Abstract
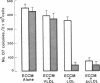
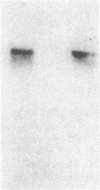
Endothelial cells are a known source of hematopoietic growth-enhancing factors, including platelet-derived growth factor (PDGF). In addition, endothelium interacts directly with plasma lipoproteins which have been shown to modulate hematopoiesis. To determine the relationship of these properties, we measured the release of an erythroid growth-enhancing factor from bovine endothelial cells under lipid-loaded and control conditions. Human bone marrow cells cultured under serum-free conditions form more erythroid, granulocyte/macrophage, and mixed hematopoietic colonies when supplemented with endothelial cell-conditioned medium (ECCM) than do controls (P less than 0.05). The activity is expressed over a wide range of erythropoietin, lymphocyte-conditioned medium (LCM), recombinant human interleukin-3, and colony-stimulating factor (CSF) concentrations, and is related to ECCM dose. In contrast, enhancing activity in ECCM prepared with 0-400 micrograms/ml acetylated low density lipoproteins (AcLDL) or native LDL is diminished to 0% in a dose-dependent fashion (relative to ECCM from unexposed cells or from cells incubated with very low density lipoproteins, P less than 0.05). Upon dilution, medium prepared from cells incubated with LDL shows a rightward shift in the dose-response curve for erythroid colony formation, while that prepared from AcLDL loaded cells demonstrates a downward shift, indicating that the inhibitory activities are kinetically distinct. Delipidation of ECCM prior to addition to marrow culture removes the inhibitory action of native LDL (P less than 0.05) but not that of AcLDL (P greater than 0.10). Immunochemical analysis suggests that the erythropoietic activity in ECCM is unrelated to that of PDGF, recombinant human CSF, and erythroid burst-promoting activity (BPA) present in LCM. This conclusion is supported by Northern blot analysis of endothelial cells using a cDNA probe for the v-sis homologue of the PDGF beta chain and by immunoprecipitation of metabolically labeled PDGF. The relative amounts of c-sis transcripts and of secreted PDGF were similar in endothelial cells incubated with or without AcLDL. We conclude that AcLDL impair the synthesis or release of an erythropoietic growth-enhancing factor(s) which is biologically distinct from PDGF and BPA present in LCM.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascensao J. L., Vercellotti G. M., Jacob H. S., Zanjani E. D. Role of endothelial cells in human hematopoiesis: modulation of mixed colony growth in vitro. Blood. 1984 Mar;63(3):553–558. [PubMed] [Google Scholar]
- Bagby G. C., Jr, Dinarello C. A., Wallace P., Wagner C., Hefeneider S., McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986 Nov;78(5):1316–1323. doi: 10.1172/JCI112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Bergstrom K. A., Burger D. A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood. 1983 Sep;62(3):663–668. [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W. Role of cholesterol metabolism in cell growth. Fed Proc. 1984 Jan;43(1):126–130. [PubMed] [Google Scholar]
- Chen S. S. Enhanced sterol synthesis in concanavalin A-stimulated lymphocytes: correlation with phospholipid synthesis and DNA synthesis. J Cell Physiol. 1979 Jul;100(1):147–157. doi: 10.1002/jcp.1041000115. [DOI] [PubMed] [Google Scholar]
- Curtiss L. K., DeHeer D. H., Edgington T. S. Influence of the immunoregulatory serum lipoprotein LDL-In on the in vivo proliferation and differentiation of antigen-binding and antibody-secreting lymphocytes during a primary immune response. Cell Immunol. 1980 Jan;49(1):1–11. doi: 10.1016/0008-8749(80)90050-7. [DOI] [PubMed] [Google Scholar]
- Dainiak N., Cohen C. M. Surface membrane vesicles from mononuclear cells stimulate erythroid stem cells to proliferate in culture. Blood. 1982 Sep;60(3):583–594. [PubMed] [Google Scholar]
- Dainiak N., Davies G., Kalmanti M., Lawler J., Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983 May;71(5):1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Feldman L., Cohen C. M. Neutralization of erythroid burst-promoting activity in vitro with antimembrane antibodies. Blood. 1985 Apr;65(4):877–885. [PubMed] [Google Scholar]
- Dainiak N., Kreczko S., Cohen A., Pannell R., Lawler J. Primary human marrow cultures for erythroid bursts in a serum-substituted system. Exp Hematol. 1985 Nov;13(10):1073–1079. [PubMed] [Google Scholar]
- Dainiak N. Role of defined and undefined serum additives to hematopoietic stem cell culture. Prog Clin Biol Res. 1985;184:59–76. [PubMed] [Google Scholar]
- Dainiak N., Sutter D., Kreczko S. L-triiodothyronine augments erythropoietic growth factor release from peripheral blood and bone marrow leukocytes. Blood. 1986 Dec;68(6):1289–1297. [PubMed] [Google Scholar]
- Davies P. F., Ross R. Mediation of pinocytosis in cultured arterial smooth muscle and endothelial cells by platelet-derived growth factor. J Cell Biol. 1978 Dec;79(3):663–671. doi: 10.1083/jcb.79.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Truskey G. A., Warren H. B., O'Connor S. E., Eisenhaure B. H. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J Cell Biol. 1985 Sep;101(3):871–879. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche F., Raines E., Powell J., Ross R., Adamson J. Platelet-derived growth factor enhances in vitro erythropoiesis via stimulation of mesenchymal cells. J Clin Invest. 1985 Jul;76(1):137–142. doi: 10.1172/JCI111936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Douay L., Barbu V., Baillou C., Najman A., Gorin N. C., Polonovski J., Duhamel G. The role of serum lipoproteins on the in vitro proliferative potential of human hematopoietic progenitors CFUC and CFUE. Exp Hematol. 1983 Jul;11(6):499–505. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feldman L., Cohen C. M., Riordan M. A., Dainiak N. Purification of a membrane-derived human erythroid growth factor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6775–6779. doi: 10.1073/pnas.84.19.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. L., DiCorleto P. E. Modified low density lipoproteins suppress production of a platelet-derived growth factor-like protein by cultured endothelial cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4774–4778. doi: 10.1073/pnas.83.13.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galelli A., Dosne A. M., Morin A., Dubor F., Chedid L. Stimulation of human endothelial cells by synthetic muramyl peptides: production of colony-stimulating activity (CSA). Exp Hematol. 1985 Dec;13(11):1157–1163. [PubMed] [Google Scholar]
- Gordon M. Y., Kearney L., Hibbin J. A. Effects of human marrow stromal cells on proliferation by human granulocytic (GM-CFC), erythroid (BFU-E) and mixed (Mix-CFC) colony-forming cells. Br J Haematol. 1983 Feb;53(2):317–325. doi: 10.1111/j.1365-2141.1983.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Heider J. G., Boyett R. L. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978 May;19(4):514–518. [PubMed] [Google Scholar]
- Heiniger H. J., Brunner K. T., Cerottini J. C. Cholesterol is a critical cellular component for T-lymphocyte cytotoxicity. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5683–5687. doi: 10.1073/pnas.75.11.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. F., Wight A., Ludwig P., Cornell R. Interrelated lipid alterations and their influence on the proliferation and fusion of cultured myogenic cells. J Cell Biol. 1978 May;77(2):334–357. doi: 10.1083/jcb.77.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W., Heiniger H. J. Biological activity of some oxygenated sterols. Science. 1978 Aug 11;201(4355):498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Nathan D. G. T cell and monocyte-derived burst-promoting activity directly act on erythroid progenitor cells. Nature. 1984 Dec 20;312(5996):775–777. doi: 10.1038/312775a0. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Reitz B. A., Volpe J. J. Effects of prior sterol depletion on neurite outgrowth in neuroblastoma cells. J Cell Physiol. 1981 Sep;108(3):475–482. doi: 10.1002/jcp.1041080322. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Burgess A. W. Direct stimulation by purified GM-CSF of the proliferation of multipotential and erythroid precursor cells. Blood. 1980 Jan;55(1):138–147. [PubMed] [Google Scholar]
- Najman A., Baillou C., Drouet X., Leblanc G., Douay L., Gorin N. C., Duhamel G. Regulation of human peripheral blood BFU-E growth in vitro by leukaemic B-lymphocytes. Br J Haematol. 1985 Aug;60(4):643–650. doi: 10.1111/j.1365-2141.1985.tb07468.x. [DOI] [PubMed] [Google Scholar]
- Quesenberry P. J., Gimbrone M. A., Jr Vascular endothelium as a regulator of granulopoiesis: production of colony-stimulating activity by cultured human endothelial cells. Blood. 1980 Dec;56(6):1060–1067. [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Sieff C. A., Tsai S., Faller D. V. Interleukin 1 induces cultured human endothelial cell production of granulocyte-macrophage colony-stimulating factor. J Clin Invest. 1987 Jan;79(1):48–51. doi: 10.1172/JCI112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroobant P., Waterfield M. D. Purification and properties of porcine platelet-derived growth factor. EMBO J. 1984 Dec 1;3(12):2963–2967. doi: 10.1002/j.1460-2075.1984.tb02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Waddell C. C., Taunton O. D., Twomey J. J. Inhibition of lymphoproliferation by hyperlipoproteinemic plasma. J Clin Invest. 1976 Oct;58(4):950–954. doi: 10.1172/JCI108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Zucker S., Michael M. S., Lysik R. M., Glucksman M. J., Reese J., Rudin A., DiStefano J. Liproprotein inhibitor of bone marrow cells in tumor-bearing rats. Cell Tissue Kinet. 1979 Jul;12(4):393–404. doi: 10.1111/j.1365-2184.1979.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Zuckerman K. S., Bagby G. C., Jr, McCall E., Sparks B., Wells J., Patel V., Goodrum D. A monokine stimulates production of human erythroid burst-promoting activity by endothelial cells in vitro. J Clin Invest. 1985 Feb;75(2):722–725. doi: 10.1172/JCI111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K. S. Human erythroid burst-forming units. Growth in vitro is dependent on monocytes, but not T lymphocytes. J Clin Invest. 1981 Mar;67(3):702–709. doi: 10.1172/JCI110086. [DOI] [PMC free article] [PubMed] [Google Scholar]