI] [PubMed] [Google Scholar]
- Matthys E., Patel Y., Kreisberg J., Stewart J. H., Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney Int. 1984 Aug;26(2):153–161. doi: 10.1038/ki.1984.149. [DOI] [PubMed] [Google Scholar]
- Parce J. W., Cunningham C. C., Waite M. Mitochondrial phospholipase A2 activity and mitochondrial aging. Biochemistry. 1978 May 2;17(9):1634–1639. doi: 10.1021/bi00602a009. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Prinzen F. W., Van der Vusse G. J., Arts T., Roemen T. H., Coumans W. A., Reneman R. S. Accumulation of nonesterified fatty acids in ischemic canine myocardium. Am J Physiol. 1984 Aug;247(2 Pt 2):H264–H272. doi: 10.1152/ajpheart.1984.247.2.H264. [DOI] [PubMed] [Google Scholar]
- Raz A., Livne A. Differential effects of lipids on the osmotic fragility of erythrocytes. Biochim Biophys Acta. 1973 Jun 22;311(2):222–229. doi: 10.1016/0005-2736(73)90269-1. [DOI] [PubMed] [Google Scholar]
- Rothman S. M. Synaptic activity mediates death of hypoxic neurons. Science. 1983 Apr 29;220(4596):536–537. doi: 10.1126/science.6836300. [DOI] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Collan Y., Kahng M. W., Trump B. F. Changes in mitochondrial lipids of rat kidney during ischemia. Biochim Biophys Acta. 1980 May 28;618(2):192–201. doi: 10.1016/0005-2760(80)90025-9. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Freudenrich C. C., Borle A. B. The effects of anoxia on cytosolic free calcium, calcium fluxes, and cellular ATP levels in cultured kidney cells. J Biol Chem. 1985 Sep 25;260(21):11619–11626. [PubMed] [Google Scholar]
- Snyder D. W., Crafford W. A., Jr, Glashow J. L., Rankin D., Sobel B. E., Corr P. B. Lysophosphoglycerides in ischemic myocardium effluents and potentiation of their arrhythmogenic effects. Am J Physiol. 1981 Nov;241(5):H700–H707. doi: 10.1152/ajpheart.1981.241.5.H700. [DOI] [PubMed] [Google Scholar]
- Soboll S., Gründel S., Schwabe U., Scholz R. Influence of fatty acids on energy metabolism. 2. Kinetics of changes in metabolic rates and changes in subcellular adenine nucleotide contents and pH gradients following addition of octanoate and oleate in perfused rat liver. Eur J Biochem. 1984 May 15;141(1):231–236. doi: 10.1111/j.1432-1033.1984.tb08180.x. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. III. The ATP dependence of the Na pump. J Gen Physiol. 1984 Oct;84(4):643–662. doi: 10.1085/jgp.84.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Soltoff S. P., Murdaugh S., Mandel L. J. Intracellular respiratory dysfunction and cell injury in short-term anoxia of rabbit renal proximal tubules. J Clin Invest. 1985 Dec;76(6):2377–2384. doi: 10.1172/JCI112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer D. A., Kreisberg J. I., Venkatachalam M. A. Lipid alterations in LLC-PK1 cells exposed to mercuric chloride. Kidney Int. 1986 Feb;29(2):530–538. doi: 10.1038/ki.1986.31. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Harding P. G., Humes H. D. Alterations in renal cortex cation homeostasis during mercuric chloride and gentamicin nephrotoxicity. Exp Mol Pathol. 1983 Aug;39(1):43–60. doi: 10.1016/0014-4800(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Schrier R. W. Nephron segment and calcium as determinants of anoxic cell death in renal cultures. Kidney Int. 1986 Jun;29(6):1172–1179. doi: 10.1038/ki.1986.124. [DOI] [PubMed] [Google Scholar]