Abstract
Lessons Learned
The concomitant use of weekly nab-paclitaxel and carboplatin with concurrent radiotherapy was demonstrated to be a safe therapeutic approach in this phase I trial of 10 evaluable patients with stage III NSCLC.
Despite the lack of systemic glucocorticoids, there were no reported infusion reactions or cases of peripheral neuropathy in this trial, both of which are known to occur with the use of paclitaxel.
Background.
Unresectable stage III non-small cell lung cancer (NSCLC) has a 5-year survival rate of 20%, and concurrent chemoradiotherapy results in significant toxicity with the use of current chemotherapeutic agents. nab-Paclitaxel was approved by the U.S. Food and Drug Administration in October 2012 for use along with carboplatin in advanced NSCLC. This study was undertaken to determine the maximum tolerated dose and dose-limiting toxicities (DLTs) of weekly nab-paclitaxel given in combination with carboplatin and concurrent radiotherapy in patients with unresectable stage III NSCLC.
Methods.
Escalating doses of once-weekly nab-paclitaxel were given along with once-weekly carboplatin area under the plasma concentration time curve (AUC) of 2 and concurrent radiotherapy 66 Gy in 33 fractions, followed by 2 cycles of carboplatin and nab-paclitaxel consolidation chemotherapy.
Results.
Eleven patients were enrolled and received treatment per protocol, with 10 evaluable for efficacy and toxicity. At dose level 1 (nab-paclitaxel 60 mg/m2), 2 DLTs were observed: esophagitis and radiation dermatitis. Six patients were enrolled at dose level 0 (nab-paclitaxel 40 mg/m2) with no DLTs. Nine of 10 evaluable patients had a partial response.
Conclusion.
Concurrent chemoradiotherapy with nab-paclitaxel 40 mg/m2 and carboplatin AUC 2 is a safe and well-tolerated therapeutic regimen in patients with stage III NSCLC. A separate phase I/II study to evaluate the efficacy of this regimen is under way.
Abstract
摘要
背景. 不可切除的III期非小细胞肺癌(NSCLC)5年生存率为20%,而使用当前的化疗药物实行同期放化疗可导致明显的毒性。美国食品和药物监督管理局(FDA)在2012年批准白蛋白结合型紫杉醇可与卡铂联合治疗进展期NSCLC。本研究旨在确定每周一次白蛋白结合型紫杉醇联合卡铂及同期放疗在不可切除的III期NSCLC患者中的最大耐受剂量和剂量限制毒性(DLT)。
方法. 白蛋白结合型紫杉醇每周一次给药,剂量逐渐上调;卡铂给药剂量为血浆浓度时间曲线(AUC)= 2;同期放疗为66 Gy剂量分33次照射。随后给予2周期卡铂和白蛋白结合型紫杉醇巩固化疗。
结果. 研究共纳入11例患者,并按方案给予治疗。其中10例可评价有效性和毒性。在剂量水平为1(白蛋白结合型紫杉醇60 mg/m2)时,观察到2起DLT:食管炎和放射性皮炎。剂量水平为0(白蛋白结合型紫杉醇40 mg/m2)时纳入的6例患者中无一发生DLT。10例可评价患者中有9例达到部分缓解。
结论. 对于III期NSCLC患者,白蛋白结合型紫杉醇40 mg/m2和卡铂AUC 2联合同期放化疗是安全且耐受良好的治疗方案。另一项I/II期研究正在评价这一方案的有效性。The Oncologist 2015;20:491–492
Author Summary
Discussion
In this phase I trial with 10 evaluable patients with stage III NSCLC, the concomitant use of weekly nab-paclitaxel and carboplatin with concurrent radiotherapy was demonstrated to be a safe therapeutic approach (Table 1). The maximum tolerated dose was nab-paclitaxel 40 mg/m2 with carboplatin AUC 2 along with daily radiotherapy to a dose of 66 Gy in 33 fractions. There was no DLT at this dose level. Adverse events (AEs) were common but expected, given this modality of therapy and stage of disease. In fact, the range and grade of AEs were similar to previous trials using concurrent chemoradiotherapy for stage III disease [1, 2].
Table 1.
Individual patient outcomes
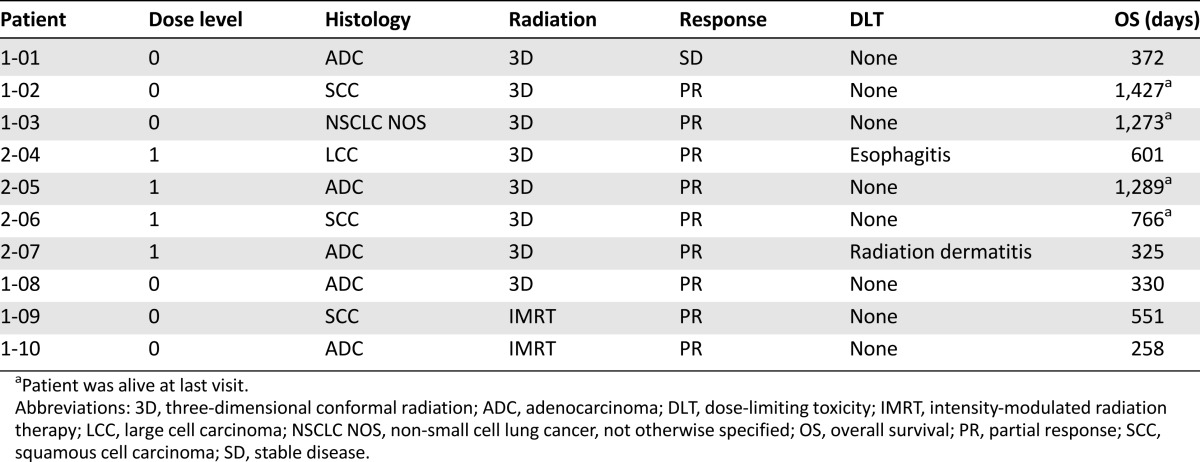
Because of the nature of a phase I trial and the small number of patients enrolled, it is not appropriate to draw meaningful conclusions concerning overall response rate (ORR) or survival. In this trial, however, 30% of the patients were alive ∼3 years after enrollment, a result similar to the acknowledged survival pattern for patients with stage III NSCLC [1, 2]. In addition, the ORR in this small phase I trial was 90%, with 9 patients experiencing a partial response by RECIST. Phase III trials of combination chemoradiotherapy in stage III disease have reported ORRs between 50% and 80% [5–7].
There is significant room for improvement in the treatment of stage III NSCLC. To that end, numerous cytotoxic agents and novel therapies have been tested or are being evaluated in this cohort of patients. Its effectiveness in this population will require further investigation, and a phase I/II trial is ongoing comparing radiotherapy given concurrently with either carboplatin plus nab-paclitaxel or carboplatin plus paclitaxel (ClinicalTrials.gov identifier NCT01757288).
Supplementary Material
Footnotes
Access the full results at: Lammers-15-30.theoncologist.com
ClinicalTrials.gov Identifier: NCT00544648
Sponsor(s): Vanderbilt-Ingram Cancer Center
Principal Investigator: Vicki Keedy
IRB Approved: Yes
For Further Reading: Eva Branden, Gunnar Hillerdal, Karl Kolbeck et al. Pemetrexed and Gemcitabine Versus Carboplatin and Gemcitabine in Non-Small Cell Lung Cancer: A Randomized Noninferiority Phase II Study in One Center. The Oncologist 2015;20:365.
Abstract
Background. The standard treatment for non-small cell lung cancer (NSCLC) stages IIIb and IV is a platinum compound combined with a third-generation cytotoxic agent. We decided to conduct a phase II study to assess whether the platinum compound could be replaced with pemetrexed with similar results and without an increase in side effects.
Methods. Consecutive eligible patients were randomized to either the standard arm of gemcitabine plus carboplatin (GC) or the experimental arm of gemcitabine plus pemetrexed (GP).
Results. Fifty evaluable patients were enrolled in the GC arm, and 44 received GP. There were 10 partial responses in the GC arm and 16 in the GP arm. With GC, mean survival was 9 months compared with 15 months with GP. The side effects were similar in both groups.
Conclusion. Pemetrexed can replace platinum compounds in the first-line treatment of stage IIIb and IV NSCLC without increasing the side effects. A trend toward better survival was observed in the patients receiving pemetrexed instead of a platinum compound, and this should be studied further.
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


