Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cyclic AMP-dependent protein kinase (PKA) regulated Cl− channel crucial for epithelial cell regulation of salt and water transport. Previous studies showed that ezrin, an actin binding and A-kinase anchoring protein (AKAP), facilitates association of PKA with CFTR. We used immunohistochemistry and immunogold transmission electron microscopy to localize CFTR, ezrin, and PKA type II regulatory (RII) and catalytic (C) subunits in striated duct cells of human parotid and submandibular glands. Immunohistochemistry localized the four proteins mainly to the apical membrane and apical cytoplasm of striated duct cells. In acinar cells, ezrin localized to the luminal membrane, and RII was present in secretory granules as previously described. Immunogold labeling showed that CFTR, PKA RII and C subunits were localized to the luminal membrane and associated with apical granules and vesicles of striated duct cells. Ezrin was present along the luminal membrane, on microvilli and along the junctional complexes between cells. Double labeling showed specific protein associations with apical granules and vesicles and along the luminal membrane. Ezrin, CFTR and PKA RII and C subunits are co-localized in striated duct cells, suggesting the presence of signaling complexes that serve to regulate CFTR activity.
Keywords: parotid gland, submandibular gland, immunohistochemistry, transmission electron microscopy, protein kinase A
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cyclic AMP-mediated Cl− channel that regulates equilibrium transport of salt and water (1, 2). CFTR is present in the luminal or apical cell membrane of airway epithelial cells, pancreatic ductal cells, intestinal epithelial cells, sweat gland duct cells, and epididymal and vas deferens epithelial cells (3). The insertion of CFTR into the cell membrane occurs via vesicular transport from the Golgi complex to the apical region of the cell (4). Numerous mutations in the CFTR gene lead to misfolded protein products that are degraded in the endoplasmic reticulum, fail to reach the cell membrane, or have little or no channel activity (4). These gene mutations cause the inherited chronic disease, cystic fibrosis, characterized by a defective regulation of epithelial electrolyte and water homeostasis, increased mucus viscosity, chronic infections, especially of the lung, and early death (5).
In rodent and human salivary glands CFTR has been localized to the luminal membrane of striated duct cells (6-13), and occasionally in acinar secretory cells (8, 11). Striated duct cells modify the primary saliva produced by the acinar cells by reabsorption of Na+ and Cl−, and secretion of K+ and HCO3−. Studies of saliva secretion in mice with a deletion in Cftr corresponding to the human ΔF508 mutation indicate that NaCl reabsorption by striated duct cells requires both CFTR and the epithelial sodium channel, ENaC (13). The activity of ENaC is dependent on CFTR in sweat gland ducts (14), and studies using fluorescence resonance energy transfer (FRET) and co-immunoprecipitation place ENaC and CFTR in close physical proximity (15).
The cellular processes leading to CFTR activation and opening of the channel involve binding of cyclic adenosine-3’5’-monophosphate (cyclic AMP) to the regulatory subunits (RII) of type II cyclic AMP-dependent protein kinase (PKA) and phosphorylation of serine residues in the regulatory domain of CFTR by the catalytic (C) subunits of PKA (16, 17). Although the cyclic AMP-PKA signaling pathway has been well studied in salivary glands (18-21), there is little understanding of how PKA is localized and how it exerts its regulatory function on CFTR in intact salivary gland cells. Generally, PKA is localized in discrete cellular compartments by anchoring proteins (A-kinase anchoring proteins, AKAPs), allowing activation of specific signaling pathways (22). In human salivary glands, PKA RII subunits are associated with small vesicles in the apical cytoplasm of striated duct cells (23), suggesting that an AKAP may be present and part of a signaling complex involved in regulating CFTR activity. In parietal cells of the gastric mucosa, the protein ezrin, which has a PKA RII binding domain in its central α-helical domain (24), is required for histamine-stimulated acid secretion (25, 26). Ezrin is a member of the ezrin-radixin-moesin (ERM) family of proteins, which bind filamentous actin, are associated with adherens junctions and involved in cortical membrane-cytoskeleton linkages, and participate in signal transduction (27, 28). These data suggest that ezrin functions in anchoring PKA to specialized regions of the cells and may be the AKAP involved in a PKA-AKAP-CFTR complex mediating efficient and localized activation of CFTR by PKA (24, 29).
We hypothesized that ezrin is an AKAP in human salivary gland duct cells, anchoring PKA to CFTR via the RII subunit. The objective of this study was to identify PKA, CFTR and ezrin in human salivary glands, employing immunohistochemistry and immunogold electron microscopy, in order to determine if they are co-localized and could function as a signaling complex that regulates CFTR activity in striated duct cells.
Materials and methods
Tissue Preparation
Normal human salivary gland tissues (4 parotid glands and 7 submandibular glands) were obtained from 11 consenting patients undergoing surgery at the Otorhinolaryngology Clinic at the University of Cagliari, Cagliari, Italy. All procedures were approved by the Human Experimentation Committee, University of Cagliari. The use of these samples at the University of Connecticut Health Center (UCHC) was approved by the UCHC Institutional Review Board. For light microscopic immunohistochemistry, the tissue samples were fixed overnight in 4% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, then stored in 1% paraformaldehyde in cacodylate buffer. The tissues were embedded in paraffin and 5-μm sections were collected on coated slides. For electron microscopic studies, the tissue samples were cut into small pieces, fixed for 2-3 h in 3% paraformaldehyde-0.1% glutaraldehyde in cacodylate buffer, then stored in 1% paraformaldehyde. The samples were embedded in LR White resin at 50°C overnight, and thin sections were cut with a diamond knife and collected on formvar-coated 200-400 mesh nickel specimen grids.
Immunolabeling and Microscopy
For light microscopic immunohistochemistry, the sections were deparaffinized and rehydrated, subjected to antigen retrieval in 5% urea/0.05 M β-mercaptoethanol at 95°C for 10 min and treated with 0.3% H2O2 in 85% methanol to block endogenous peroxidase activity.
Non-specific binding was blocked with 1% bovine serum albumin (BSA)/5% normal serum in phosphate buffered saline (PBS), then the sections were incubated for 1 h at room temperature with the primary antibody diluted in the blocking buffer. The antibodies used and their dilutions are shown in Table 1. Bound antibodies were detected with biotinylated secondary antibody, followed by an ABC reagent (Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine-H2O2 for color development. The sections were lightly stained with hematoxylin, dehydrated and coverslipped with DPX. Controls included omission of the primary antibody and substitution of the primary with non-immune IgG. The sections were examined and photographed in a Leica Orthoplan brightfield microscope equipped with a Nikon digital camera. The images were transferred to a Macintosh G4 computer, and a background white correction was made using Adobe Photoshop version 7.0.1
Table 1.
Antibodies Used for Light and Electron Microscopic Immunolabeling
| Antibody | Source | Catalog No. | Dilution – LM | Dilution – EM |
|---|---|---|---|---|
| Rabbit anti-CFTR | Chemicon | AB3555 | 1:40 – 1:75 | 1:5 – 1:20 |
| Mouse monoclonal anti-CFTR | R&D Systems | MAB1660 | --- | 1:10 – 1:25 |
| Mouse monoclonal anti-ezrin | Sigma-Aldrich | E8897 | --- | 1:20 |
| Rabbit anti-ezrin | Abcam | ab41672 | 1:150 – 1:400 | 1:10 – 1:100 |
| Mouse monoclonal anti-PKA C subunit | Abcam | ab64596 | 1:50 – 1:100 | 1:10 – 1:30 |
| Rabbit anti-PKA RII subunit | M. Mednieks | Ref. no. 30, 31 | 1:500 – 1:1000 | 1:250 – 1:500 |
Electron microscopic immunogold labeling was performed as described previously (32). To block non-specific binding, the thin sections were treated with BSA and normal serum in PBS, or a combination of ovalbumin, fish gelatin, Tween 20 and Tris buffer with 0.5 M NaCl. Primary antibodies were diluted in the same blocking solution, and incubated overnight at 4°C. Dilutions of the primary antibodies were selected based on optimal conditions that minimized background and maximized protein labeling. The bound primary antibodies were detected by gold-labeled goat anti-mouse IgG (10 nm diameter gold particles) or goat anti-rabbit IgG (15 nm diameter) (Auroprobe, GE Healthcare, City?, UK; or Aurion, Electron Microscopy Sciences, Hatfield, PA, USA), which were incubated with the thin sections for 1 h at room temperature. Control incubations were performed as described for light microscopy.
The labeled thin sections were stained with uranyl acetate and lead citrate, and then examined and photographed in a JEOL 100CX or Philips CM10 transmission electron microscope at 60 kV. The negatives were scanned at 1200 ppi on an Epson Perfection V750 Pro scanner, and levels and contrast were adjusted in Photoshop.
Semiquantitative Analyses
For estimation of the immunogold labeling density x sections from y patient samples were investigated. All labeled regions of striated ducts visible in the thin sections were photographed. The labeling density (gold particles/unit length) of the apical, lateral and basal cell membranes for the four proteins (CFTR, ezrin, PKA C and RII subunits) was determined using a grid overlay technique. Using Photoshop, a grid pattern with a spacing equivalent to 0.5 μm was placed over each high magnification TEM image. The total number of intersections of the plasma membrane segment with the horizontal and vertical grid lines was counted for each micrograph. The number of membrane-associated gold particles, defined as those lying within 30 nm of either side of the membrane, was counted. The distance of 30 nm was based on the estimated size of the primary antibody-immunoglobulin-gold complex. The labeling density was calculated as N/(π/4)•I•d, where N = the number of gold particles, I = the number of membrane intersections, and d = the grid spacing (33). A total of 7-21 micrographs were counted for each protein. The mean value of the immunogold labeling of each of the three membrane domains of interest was calculated from all micrographs for each protein. These mean values were subjected to statistical analyses using the ANOVA and t-test functions in Microsoft Excel.
The labeling of small granules and vesicles in the apical cytoplasm of the duct cells also was determined. The total number of granules and vesicles, and the number of labeled granules and vesicles (gold particles lying over or within 30 nm of the granule or vesicle), were counted in each micrograph, and the percentage of labeled granules and vesicles was calculated. A total of 3-11 micrographs were counted for each protein.
Results
Light Microscopic Immunohistochemistry
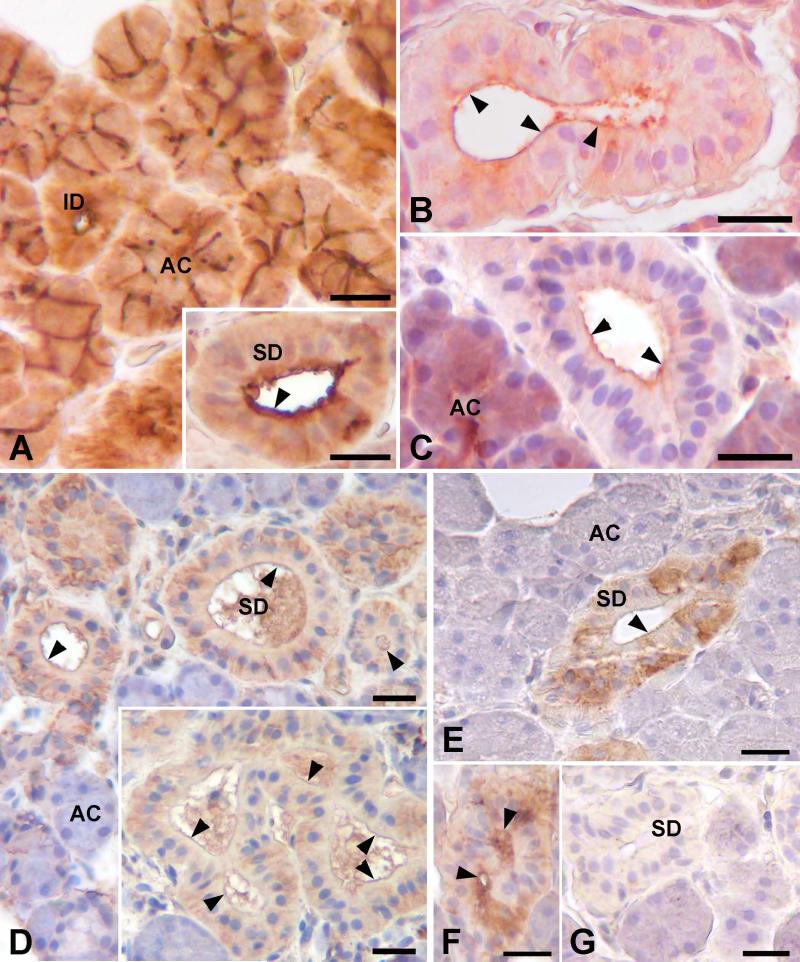
Reactivity for ezrin, PKA RII and C subunits, and CFTR was seen in striated ducts of the parotid and submandibular glands (Fig. 1). Ezrin immunolabeling was seen mainly along the luminal membranes of the acinar cells and the striated and intercalated duct cells. Additionally, the intercellular canaliculi between adjacent acinar cells were strongly labeled (Fig. 1A). The type II regulatory (RII) subunits of PKA were present in the apical cytoplasm, and occasionally in the perinuclear and basal regions of the striated duct cells (Fig. 1B, C). Increased labeling intensity was frequently seen along the luminal membrane. In acinar cells RII reactivity was strongest in the apical region; previous studies have shown that this reactivity is associated with secretory granules (23). Reactivity for the catalytic (C) subunits of PKA was observed along the luminal membrane of striated duct cells (Fig. 1D). Additionally, the basal and perinuclear regions of most duct cells showed PKA C reactivity. Reactivity for CFTR was seen in striated duct cells, but not in intercalated duct cells or acinar cells (Fig. 1E, F). Labeling was present in the apical, perinuclear and basal regions of the striated duct cells, and frequently along the luminal membrane. The endothelium of arterioles small arteries, and some capillaries and venules also showed reactivity for CFTR, as described by others (34). Control sections incubated without primary antibody or with non-immune IgG instead of primary antibody (Fig. 1G) showed no reactivity in any structures.
Figure 1.
Light microscopic localization of ezrin, PKA and CFTR in human salivary glands. A: SMG; ezrin reactivity is present around the lumen and along the intercellular canaliculi in secretory acini (AC), and around the lumen of intercalated duct (ID) cells. Inset: Parotid; ezrin is present along the luminal surface of striated duct cells (SD). B, C: In striated duct cells, RII subunits of PKA are present along the luminal membrane (arrowheads) and in the apical cytoplasm; in some cells reactivity also is present in the basal cytoplasm. In acinar cells, RII is present mainly in the apical region. B, SMG; C, parotid gland. D: SMG; C subunits of PKA are present along the luminal membrane (arrowheads) and in the basal cytoplasm of striated duct cells. E: SMG; CFTR is present along the luminal membrane (arrowheads) of striated duct cells, in the apical cytoplasm, and in the basal cytoplasm of some cells. No reactivity is present in the acini. F: Parotid; CFTR reactivity is present along the luminal membrane (arrowheads) and in the apical cytoplasm of striated duct cells. G: SMG; no labeling is seen in control sections incubated with non-immune IgG instead of primary antibody. Scale bars = 20 μm.
Electron Microscopic Immunogold Labeling
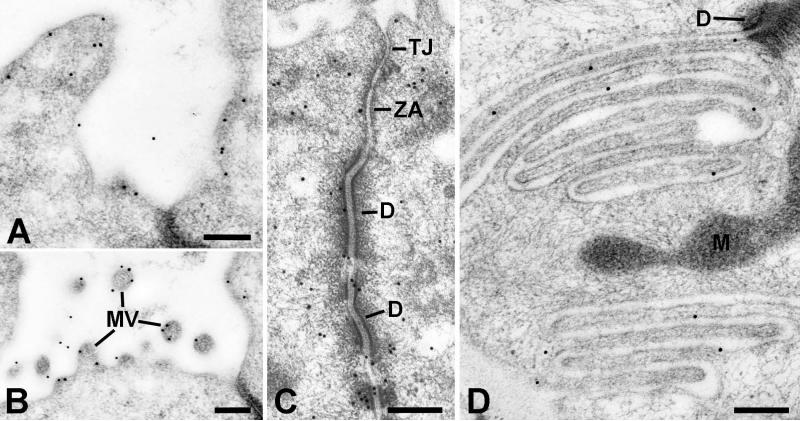
Gold particles representing ezrin were present along the luminal, lateral and basal plasma membranes of striated duct cells (Fig. 2). At the luminal surface, gold particles were mainly along the cytoplasmic face of the membrane, associated with the cortical actin network, and with the microvilli (Fig. 2A, B). Numerous gold particles were present near the junctional complexes and their cytoskeletal components (Fig. 2C). Gold particles also were present in regions of membrane infoldings and interdigitation along the lateral and basal membranes (Fig. 2D). Ezrin also was associated with the membranes of small apical vesicles and granules.
Figure 2.
Electron microscopic localization of ezrin in striated duct cells. Gold particles indicating ezrin reactivity are associated with the luminal membrane (A) and microvilli (MV) (B), the lateral membranes especially in relation to intercellular junctions and associated cytoskeletal components (C), and the basal membrane infoldings (D). Tight junction (TJ); zonula adherens (ZA); desmosome (D); mitochondrion (M). Scale bars = 0.25 μm.
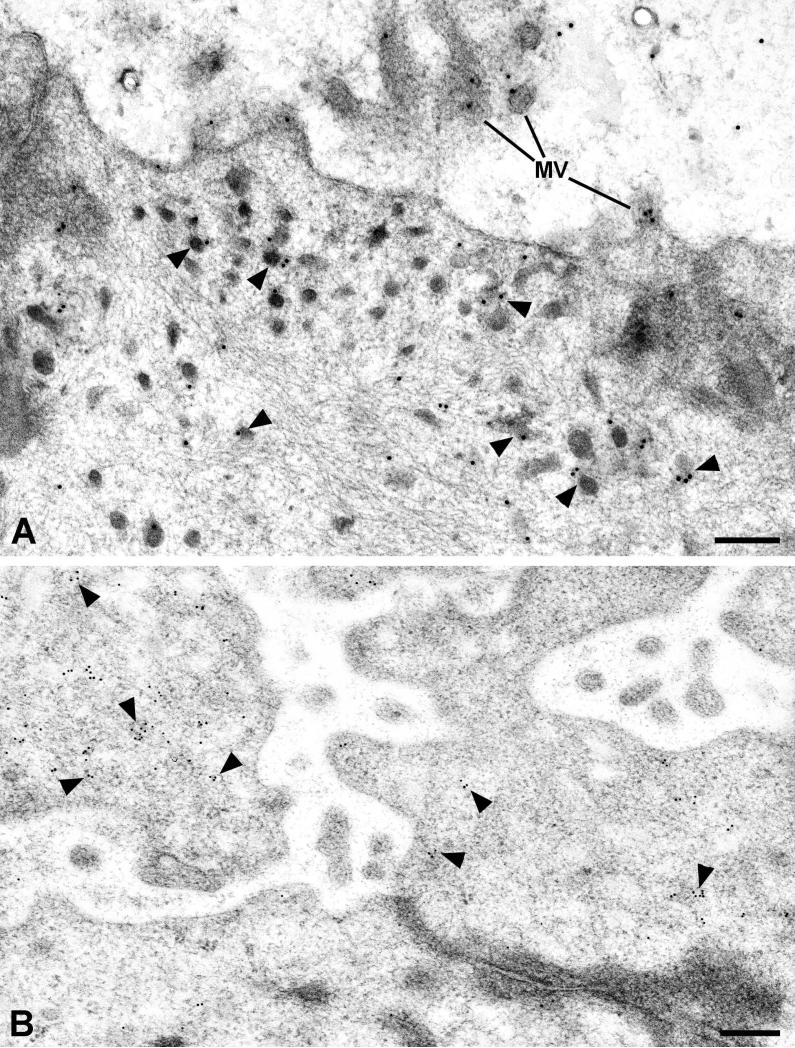
The RII subunits of PKA were present throughout the cytoplasm of the striated duct cells but were more concentrated in the apical region (Fig. 3A). Additionally, gold particles representing RII were associated with small apical granules and vesicles, as well as along the luminal membrane and associated with microvilli. The C subunits of PKA were also found throughout the cytoplasm of the striated duct cells, but with an increased concentration of gold particles associated with small vesicles in the apical cytoplasm of the duct cells (Fig. 3B).
Figure 3.
Electron microscopic localization of PKA in striated duct cells. A: RII subunits of PKA are present along the luminal membrane and microvilli (MV), and are associated with apical granules and vesicles (arrowheads). B: Catalytic (C) subunits of PKA are mainly associated with apical granules and vesicles. Scale bars = 0.25 μm.
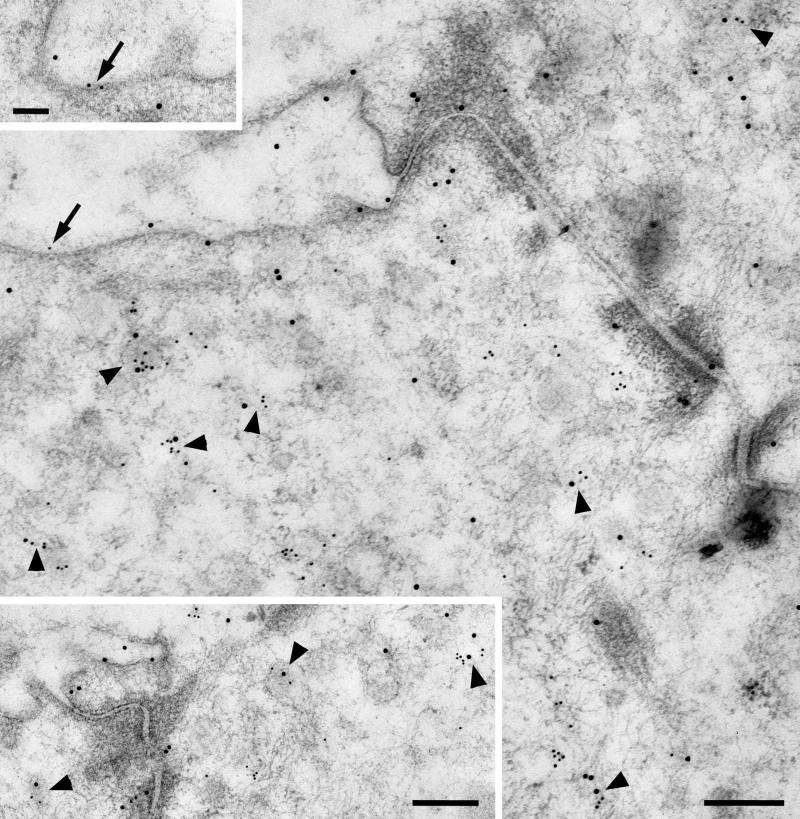
Gold particles indicating the presence of CFTR were mainly associated with small granules and vesicles in the apical cytoplasm of striated duct cells (Fig. 4). Some gold particles were associated with the luminal membrane (Fig. 4; see also Fig. 7), and to a lesser extent with the lateral and basal plasma membranes. Gold particles also were observed over saccules and vesicles of the Golgi complex (not shown). The localization of CFTR in these cytoplasmic organelles correlates with its distribution observed by light microscopic immunohistochemistry.
Figure 4.
CFTR is mainly associated with apical granules and vesicles of striated duct cells. The arrows indicate particles associated with the apical membrane. Scale bar = 0.25 μm.
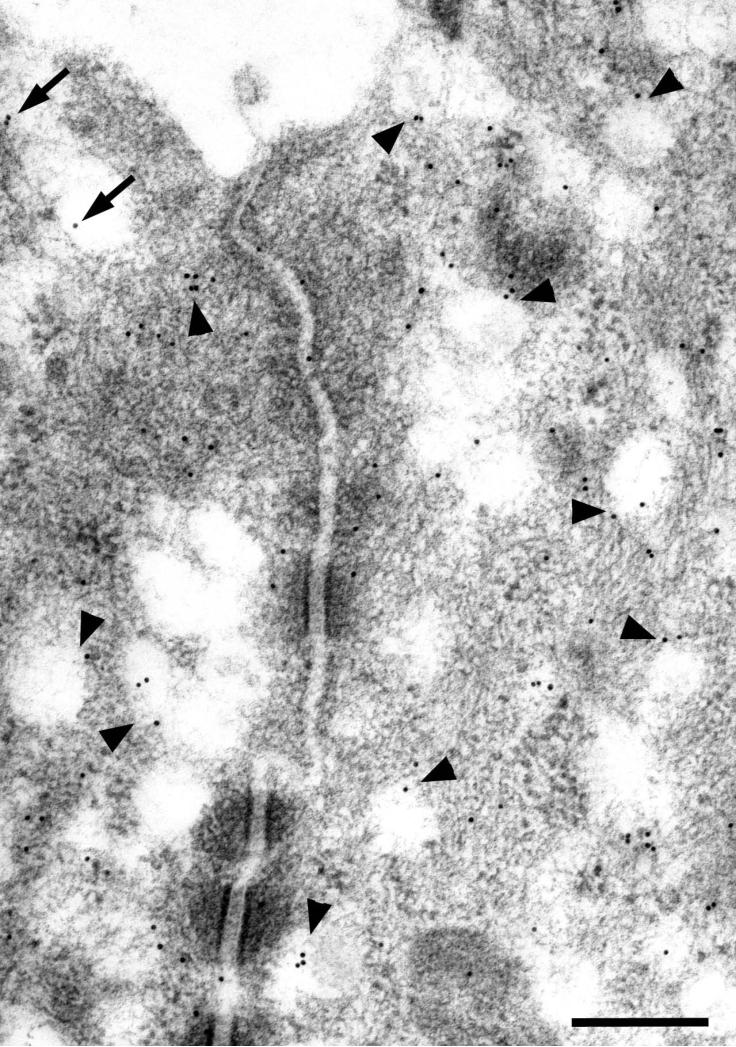
Figure 7.
Double labeling for RII (15 nm gold particles) and CFTR (10 nm gold particles) in parotid striated duct cells. RII is present along the luminal membrane and associated with apical granules and vesicles. CFTR is mainly associated with granules and vesicles; gold particles representing CFTR also are associated with the luminal membranes (arrows). Arrowheads indicate granules and vesicles labeled for both RII and CFTR. Scale bars = 0.25 μm; upper inset = 0.1 μm.
Thin sections incubated without primary antibody or with non-immune IgG instead of primary antibody served as controls (not shown). Only a few randomly scattered gold particles were seen on the sections, with no consistent relationship to cell membranes or organelles.
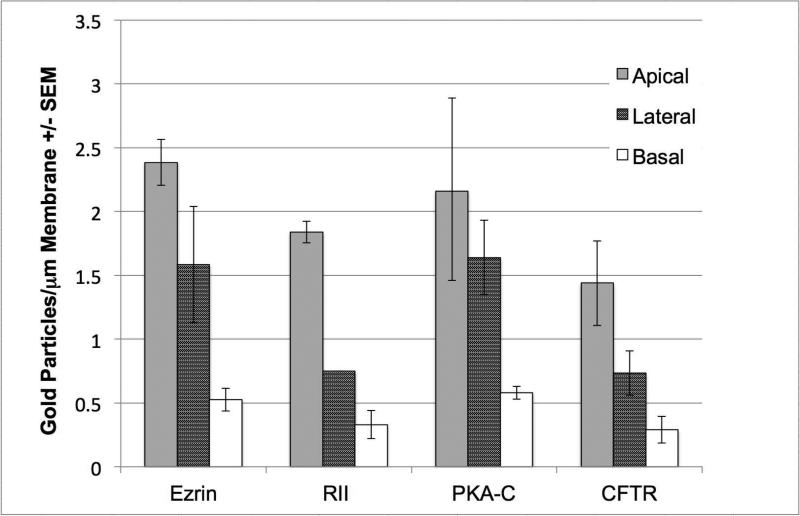
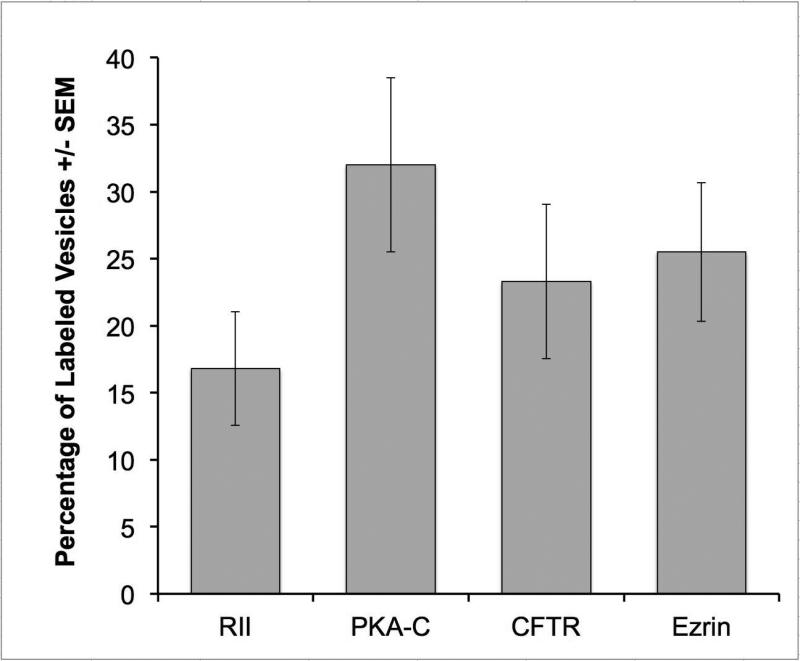
Semiquantitative analysis of the labeling density (gold particles /μm2) of striated duct cell membranes showed that the apical membrane had the highest labeling density for each of the four proteins, followed by the lateral membranes and the basal membranes (Fig. 5). The differences in calculated labeling density among the three membrane domains were significant for ezrin (p<0.001), PKA RII (p<0.001) and CFTR (p<0.05), but not for PKA C (p=0.58), as determined by ANOVA. The percentage of apical granules and vesicles labeled for each of the four proteins ranged from ~ 17% to 32% (Fig. 6). There was no difference in the percentage of vesicles labeled as determined by ANOVA (p=0.25).
Figure 5.
Immunogold labeling of striated duct cell plasma membranes. Quantitative analysis showed that the labeling density of all four proteins was greatest for the apical membrane, and that a gradual decrease in density occurs from apical to lateral to basal membranes.
Figure 6.
Percentage (± SEM) of apical granules/vesicles in striated duct cells labeled for RII, PKA-C, CFTR or ezrin.
In sections that were double labeled for PKA RII and CFTR using 15 nm and 10 nm gold particles, respectively, some small apical granules and vesicles were labeled for both proteins (Fig. 7). Other double labeling combinations (ezrin – PKA C; PKA RII – PKA C) showed some granules and vesicles also were labeled for both proteins. The percentage of double labeled vesicles was small (PKA RII – CFTR, 13%; ezrin – PKA C, 12.7%; PKA RII – PKA C, 4.5%); however, the actual proportion of vesicles with two or more of these proteins may be greater, considering that the labeling efficiencies of the various antibodies on plastic sections are less than 100%, and not all of the proteins associated with a single vesicle are exposed at the section surface and accessible to the antibodies.
Discussion
The present results indicate that ezrin, CFTR and PKA RII and C subunits are localized to the luminal membrane and apical granules and vesicles of striated duct cells of human salivary glands. CFTR is required for the reabsorption of Cl− (and for ENaC channel expression and activity) by striated duct cells (11, 13), and Na+ and Cl− concentrations are increased in saliva of cystic fibrosis patients (35, 36). Ezrin is an actin binding protein and has been shown to function as an AKAP, binding to the regulatory subunits of both type I and type II PKA (RI and RII) (24, 37). Additionally, ezrin binds to the C-terminus of CFTR via the PDZ domain-containing protein ezrin/radixin/moesin binding protein of 50 kDa/Na+/H+ exchanger regulatory factor 1 (EBP50/NHERF1) (3, 38, 39). CFTR is regulated by phosphorylation of its regulatory domain by type II PKA, and its localization and function are dependent upon interactions with ezrin, EBP50/NHERF1 and the actin cytoskeleton (3, 40, 41).
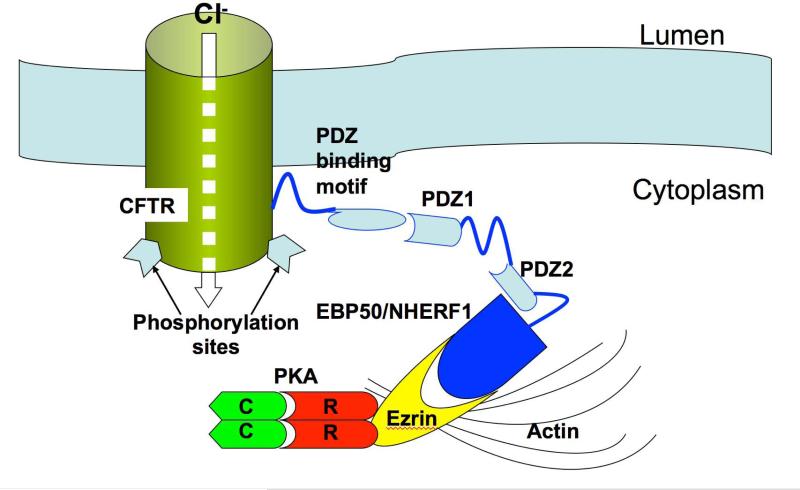
Our results show that four of the five components constituting the molecular complex required for Cl− reabsorption are present in the apical cytoplasm of salivary gland striated duct cells (Fig. 8). The localization of CFTR in striated duct cells of human salivary glands is consistent with previous studies in rats, where it was localized to the luminal membrane and apical vesicles and granules (6, 9). The presence of CFTR in elements of the Golgi complex and apical vesicles and granules most likely represents its intracellular synthetic and transport pathways. The apical granules of striated duct cells are components of the exocytic pathway; in rodents and primates they have been shown to contain kallikrein (42, 43), which is secreted into saliva by the duct cells. While some apical vesicles also may be components of the exocytic pathway, it is clear that some of them are endocytic in nature. Native and cationized ferritin infused into the duct system are taken up in small vesicles and tubules by striated duct cells (44), and apical vesicles label with antibodies to molecular markers of endosomes (6). Most of the internalized CFTR appears to be recycled back to the plasma membrane (45).
Figure 8.
Schematic depicting possible CFTR-PKA-ezrin interaction at the apical membrane of striated duct cells allowing for rapid and efficient activation of the CFTR channel. PKA is anchored to ezrin via its RII subunits; ezrin in turn binds to EBP50/NHERF1, which has 2 PDZ domains that interact with the PDZ binding motif at the C-terminus of CFTR. Ezrin also binds to actin, which stabilizes the entire complex near the luminal membrane. Not shown are the heterotrimeric Gs protein (48) and other components of signaling pathways demonstrated (in other systems) or presumed to be involved with CFTR localization and activation (β-adrenergic receptor, P2Y2 receptor, adenylate cyclase, protein kinase C, etc.).
Ezrin was shown to be present at the luminal surface of acinar cells and ducts of human labial salivary glands (46). In the acinar cells ezrin was mainly associated with microvilli on the luminal cell surface. Our results confirm the localization of ezrin to the luminal surface of the acinar cells. Additionally, we show that it is associated with the cytoskeleton of the luminal and basolateral membranes, the microvilli and the junctional complexes of striated duct cells. This distribution is consistent with the actin binding function of ezrin. Furthermore, its association with the luminal membrane places it in the location necessary for anchoring PKA in position to phosphorylate luminal membrane proteins, such as CFTR.
Protein kinase A functions to regulate many cellular processes. A-kinase anchoring proteins serve to localize PKA to specific cytoplasmic regions and ensure specificity of substrate phosphorylation (22). The presence of PKA RII and C subunits in several cytoplasmic regions of striated duct cells presumably reflects binding to other AKAPs and their role in a variety of cellular functions. We previously demonstrated that PKA RII is present in the secretory granules of human salivary gland acinar cells (23), and is secreted into saliva (47). The present results confirm that PKA RII is associated with small secretory granules and vesicles in the apical cytoplasm of human striated duct cells (23), as well as with the apical cell membrane. We now show that PKA C subunits also are associated with these small granules and vesicles. These results indicate that the PKA holoenzyme is appropriately localized to function in the regulation of electrolyte reabsorption by striated duct cells.
Activation of PKA, resulting in dissociation of its R and C subunits and leading to substrate phosphorylation, requires increased local concentration of cyclic AMP, its binding to the R subunits and dissociation of the holoenzyme. We recently have shown that the alpha subunit of the heterotrimeric Gs signaling protein is present in the apical region of mouse striated duct cells (48), and previous studies demonstrated that stimulation by isoproterenol, norepinephrine and forskolin resulted in increased cyclic AMP content in isolated rabbit striated ducts (49, 50). Thus, in some mammalian species, and probably in humans, striated duct cells express the components of the cyclic AMP signaling pathway, β-adrenergic receptors, Gs, and adenylate cyclase, necessary to increase cyclic AMP levels and activate PKA. Immunohistochemical studies of human sweat gland duct cells (51) and airway epithelial cells (52, 53) have shown that several components of the cyclic AMP-PKA signaling pathway are present with CFTR along the apical cell membranes.
Other proteins also may participate in the regulation of CFTR in striated duct cells. Regulation of CFTR may occur by extracellular ATP activation of P2Y2 receptors and phosphorylation by protein kinase C, both of which are present in salivary gland duct cells (12, 54, 55). Shank2, a PDZ domain-containing protein, binds to the C-terminal of CFTR and to the cyclic AMP-specific phosphodiesterase 4D (PDE4D) (3, 56, 57). Modulation of cyclic AMPPKA signaling by Shank2-PDE4D may provide an additional mechanism for regulation of CFTR activity. However, neither of these proteins, or other kinases and phosphatases shown to associate with and regulate CFTR activity (58), have been demonstrated in human striated duct cells.
Mutations involving CFTR or its interacting partners causing mislocalization and/or altered activity result in altered salivary gland function. Patients with cystic fibrosis exhibit changes in salivary electrolyte and protein composition (36, 59). The present results provide new information on the localization and compartmentalization of signaling proteins that lead to the selective activation of CFTR in striated duct cells of human salivary glands. Further studies should explore the localization and function of other scaffolding and signaling proteins that may regulate CFTR localization and activity in these cells.
Acknowledgments
We are grateful to Professor Alessandro Riva and Dr. Marco Piludu, University of Cagliari, for their generosity in providing the tissue samples. We thank Ms. Maya Yankova for excellent technical assistance. This research was supported by NIH grant T32DE007302, and the University of Connecticut Health Center.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest
References
- 1.WELSH MJ, BOAT TF, TSUI L-C, BEAUDET AL. Cystic Fibrosis. In: Seriven CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. McGraw Hill; New York, NY: 1995. pp. 3799–3876. [Google Scholar]
- 2.FRIZZELL RA, HANRAHAN JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med. 2012 doi: 10.1101/cshperspect.a009563. doi: 10.1101/cshperspect.a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LI C, NAREN AP. CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners. Integr Biol (Camb) 2010;2:161–177. doi: 10.1039/b924455g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PRANKE IM. SERMET-GAUDELUS I. Biosynthesis of cystic fibrosis transmembrane conductance regulator. Int J Biochem Cell Biol. 2014 doi: 10.1016/j.biocel.2014.03.020. http://dx.doi.org/10.1016/j.biocel.2014.03.020. [DOI] [PubMed]
- 5.CANT N, POLLOCK N, FORD RC. CFTR structure and cystic fibrosis. Int J Biochem Cell Biol. 2014 doi: 10.1016/j.biocel.2014.02.004. http://dx.doi.org/10.1016/j.biocel.2014.02.004. [DOI] [PubMed]
- 6.WEBSTER P, VANACORE L, NAIRN AC, MARINO CR. Subcellular localization of CFTR to endosomes in a ductal epithelium. Am J Physiol (Cell Physiol) 1994;267:C340–C348. doi: 10.1152/ajpcell.1994.267.2.C340. [DOI] [PubMed] [Google Scholar]
- 7.ZEIHER BG, EICHWALD E, ZABNER J, SMITH JJ, PUGA AP, MCCRAY PB, JR, CAPECCHI MR, WELSH MJ, THOMAS KR. A mouse model for the ΔF508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ZENG W, LEE MG, YAN M, DIAZ J, BENJAMIN I, MARINO CR, KOPITO R, FREEDMAN S, COTTON C, MUALLEM S, THOMAS P. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol (Cell Physiol) 1997;1997;273:C442–C455. doi: 10.1152/ajpcell.1997.273.2.C442. [DOI] [PubMed] [Google Scholar]
- 9.BANNYKH SI, BANNYKH GI, FISH KN, MOYER BD, RIORDAN JR, BALCH WE. Traffic pattern of cystic fibrosis transmembrane regulator through the early exocytic pathway. Traffic. 2000;1:852–870. doi: 10.1034/j.1600-0854.2000.011105.x. [DOI] [PubMed] [Google Scholar]
- 10.KULAKSIZ H, REHBERG E, STREMMEL W, CETIN Y. Guanylin and functional coupling proteins in the human salivary glands and gland tumors. Am J Pathol. 2002;161:655–664. doi: 10.1016/S0002-9440(10)64221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ISHIBASHI K, YAMAZAKI J, OKAMURA K, TENG Y, KITAMURA K, ABE K. Roles of CLCA and CFTR in electrolyte re-absorption from rat saliva. J Dent Res. 2006;85:1101–1105. doi: 10.1177/154405910608501207. [DOI] [PubMed] [Google Scholar]
- 12.ISHIBASHI K, OKAMURA K, YAMAZAKI J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl− reabsorption in rat submandibular gland. Am J Physiol (Integr Comp Physiol) 2008;294:R1729–1736. doi: 10.1152/ajpregu.00758.2007. [DOI] [PubMed] [Google Scholar]
- 13.CATALÁN MA, NAKAMOTO T, GONZALEZ-BEGNE M, CAMDEN JM, WALL SM, CLARKE LL, MELVIN JE. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol. 2010;588:713–724. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.REDDY MM, LIGHT MJ, QUINTON PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- 15.BERDIEV BK, CORMET-BOYAKA E, TOUSSON A, QADRI YJ, OOSTERVELDHUT HM, HONG JS, GONZALES PA, FULLER CM, SORSCHER EJ, LUKACS GL, BENOS DJ. Molecular proximity of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel assessed by fluorescence resonance energy transfer. J Biol Chem. 2007;282:36481–36488. doi: 10.1074/jbc.M708089200. [DOI] [PubMed] [Google Scholar]
- 16.RIORDAN JAR, ROMMENS JM, KEREM B, ALON N, ROZMAHEL R, GRZELCZAK Z, ZIELENSKI J, LOK S, PLAVSIC N, CHOU J-L, DRUMM ML, IANNUZZI MC, COLLINS FS, TSUI L-C. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:166–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 17.ANDERSON MP, BERGER HA, RICH DP, GREGORY RJ, SMITH AE, WELSH MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 18.BDOLAH A, SCHRAMM M. The function of 3′5′ cyclic AMP in enzyme secretion. Biochem Biophys Res Commun. 1965;18:452–454. doi: 10.1016/0006-291x(65)90730-8. [DOI] [PubMed] [Google Scholar]
- 19.BUTCHER FR, PUTNEY JW., JR Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. [PubMed] [Google Scholar]
- 20.MEDNIEKS MI, HAND AR. Cyclic AMP-dependent protein kinase in stimulated rat parotid gland cells: compartmental shifts after in vitro treatment with isoproterenol. Eur J Cell Biol. 1982;28:264–271. [PubMed] [Google Scholar]
- 21.HORIO B, DOWD F, WATSON E, MEDNIEKS M, WARREN J. Isoproterenol-induced amylase release in rabbit parotid acini: relation of protein phosphorylation, cyclic AMP and related kinase activity to changes in secretory rate. J Pharmacol Exp Ther. 1984;229:608–614. [PubMed] [Google Scholar]
- 22.WELCH EJ, JONES BW, SCOTT JD. Networking with AKAPs. Context-dependent regulation of anchored enzymes. Mol Intervent. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.PILUDU M, MEDNIEKS MI, HAND AR. Cyclic AMP – receptor proteins in human salivary glands. Eur J Morphol. 2002;40:219–225. doi: 10.1076/ejom.40.4.219.16696. [DOI] [PubMed] [Google Scholar]
- 24.DRANSFIELD D, BRADFORD A, SMITH J, MARTIN M, ROY C, MANGEAT P, GOLDENRING J. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.URUSHIDANI T, FORTE JG. Signal transduction and activation of acid secretion in the parietal cell. J Memb Biol. 1997;159:99–111. doi: 10.1007/s002329900274. [DOI] [PubMed] [Google Scholar]
- 26.DING X, DENG H, WANG D, ZHOU J, HUANG Y, ZHAO X, YU X, WANG M, WANG F, WARD T, AIKHIONBARE F, YAO X. Phospho-regulated ACAP4-Ezrin interaction is essential for histamine-stimulated parietal cell secretion. J Biol Chem. 2010;285:18769–18780. doi: 10.1074/jbc.M110.129007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.BRETSCHER A, EDWARDS K, FEHON RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 28.NEISCH AL, FEHON RG. Ezrin, Radixin and Moesin: key regulators of membrane-cortex interactions and signaling. Curr Opin Cell Biol. 2011;23:377–382. doi: 10.1016/j.ceb.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SUN F, HUG MJ, BRADBURY NA, FRIZZELL RA. Protein kinase A associates with cystic fibrosis transmembrane conductance regulator via an interaction with ezrin. J Biol Chem. 2000;275:14360–14366. doi: 10.1074/jbc.275.19.14360. [DOI] [PubMed] [Google Scholar]
- 30.SZCZEPANSKI A, MEDNIEKS MI, HAND AR. Expression and distribution of parotid secretory proteins in experimental diabetes. Eur J Morphol. 1998;36(Suppl):240–246. [PubMed] [Google Scholar]
- 31.MEDNIEKS M, LIN M, HAND AR. Immunocytochemical analysis of cyclic AMP receptor proteins in the developing rat parotid gland. Arch Oral Biol 2008. 2008;53:429–436. doi: 10.1016/j.archoralbio.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 32.HAND AR. Electron Microscopy. In: Glasel JA, Deutscher MP, editors. Introduction to biophysical methods for protein and nucleic acid research. Academic Press; San Diego, CA: 1995. pp. 205–260. [Google Scholar]
- 33.GRIFFITHS G. Fine structure immunocytochemistry. Springer-Verlag; Berlin, Heidelberg: 1993. [Google Scholar]
- 34.TOUSSON A, VAN TINE BA, NAREN AP, SHAW GM, SCHWIEBERT LM. Characterization of CFTR expression and chloride channel activity in human endothelia. Am J Physiol (Cell Physiol) 1998;275:C1555–C1564. doi: 10.1152/ajpcell.1998.275.6.C1555. [DOI] [PubMed] [Google Scholar]
- 35.JOHNSTON WH. Salivary electrolytes in fibrocystic disease of the pancreas. Arch Dis Child. 1956;31:477–480. doi: 10.1136/adc.31.160.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GONÇALVES AC, MARSON FA, MENDONÇA RM, RIBEIRO JD, RIBEIRO AF, PASCHOAL IA, LEVY CE. Saliva as a potential tool for cystic fibrosis diagnosis. Diagn Pathol. 2013;8:46. doi: 10.1186/1746-1596-8-46. doi: 10.1186/1746-1596-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.RUPPELT A, MOSENDEN R, GRÖNHOLM M, AANDAHL EM, TOBIN D, CARLSON CR, ABRAHAMSEN H, HERBERG FW, CARPÉN O, TASKÉN K. Inhibition of T cell activation by cyclic adenosine 5′-monophosphate requires lipid raft targeting of protein kinase A type I by the A-kinase anchoring protein ezrin. J Immunol. 2007;179:5159–5168. doi: 10.4049/jimmunol.179.8.5159. [DOI] [PubMed] [Google Scholar]
- 38.SHORT DB, TROTTER KW, RECZEK D, KREDA SM, BRETSCHER A, BOUCHER RC, STUTTS MJ, MILGRAM SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 39.LEE MG, CHOI JY, LUO W, STRICKLAND E, THOMAS PJ, MUALLEM S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl−/HCO −3 exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- 40.RAGHURAM V, MAK D-OD, FOSKETT JK. Regulation of cystic fibrosis transmembrane conductance regulator single-channel gating by bivalent PDZ-domain-mediated interaction. Proc Natl Acad Sci USA. 2001;98:1300–1305. doi: 10.1073/pnas.031538898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MONTERISI S, CASAVOLA V, ZACCOLO M. Local modulation of cystic fibrosis conductance regulator: cytoskeleton and compartmentalized cAMP signalling. Brit J Pharmacol. 2013;169:1–9. doi: 10.1111/bph.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SIMSON JA, FENTERS R, CHAO J. Electron microscopic immunostaining of kallikrein in rat submandibular glands. J Histochem Cytochem. 1983;31:301–306. doi: 10.1177/31.2.6339607. [DOI] [PubMed] [Google Scholar]
- 43.YAHIRO J, NAGATO T. Distribution of kallikrein in striated duct cells of monkey submandibular glands. Arch Oral Biol. 2002;47:631–635. doi: 10.1016/s0003-9969(02)00056-0. [DOI] [PubMed] [Google Scholar]
- 44.COLEMAN R, HAND AR. Endocytosis of native and cationized ferritin by intralobular duct cells of the rat parotid gland. Cell Tissue Res. 1987;249:577–586. doi: 10.1007/BF00217329. [DOI] [PubMed] [Google Scholar]
- 45.GENTZSCH M, CHANG XB, CUI L, WU Y, OZOLS VV, CHOUDHURY A, PAGANO RE, RIORDAN JR. Endocytic trafficking routes of wild type and DeltaF508 cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2004;15:2684–2696. doi: 10.1091/mbc.E04-03-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.PEREZ P, AGUILERA S, OLEA N, ALLIENDE C, MOLINA C, BRITO M, BARRERA M-J, LEYTON C, ROWZEE A, GONZÁLEZ M-J. Aberrant localization of ezrin correlates with salivary acini disorganization in Sjögren's syndrome. Rheumatol. 2010;49:915–923. doi: 10.1093/rheumatology/keq033. [DOI] [PubMed] [Google Scholar]
- 47.MEDNIEKS MI, HAND AR. Cyclic AMP binding proteins in saliva. Experientia. 1984;40:945–947. doi: 10.1007/BF01946451. [DOI] [PubMed] [Google Scholar]
- 48.HAND AR, ELDER KO, NORRIS RP. Redistribution of Gαs in mouse salivary glands following β-adrenergic stimulation. Arch Oral Biol. doi: 10.1016/j.archoralbio.2015.01.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.EVANS RL, LAU KR, CASE RM. Structural and functional characterization of striated ducts isolated from the rabbit mandibular salivary gland. Exp Physiol. 1993;78:49–64. doi: 10.1113/expphysiol.1993.sp003670. [DOI] [PubMed] [Google Scholar]
- 50.EVANS RL, PERROTT MN, LAU KR, CASE RM. Elevation of intracellular cAMP by noradrenaline and vasoactive intestinal peptide in striated ducts isolated from the rabbit mandibular salivary gland. Arch Oral Biol. 1996;41:689–694. doi: 10.1016/s0003-9969(96)00028-3. [DOI] [PubMed] [Google Scholar]
- 51.REDDY MM, SUN D, QUINTON PM. Apical heterotrimeric G-proteins activate CFTR in the native sweat duct. J Memb Biol. 2001;179:51–61. doi: 10.1007/s002320010036. [DOI] [PubMed] [Google Scholar]
- 52.BOSSARD F, SILANTIEFF E, LAVAZAIS-BLANCOU E, ROBAY A, SAGAN C, ROZEC B, GAUTHIER C. β1, β2, and β3 adrenoceptors and Na+/H+ exchanger regulatory factor 1 expression in human bronchi and their modifications in cystic fibrosis. Am J Respir Cell Mol Biol. 2011;44:91–98. doi: 10.1165/rcmb.2009-0372OC. [DOI] [PubMed] [Google Scholar]
- 53.NAREN AP, COBB B, LI C, ROY K, NELSON D, HEDA GD, LIAO J, KIRK KL, SORSCHER EJ, HANRAHAN J, CLANCY JP. A macromolecular complex of β2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA. 2003;100:342–346. doi: 10.1073/pnas.0135434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.TÖRNWALL J, KONTTINEN YT, TUOMINEN RK, TÖRNWALL M. Protein kinase C expression in salivary gland acinar epithelial cells in Sjögren’s syndrome. Lancet. 1997;349:1814–1815. doi: 10.1016/s0140-6736(05)61694-7. [DOI] [PubMed] [Google Scholar]
- 55.TURNER JT, LANDON LA, GIBBONS SJ, TALAMO BR. Salivary Gland P2 Nucleotide Receptors. Crit Rev Oral Biol Med. 1999;10:210–224. doi: 10.1177/10454411990100020701. [DOI] [PubMed] [Google Scholar]
- 56.KIM JY, HAN W, NAMKUNG W, LEE JH, KIM KH, SHIN H, KIM E, LEE MG. Inhibitory regulation of cystic fibrosis transmembrane conductance regulator anion-transporting activities by Shank2. J Biol Chem. 2004;279:10389–10396. doi: 10.1074/jbc.M312871200. [DOI] [PubMed] [Google Scholar]
- 57.LEE JH, RICHTER W, NAMKUNG W, KIM KH, KIM E, CONTI M, LEE MG. Dynamic regulation of cystic fibrosis transmembrane conductance regulator by competitive interactions of molecular adaptors. J Biol Chem. 2007;282:10414–10422. doi: 10.1074/jbc.M610857200. [DOI] [PubMed] [Google Scholar]
- 58.KUNZELMANN K, MEHTA A. CFTR: a hub for kinases and crosstalk of cAMP and Ca2+. FEBS J. 2013;280:4417–4429. doi: 10.1111/febs.12457. [DOI] [PubMed] [Google Scholar]
- 59.LIVNAT G, BENTUR L, KUZMISNSKY E, NAGLER RM. Salivary profile and oxidative stress in children and adolescents with cystic fibrosis. J Oral Pathol Med. 2010;39:16–21. doi: 10.1111/j.1600-0714.2009.00813.x. [DOI] [PubMed] [Google Scholar]