Abstract
Lessons Learned
The pharmacokinetic results of this phase Ib study of ramucirumab combined with paclitaxel as second-line therapy in Japanese patients with metastatic gastric or gastro-esophageal junction adenocarcinoma are in line with previous ramucirumab studies.
This combination at the doses and schedule given did not result in any dose-limiting toxicities and appeared to be safe and well tolerated.
Background.
This phase Ib study evaluated the tolerability and pharmacokinetics of ramucirumab, an anti-VEGFR-2 antibody, combined with paclitaxel as second-line therapy in Japanese patients with metastatic gastric or gastroesophageal junction adenocarcinoma after first-line therapy with fluoropyrimidines and/or platinum.
Methods.
Patients received ramucirumab 8 mg/kg on days 1 and 15 and paclitaxel 80 mg/m2 on days 1, 8, and 15 of a 28-day cycle. Safety analyses included all patients (n = 6).
Results.
No dose-limiting toxicities occurred in the first cycle. All patients experienced ≥1 treatment-emergent adverse event (TEAE); 5 patients experienced grade ≥3 TEAEs. There were two deaths caused by disease progression. The best overall responses were stable disease (n = 5) and partial response (n = 1). Patients received ramucirumab and paclitaxel for a median of 12.5 weeks (range: 11.4–42.7 weeks) and 12.2 weeks (range: 11.0–41.0 weeks), respectively. Following a single dose of ramucirumab IV infusion 8 mg/kg, clearance was ∼0.017 L/hour, half-life (t1/2) was 138 to 225 hours, and steady-state volume of distribution (Vss) was ∼3 L.
Conclusion.
The ramucirumab/paclitaxel combination appears to be well-tolerated in Japanese patients with advanced gastric adenocarcinomas. These results are in line with previous ramucirumab pharmacokinetic studies as anticipated.
Abstract
摘要
背景. 本项Ib期研究纳入了患有转移性胃腺癌或胃食管交界部腺癌的日本患者,评价了抗VEGFR-2抗体雷莫芦单抗联合紫杉醇作为氟尿嘧啶和/或铂类一线治疗失败后的二线治疗的耐受性和药代动力学特征。
方法. 给予患者雷莫芦单抗8 mg/kg(第1、15天)和紫杉醇80 mg/m2(第1、8、15天)治疗,28天为一周期。安全性分析纳入了所有患者(n = 6)。
结果. 第一个周期中未发生剂量限制毒性事件。所有患者均发生≥ 1次治疗后出现的不良事件(TEAE),5例患者发生了≥ 3级TEAE。2例患者死于疾病进展。最佳总体治疗反应为疾病稳定(n = 5)和部分缓解(n = 1)。中位治疗时间分别为雷莫芦单抗12.5周(范围:11.4 ∼ 42.7周)和紫杉醇12.2周(范围:11.0 ∼ 41.0周)。单次静脉注射雷莫芦单抗8 mg/kg后,清除率约为0.017 L/小时,半衰期(t1/2)为138 ∼ 225小时,稳态分布容积(VSS)约为3 L。
结论. 雷莫芦单抗联合紫杉醇方案在日本进展期胃腺癌患者中耐受良好。如同预期,这些结果与既往雷莫芦单抗药代动力学研究的结果一致。The Oncologist 2015;20:493–494
Author Summary
Discussion
The primary objective of this study was to confirm the recommended dose of ramucirumab in combination with paclitaxel and assess pharmacokinetics (PK) of ramucirumab in Japanese patients with advanced gastric adenocarcinomas who failed standard therapy with fluoropyrimidines and/or platinum. Exploratory objectives included pharmacodynamics and antitumor activity. Ramucirumab is a recombinant human monoclonal antibody against human vascular endothelial growth factor receptor-2 (VEGFR-2) preventing ligand binding and receptor-mediated pathway activation in endothelial cells [1, 2]. Inhibition of VEGFR-2 in gastric cancer xenografts (thymidylate kinase-1 cell line) is associated with reduced tumor growth [1]. Weekly administration of paclitaxel (at a dose of 80 mg/m2) has been extensively studied as second-line chemotherapy for gastric cancer and is considered standard care [3–10].
Ramucirumab plus paclitaxel is approved by the U.S. Food and Drug Administration (FDA) for second-line treatment in gastric cancer based on the 2.2-month overall survival advantage seen in the RAINBOW trial (trial was powered to detect a 2.3-month difference) [11]. In the current study, 8 mg/kg ramucirumab was administered on days 1 and 15 combined with 80 mg/m2 paclitaxel on days 1, 8, and 15 in a 28-day cycle. Patients received ramucirumab and paclitaxel for a median of 12.5 weeks (range: 11.4–42.7 weeks) and 12.2 weeks (range: 11.0–41.0 weeks), respectively.
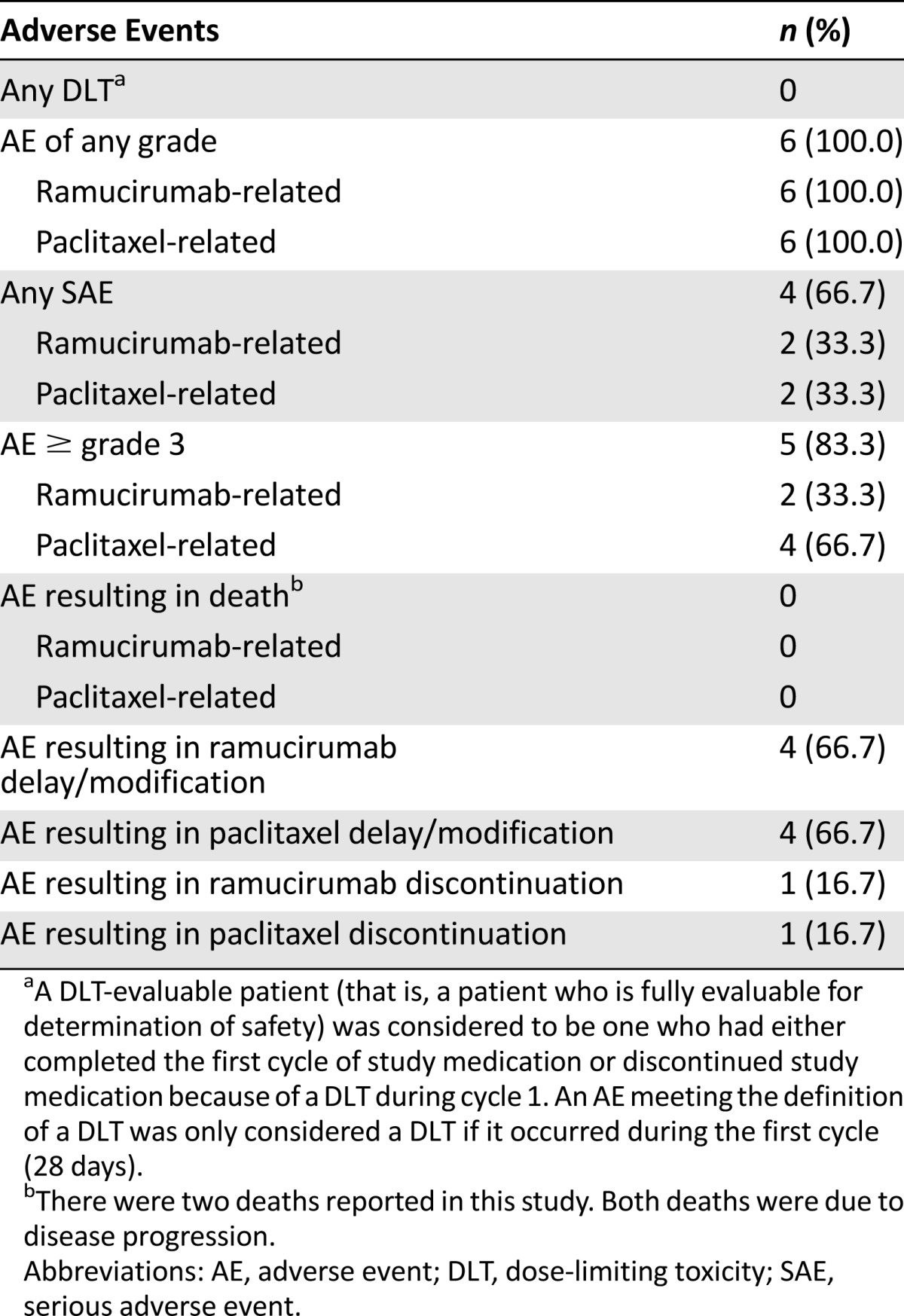
Safety analyses included all treated patients (n = 6) (Table 1). All patients (n = 6) experienced ≥1 treatment-emergent adverse event (TEAE) of any grade (grade ≥3 in 5 patients), ramucirumab-related TEAEs, and paclitaxel-related TEAEs. There were no ramucirumab- or paclitaxel-related grade ≥4 TEAEs. Five patients discontinued because of progressive disease (PD), and one patient discontinued because of a TEAE (meningism) not related to ramucirumab or paclitaxel. The two deaths reported were due to PD and were not study drug-related. Seven serious adverse events (SAEs) occurred in four patients. Ramucirumab- or paclitaxel-related SAEs included pneumonia in two patients and gastrointestinal hemorrhage in one patient.
Table 1.
Treatment-emergent adverse events (safety population, N = 6)
Following a single IV infusion of 8 mg/kg ramucirumab, PK analysis indicated a half-life ranging from 138 to 225 hours. Following multiple doses of 8 mg/kg ramucirumab, steady state was approximately achieved on cycle 2, day 1, and the accumulation ratio calculated using area under the concentration-time curve (RA, AUC) was approximately 1.5. Geometric mean of steady state Cmin ranged from 44.2 µg/mL (% coefficient of variation [CV]: 21%) to 66.6 µg/mL (% CV: 25%) between cycle 2, day 1 and cycle 3, day 1. Trend plots for pharmacodynamic data revealed increasing levels of VEGF-D following the first ramucirumab infusion. No apparent trends were identified for VEGF-C, soluble neuropilin-1, or VEGFR-1.
No dose-limiting toxicities (DLTs) were observed within the first 28-day cycle, which was the DLT-observation period. Four patients experienced SAEs in later cycles, and predefined dose-modification strategies were used. Limitations of this study included the small sample size and uncontrolled design. Because of the small sample size, no efficacy conclusions could be drawn. In conclusion, the combination of ramucirumab and paclitaxel at the doses and schedule given did not result in any DLTs and appeared to be safe and well-tolerated in Japanese patients with advanced gastric adenocarcinomas.
Supplementary Material
Footnotes
Access the full results at: Satoh-14-440.theoncologist.com
ClinicalTrials.gov Identifier: NCT01253525
Sponsor(s): Eli Lilly and Company
Principal Investigator: Toshihiko Doi
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


