Abstract
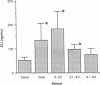
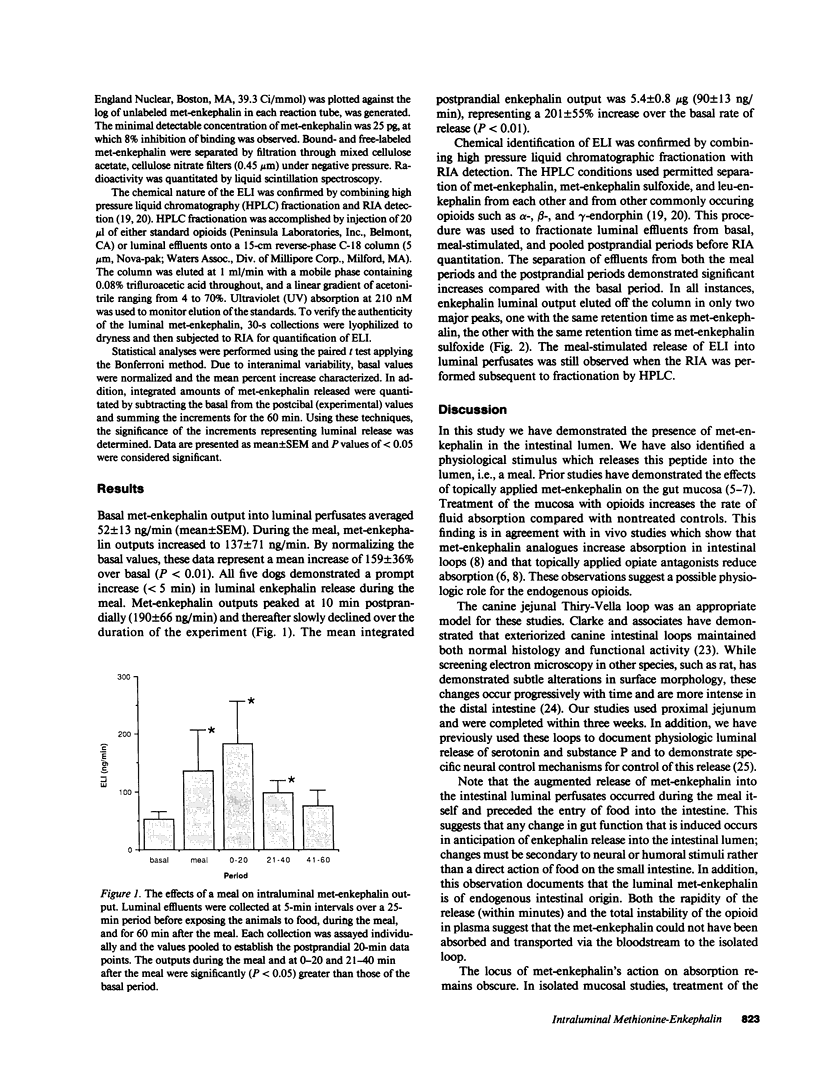
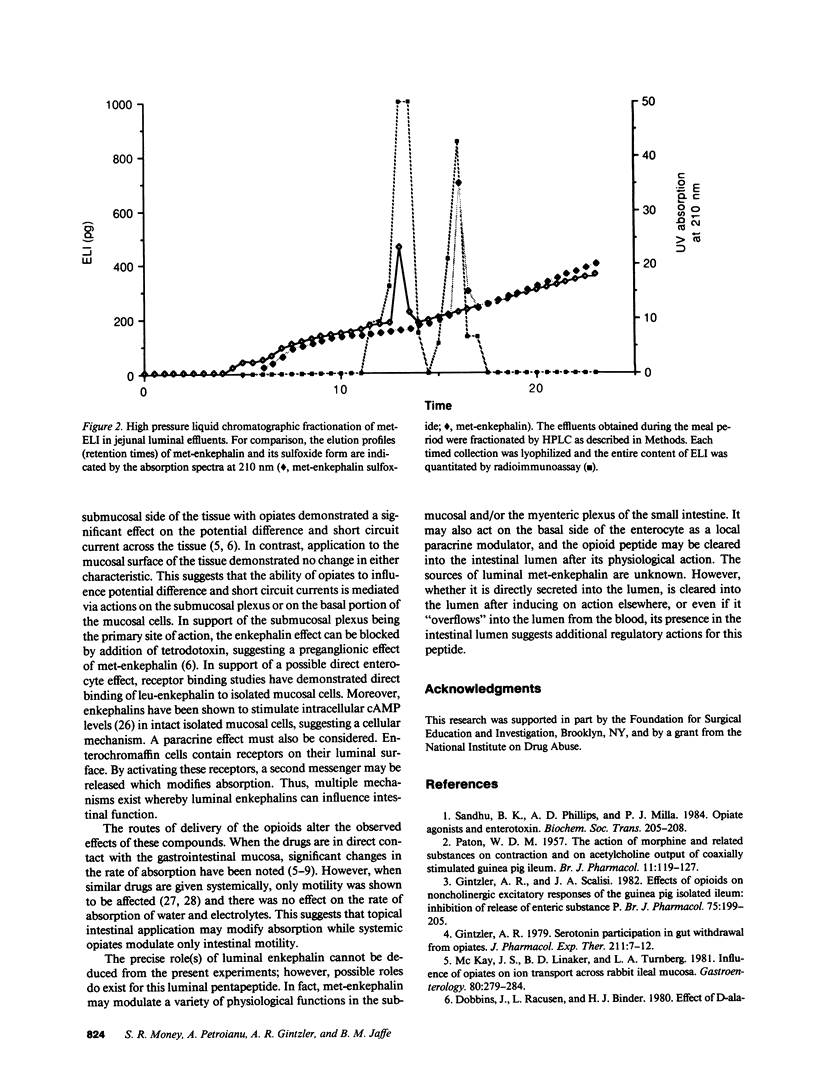
Application of enkephalins to the luminal surface of the bowel augments intestinal absorption. However, to date, endogenous enkephalins have not been demonstrated within intestinal luminal fluid. To determine whether enkephalins are present in the intestinal lumen, five adult dogs had 25-cm chronic jejunal Thiry-Vella loops constructed. Dogs were studied in the awake, fasted state. Jejunal loops were perfused with isoosmotic, neutral Krebs buffer containing protease inhibitors. After basal sampling, the dogs received a high fat meat meal. Collections were made during the meal and for 60 min postprandially. Luminal met-enkephalin levels were determined by radioimmunoassay and confirmed by HPLC. HPLC separation of luminal samples demonstrated two immunoreactive peaks which co-eluted with pure met-enkephalin and met-enkephalin-sulfoxide. Basal met-enkephalin outputs averaged 52 +/- 13 ng/min. The meal significantly increased mean luminal met-enkephalin output to 137 +/- 71 ng/min. During the initial 20-min postprandial period, output remained elevated (180 +/- 73 ng/min), after which it returned to basal levels. We conclude that met-enkephalin is present in the jejunal lumen, and that luminal release of this opioid is augmented by a meal.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark S. J., Smith T. W. Peristalsis abolishes the release of methionine-enkephalin from guinea-pig ileum in vitro. Eur J Pharmacol. 1981 Mar 26;70(3):421–424. doi: 10.1016/0014-2999(81)90175-8. [DOI] [PubMed] [Google Scholar]
- Clark S. J., Smith T. W. The release of Met-enkephalin from the guinea-pig ileum at rest and during peristaltic activity. Life Sci. 1983;33 (Suppl 1):465–468. doi: 10.1016/0024-3205(83)90542-8. [DOI] [PubMed] [Google Scholar]
- Clarke A. M., Miller M., Shields R. Intestinal transport of sodium, potassium, and water in the dog during sodium depletion. Gastroenterology. 1967 May;52(5):846–858. [PubMed] [Google Scholar]
- Daniel E. E., Costa M., Furness J. B., Keast J. R. Peptide neurons in the canine small intestine. J Comp Neurol. 1985 Jul 8;237(2):227–238. doi: 10.1002/cne.902370207. [DOI] [PubMed] [Google Scholar]
- Dashwood M. R., Debnam E. S., Bagnall J., Thompson C. S. Autoradiographic localisation of opiate receptors in rat small intestine. Eur J Pharmacol. 1985 Jan 2;107(2):267–269. doi: 10.1016/0014-2999(85)90068-8. [DOI] [PubMed] [Google Scholar]
- Dobbins J., Racusen L., Binder H. J. Effect of D-alanine methionine enkephalin amide on ion transport in rabbit ileum. J Clin Invest. 1980 Jul;66(1):19–28. doi: 10.1172/JCI109830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara A., Zinner M. J., Jaffe B. M. Intraluminal release of serotonin, substance P, and gastrin in the canine small intestine. Dig Dis Sci. 1987 Mar;32(3):289–294. doi: 10.1007/BF01297056. [DOI] [PubMed] [Google Scholar]
- Fogel R., Kaplan R. B. Role of enkephalins in regulation of basal intestinal water and ion absorption in the rat. Am J Physiol. 1984 Apr;246(4 Pt 1):G386–G392. doi: 10.1152/ajpgi.1984.246.4.G386. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Miller R. J. Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):653–664. doi: 10.1016/0306-4522(83)90001-5. [DOI] [PubMed] [Google Scholar]
- Gintzler A. R., Scalisi J. A. Effects of opioids on noncholinergic excitatory responses of the guinea-pig isolated ileum: inhibition of release of enteric substance P. Br J Pharmacol. 1982 Jan;75(1):199–205. doi: 10.1111/j.1476-5381.1982.tb08773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintzler A. R. Serotonin participation in gut withdrawal from opiates. J Pharmacol Exp Ther. 1979 Oct;211(1):7–12. [PubMed] [Google Scholar]
- Glass J., Chan W. C., Gintzler A. R. Direct analysis of the release of methionine-enkephalin from guinea pig myenteric plexus: modulation by endogenous opioids and exogenous morphine. J Pharmacol Exp Ther. 1986 Dec;239(3):742–747. [PubMed] [Google Scholar]
- Glass J., Clouet D., Gintzler A. R. Short-term nerve stimulation increases enkephalin production and content in the guinea pig myenteric plexus. Brain Res. 1986 Apr 30;372(1):180–184. doi: 10.1016/0006-8993(86)91475-7. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Cullen J., Dowling R. H. Intestinal structure and function after small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):731–742. doi: 10.1042/cs0430731. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Jaffe B. M., Ferrara A., Sherlock D. J. Comparative effects of ketanserin, atropine and methysergide on the gastrointestinal effects of hyperserotoninemia in the awake dog. J Pharmacol Exp Ther. 1986 Aug;238(2):536–541. [PubMed] [Google Scholar]
- Kachur J. F., Miller R. J. Characterization of the opiate receptor in the guinea-pig ileal mucosa. Eur J Pharmacol. 1982 Jul 9;81(2):177–183. doi: 10.1016/0014-2999(82)90435-6. [DOI] [PubMed] [Google Scholar]
- Kachur J. F., Miller R. J., Field M. Control of guinea pig intestinal electrolyte secretion by a delta-opiate receptor. Proc Natl Acad Sci U S A. 1980 May;77(5):2753–2756. doi: 10.1073/pnas.77.5.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. K., Coupar I. M. Opiate receptor-mediated inhibition of rat jejunal fluid secretion. Life Sci. 1980 Dec 15;27(24):2319–2325. doi: 10.1016/0024-3205(80)90500-7. [DOI] [PubMed] [Google Scholar]
- López-Ruiz M. P., Arilla E., Gómez-Pan A., Prieto J. C. Interaction of Leu-enkephalin with isolated enterocytes from guinea pig: binding to specific receptors and stimulation of cAMP accumulation. Biochem Biophys Res Commun. 1985 Jan 16;126(1):404–411. doi: 10.1016/0006-291x(85)90620-5. [DOI] [PubMed] [Google Scholar]
- McKay J. S., Linaker B. D., Turnberg L. A. Influence of opiates on ion transport across rabbit ileal mucosa. Gastroenterology. 1981 Feb;80(2):279–284. [PubMed] [Google Scholar]
- Nishimura E., Buchan A. M., McIntosh C. H. Autoradiographic localization of opioid receptors in the rat stomach. Neurosci Lett. 1984 Sep 7;50(1-3):73–78. doi: 10.1016/0304-3940(84)90465-8. [DOI] [PubMed] [Google Scholar]
- PATON W. D. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957 Mar;12(1):119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Bloom S. R., Sullivan S. N., Facer P., Pearse A. G. Enkephalin-like immunoreactivity in the human gastrointestinal tract. Lancet. 1977 May 7;1(8019):972–974. doi: 10.1016/s0140-6736(77)92277-2. [DOI] [PubMed] [Google Scholar]
- Sandhu B. K., Phillips A. D., Milla P. J. Opiate agonists and enterotoxins. Biochem Soc Trans. 1984 Apr;12(2):205–208. doi: 10.1042/bst0120205. [DOI] [PubMed] [Google Scholar]
- Schiller L. R., Davis G. R., Santa Ana C. A., Morawski S. G., Fordtran J. S. Studies of the mechanism of the antidiarrheal effect of codeine. J Clin Invest. 1982 Nov;70(5):999–1008. doi: 10.1172/JCI110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinayek R., Brown D. R., Miller R. J. Tolerance and cross-tolerance to the antisecretory effects of enkephalins on the guinea-pig ileal mucosa. J Pharmacol Exp Ther. 1985 Mar;232(3):781–785. [PubMed] [Google Scholar]